Abstract
M2-polarized macrophages, also known as alternatively activated macrophages, have long been associated with pulmonary fibrosis; however, the mechanism has not been fully defined. Gab1 and Gab2 proteins belong to the Gab family of adaptors and are integral components of the signal specificity in response to various extracellular stimuli. In this report, we found that levels of both Gab1 and Gab2 were elevated in M2-polarized macrophages isolated from bleomycin-induced fibrotic lungs. In vitro Gab1/2 deficiency in bone marrow-derived macrophages abrogated IL-4–mediated M2 polarization. Furthermore, in vivo conditional removal of Gab1 (Gab1MyKO) and germ line knock-out of Gab2 (Gab2−/−) in macrophages prevented a bias toward the M2 phenotype and attenuated bleomycin-induced fibrotic lung remodeling. In support of these observations, Gab1/2 were involved in responses predominated by IL-4 signaling, an essential determinant for macrophage M2 polarization. Further investigation revealed that both Gab1 and -2 are recruited to the IL-4 receptor, synergistically enhancing downstream signal amplification but conferring IL-4 signal preference. Mechanistically, the loss of Gab1 attenuated AKT activation, whereas the absence of Gab2 suppressed STAT6 activation in response to IL-4 stimulation, both of which are commonly attributed to M2-driven pulmonary fibrosis in mice. Taken together, these observations define a non-redundant role of Gab docking proteins in M2 polarization, adding critical insights into the pathogenesis of idiopathic pulmonary fibrosis.
Keywords: adaptor protein, Akt PKB, macrophage, pulmonary fibrosis, STAT transcription factor, AKT signaling, Gabs, M2 polarization, STAT6 signaling
Introduction
Idiopathic pulmonary fibrosis (IPF)3 is a chronic interstitial disease of unknown etiology that is characterized by alveolar inflammation, a Th2-cell cytokine milieu, and excess collagen deposition, leading to respiratory failure and death (1–3). However, the mechanisms underlying this devastating progression remain largely elusive. Macrophages are the predominant innate immune cells in the lung, serving as important regulators of lung injury and repair (4). In response to injury, macrophages demonstrate remarkable plasticity and are capable of acquiring alternatively activated polarization (M2), which is characterized by producing matrix metalloproteases and secreting chemokines, regulating Th2 and fibrotic progression (4–6). It has been demonstrated that M2 markers associate with pulmonary fibrosis in a mouse model, suggesting an important role for M2 macrophages in the development of pulmonary fibrosis (4, 7–9). The M2 marker Arg1 (arginase 1) regulates the production of proline, which is required for collagen synthesis, yet recent studies indicate that Arg1 is not essential for regulation of the proline–collagen pathway (10). Additional bleomycin (BLM)-induced mouse experiments indicate that FIZZ1 (found in inflammatory zone 1) promotes the differentiation and survival of myofibroblasts during fibrogenesis. FIZZ1-deficient mice exhibit ameliorated fibrosis in lung tissue (11, 12). Recent clinical findings indicate that the expression of Ym1 (chitinase-3–like protein 3) and CD206 (mannose receptor) are also elevated in alveolar macrophages isolated from patients with IPF (13, 14). Taken together, these findings highlight the important role of M2 macrophages in the development of pulmonary fibrosis.
M2 polarization is driven by the products of Th2 cells, among which IL-4 is well demonstrated to be an important determinant controlling the process (14–16). Upon IL-4 stimulation, engagement of the IL-4 receptor (IL-4R) by IL-4 subsequently activates its two major downstream signals, JAK1/STAT6 and PI3K/AKT, in M2-polarized macrophages (17, 18). The outcome of this process results in enhanced levels of Arg1, FIZZ1, Ym1, and CD206, which are also clinically featured in IPF (8, 14, 19). Nevertheless, much work is needed to unveil the signal integration in M2-driven fibrosis.
The Grb2-associated binder (Gab) proteins, which belong to the Gab/DOS family of docking proteins, function as important elements in signal integration during the assembly of downstream signaling complexes (20, 21). The Gab proteins, Gab1, Gab2, and Gab3, have been identified in mammals, DOS (daughter of sevenless) has been identified in Drosophila, and Soc1 (suppressor-of-clear) has been identified in Caenorhabditis elegans (22–24). All Gab docking molecules are characterized by a highly conserved pleckstrin homology domain and a proline-rich domain. Although lacking enzymatic activity itself, Gab contains multiple tyrosine-based activation motifs for binding kinase and phosphatase, through which it confers signal diversity and specificity (20, 21, 25–27).
Gab1 and Gab2 (Gab1/2) are widely expressed in various cell types, including immune cells such as T cells, macrophages, and mast cells (28). In response to multiple extracellular signals, Gab1/2 are rapidly phosphorylated and coordinate the signaling cascades, leading to the altered cellular functions or phenotypes. Gab1/2 have a potential functional redundancy due to their homology and similar expression patterns (29–33). Recent studies have demonstrated that cardiomyocyte-specific Gab1/2 KO mice show high mortality after birth due to heart failure, suggesting the crucial roles of Gab1/2 in the maintenance of cardiac functions (29). In addition, both Gab1 and Gab2 are reported to participate in EGF-mediated MAPK/ERK signaling (33). Despite their similarity of function, many lines of evidence clearly demonstrate distinct roles for Gab1/2 under certain pathophysiological conditions. Germ line knock-out of Gab1 in mice is embryonic lethal due to defects in the heart, liver, muscle, and placenta (34, 35). In contrast, Gab2 KO mice are viable and generally healthy with only mild defects in the allergic response and osteoclastogenesis (36–38). In addition, both Gab1 and Gab2 promote endothelial cell migration in response to VEGF. However, contrary to Gab1, Gab2 depletion surprisingly results in the increased activation of AKT (39, 40). We previously reported that Gab1 enhances allergic asthma by promoting dendritic cell migration (41), whereas Gab2 is involved in asthma by positively regulating goblet cell hyperplasia and mucus secretion in the airway epithelium (42).
In the present study, we observed that the expression of Gab1 and Gab2 are enhanced in alveolar macrophages isolated from BLM-induced fibrotic mice. In vitro and in vivo investigations demonstrated that the deficiency of either Gab1 or Gab2 could result in impaired M2 polarization. Furthermore, myeloid-specific Gab1 KO mice and Gab2 KO mice exhibited significantly attenuated pulmonary fibrosis induced by BLM. However, distinct molecular mechanisms are used by Gab1 and Gab2 to regulate IL-4–induced macrophage polarization, with Gab1 positively regulating AKT signaling and Gab2 positively regulating STAT6 signaling. Our findings reveal a novel, non-redundant mechanism for Gab1/2 in modulating macrophage function as well as their regulatory roles in mediating the pathogenesis of pulmonary fibrosis.
Results
Gab1/2 are highly expressed in M2-polarized macrophage isolated from fibrotic lungs
To probe the possible role of Gab1/2 in pulmonary fibrosis, we examined the levels of Gab1/2 in alveolar macrophages harvested from BLM-treated mice, which is a widely used animal model of pulmonary fibrosis (43). It was evident that Gab1/2 expression were elevated at both the mRNA and protein levels compared with SAL-treated mice (Fig. 1 (A and B) and supplemental Fig. 1 (A and B)). These alveolar macrophages were characterized by enriched M2-polarized macrophages, which exhibited the canonical CD206 positivity and a strong up-regulation of M2 markers, including Arg1, FIZZ1, and Ym1 (Fig. 1, C and D). Both Gab1 and Gab2 are ubiquitously and relatively abundantly expressed. Nevertheless, Gab1 germ line knock-out is lethal in embryos (34, 35). Therefore, our strategy was to develop a conditionally deleted Gab1 system (Gab1MyKO) to probe Gab1 function in macrophages. In contrast, complete Gab2 knockouts appear normal and are generally healthy (36–38). Given the fact that Gab1 expression appears earlier and more abundant than Gab2, it is believed that loss of Gab2 is probably compensated somewhat by Gab1 or other Gab members. To determine whether there is a putative compensation between Gab1 and Gab2 in macrophages, which was previously reported but remains controversial, we detected the levels of Gab1/2 in Gab1MyKO and Gab2−/− mice. As shown in Fig. 1 (E and F), deletion of Gab1 in bone marrow-derived macrophages (BMDMs) resulted in unaltered Gab2 expression (Fig. 1E), whereas the loss of Gab2 resulted in a slight increase in Gab1 at both the mRNA and protein levels (Fig. 1F). This observation implies the synergistic role of Gab1/2 in macrophages. Overall, these data suggest that Gab1/2 are potentially involved in M2 polarization, which regulates the progression of pulmonary fibrosis.
Figure 1.
Elevated levels of Gab1/2 in M2-polarized macrophages. A, the mRNA expression levels of Gab1/2 (relative to β-actin) in alveolar macrophages obtained from SAL- or BLM-treated mice (n = 6/group) were assessed by qPCR. B, Gab1/2 protein levels in alveolar macrophages obtained from SAL- or BLM-treated mice were determined by immunoblotting analysis. Each sample represents three mice. C, representative results of the immunostaining of CD206 (red)/DAPI (blue) in lung sections from SAL- and BLM-challenged mice (n = 6/group). Scale bars, 25 μm. D, analysis of Arg1, FIZZ1, and Ym1 mRNA expression levels by qPCR in alveolar macrophages from SAL- and BLM-treated mice (n = 6/group). E, protein (top) and mRNA (bottom) levels of Gab2 were measured in BMDMs from Gab1f/f and Gab1MyKO mice. F, protein (top) and mRNA (bottom) levels of Gab1 were measured in BMDMs from Gab2+/− and Gab2−/− mice. Data from three independent experiments are shown. Error bars, S.D. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Loss of Gab1/2 in macrophages impairs IL-4–induced M2 polarization ex vivo
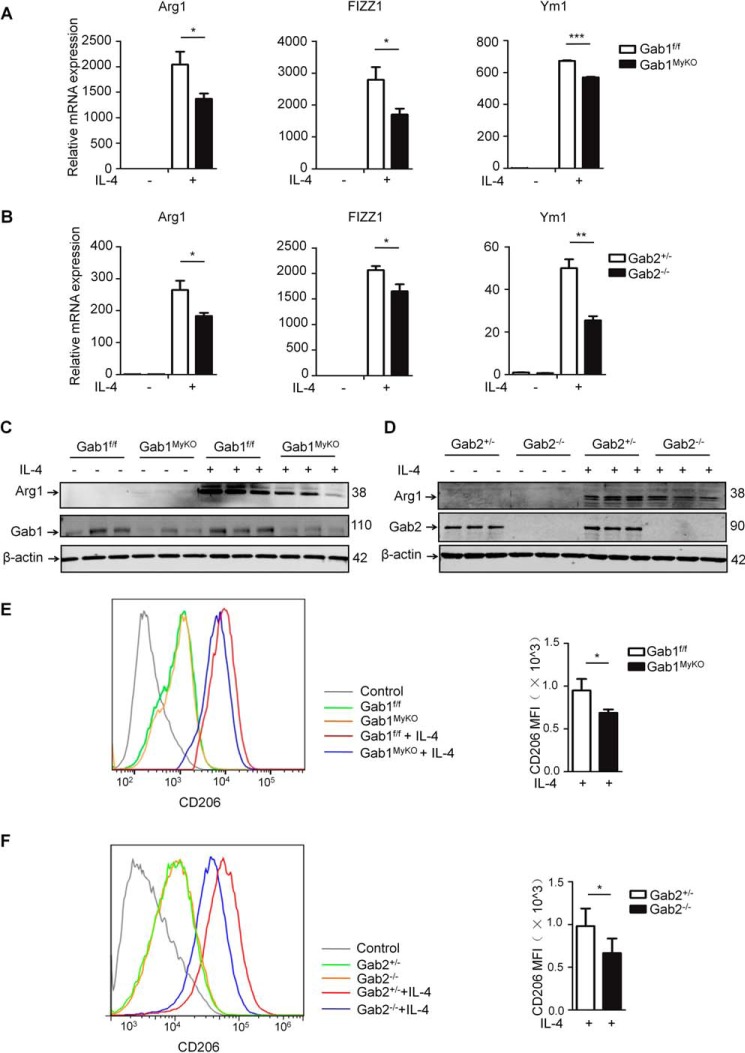
To further investigate the roles of Gab1 and Gab2 in macrophage M2 polarization, BMDMs were isolated from Gab1f/f, Gab1MyKO, Gab2+/−, and Gab2−/− mice and then stimulated with IL-4, followed by M2 assessment. As indicated in Fig. 2 (A and B), both Gab1 and Gab2 deficiency in macrophages impaired M2 activation with a substantial decrease in the expression of Arg1, FIZZ1, and Ym1 compared with the controls. Consistent with this finding, the reduction of Arg1 levels in Gab1/2 deficiency was confirmed using immunoblotting and immunofluorescence assays (Fig. 2 (C and D) and supplemental Fig. 2 (A and B)). We also found that the IL-4-mediated up-regulation of CD206 was attenuated in Gab1MyKO and Gab2−/− BMDMs (Fig. 2, E and F). Additionally, IL-4Rα expression was unaltered in Gab1MyKO and Gab2−/− BMDMs (supplemental Fig. 3, A and B), and the loss of Gab1/2 had no effect on macrophage viability, as evaluated by annexin V-propidium iodide staining (supplemental Fig. 3, C and D). Collectively, these ex vivo observations support a positive role of Gab1/2 in the control of macrophage M2 polarization.
Figure 2.
Gab1/2-deficient BMDMs exhibit reduced M2 polarization. To obtain BMDMs, BMs from Gab1f/f, Gab1MyKO, Gab2+/−, and Gab2−/− mice were cultured with M-CSF for 7 days. A and B, after stimulation with IL-4 (20 ng/ml) for 24 h, the Arg1, FIZZ1, and Ym1 mRNA expression levels in BMDMs were measured by qPCR. C and D, expression of Arg1 protein was assessed by immunoblotting analysis in IL-4-stimulated BMDMs. E and F, surface expression of CD206 was evaluated in IL-4-stimulated BMDMs by flow cytometry analysis. A representative graph (left) and statistical graph (right) are shown. Data from three independent experiments are shown. Error bars, S.D.*, p < 0.05; **, p < 0.01; ***, p < 0.001. MFI, mean fluorescence intensity.
Ablation of Gab1/2 leads to a decrease in chitin-induced M2 polarization in vivo
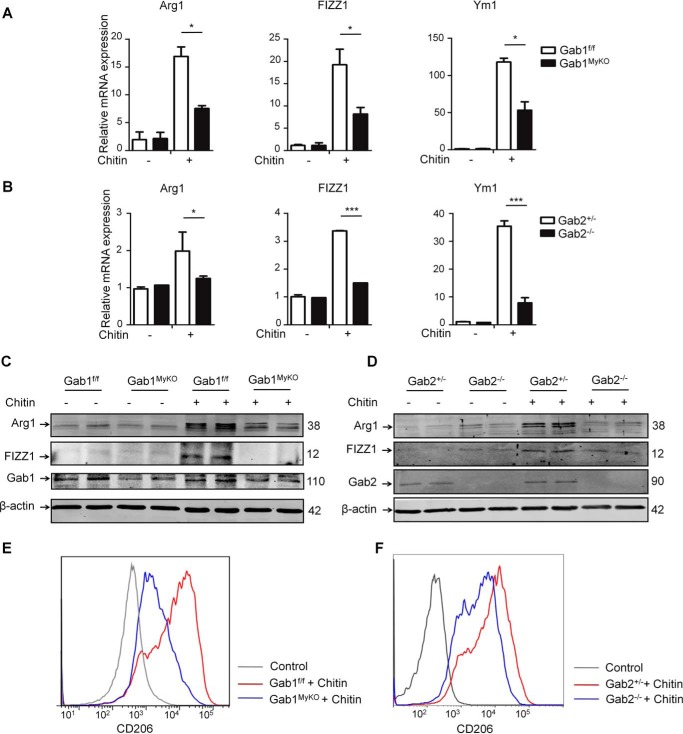
To further explore the in vivo observations related to Gab1/2 in M2 polarization, Gab1MyKO and Gab2−/− mice were administered chitin intraperitoneally. Chitin is an important component of fungi and helminths and is essential for inducing mouse M2 activation (44, 45). The in vivo levels of M2 markers were assessed at the transcriptional and protein levels in chitin-elicited macrophages isolated from the Gab1MyKO and Gab2−/− mice. We found that the up-regulation of the M2 markers induced by chitin was markedly abrogated in both Gab1- and Gab2-deficient macrophages (Fig. 3, A and B), and Arg1 and FIZZ1 expression were further analyzed using immunoblotting assay, with consistent results (Fig. 3, C and D). Furthermore, the in vivo individual deletion of Gab1/2 resulted in a reduction in CD206 following the chitin challenge compared with the corresponding controls (Fig. 3, E and F). Together, these data demonstrate that Gabs are required for M2 polarization in vivo.
Figure 3.
Crucial roles of Gab1/2 in M2 polarization in response to chitin administration. Peritoneal macrophages were harvested from Gab1f/f, Gab1MyKO, Gab2+/−, and Gab2−/− mice 2 days after chitin administration. A and B, the mRNA levels were determined by qPCR for Arg1, FIZZ1, and Ym1 in peritoneal macrophages. C and D, the protein levels of Arg1 and FIZZ1 in peritoneal macrophages were analyzed by immunoblotting analysis. E and F, surface expression of CD206 on peritoneal macrophages was determined by flow cytometry analysis. Data from three independent experiments are shown. Error bars, S.D. *, p < 0.05; ***, p < 0.001.
Gab1/2 deficiency protects mice from BLM-induced pulmonary fibrosis
M2-polarized macrophages are known to contribute to the pathogenesis of pulmonary fibrosis (15, 46), so we then determined whether the loss of Gab1 and Gab2 affects the progression of pulmonary fibrosis. To test this possibility, Gab1f/f, Gab1MyKO, Gab2+/−, and Gab2−/− mice were challenged with BLM. M2 markers were determined by quantitative PCR (qPCR) on day 21, indicating that the expression of Arg1, FIZZ1, and Ym1 were significantly decreased in purified alveolar macrophages from Gab1MyKO and Gab2−/− mice compared with the corresponding controls (Fig. 4, A and B). Cells obtained from bronchoalveolar lavage fluid (BALF) were further analyzed by flow cytometry using alveolar macrophages markers (F4/80+ CD11b−), revealing that the percentages of alveolar macrophages in the lungs were unchanged due to the loss of Gab1/2 (supplemental Fig. 4, A and B). Meanwhile, Gab1/2 deletion efficiency was confirmed by an immunoblotting assay (supplemental Fig. 4, C and D). Additionally, the total number of leukocytes in BALF was determined, and the results showed that the influx of inflammatory cells into the lungs of BLM-treated Gab1MyKO and Gab2−/− mice remained unaltered compared with the corresponding controls (supplemental Fig. 4, E and F). Fibrotic injury can be evaluated by assessing excess and aberrant collagen deposition in a BLM-induced model of pulmonary fibrosis (43). We thus collected the whole lungs from BLM-challenged transgenic mice. Collagen accumulation was determined by Masson's trichrome staining. Histological analysis indicated a substantial decrease in collagen deposition and less fibrotic thickening in the alveolar walls in the Gab1MyKO and Gab2−/− mice compared with the corresponding controls. In addition, hydroxyproline assays also showed reduced collagen expression (Fig. 4, C and D). The attenuated pulmonary fibrosis in Gab1MyKO and Gab2−/− mice was further supported by the reduced expression of fibrotic markers, such as fibronectin, α-SMA, and Col I (Fig. 4, E and F). To investigate whether the protective effect observed in Gab1MyKO and Gab2−/− mice was dependent on the impaired M2 polarization, BMDMs originated from Gab1f/f, Gab1MyKO, Gab2+/−, and Gab2−/− mice were treated with IL-4 for the induction of M2 macrophages and administered into clodronate liposome-treated mice by the intratracheal route 7 days after BLM induction. Compared with the relative controls, animals that received Gab1MyKO BMDMs or Gab2−/− BMDMs exhibited reduced lung fibrosis, as determined by Masson's trichrome staining and hydroproline assays (Fig. 4, G and H). Together, these results indicate that high levels of Gabs in macrophages predispose mice to pulmonary fibrosis and, vice versa, that Gab1/2 deficiency protects mice from M2-associated pulmonary fibrosis.
Figure 4.
Gab1/2 deficiency attenuates BLM-induced lung fibrosis. Gab1f/f, Gab1MyKO, Gab2+/−, and Gab2−/− mice were intratracheally injected with BLM and sacrificed 21 days after challenge for functional analysis. A and B, expression levels of M2 markers in alveolar macrophages were measured by qPCR. C and D, the severity of lung fibrosis was analyzed in mice. The left panel presents representative images of Masson's trichrome staining in lung sections as shown at a magnification of ×20. The right panel displays hydroxyproline content in the lungs after acid hydrolysis. Scale bars, 50 μm. E and F, the expression levels of fibrotic markers, including fibronectin, α-SMA, and Col I, in the lungs were determined by immunoblotting analysis. G and H, intratracheally adoptive transfer of IL-4-activated Gab1f/f, Gab1MyKO (G), Gab2+/−, or Gab2−/− (H) BMDMs into Gab1f/f (G) or Gab2+/− mice (H) for functional analysis. The left panel presents representative images of Masson's trichrome staining in lung sections as shown at a magnification of ×20. The right panel displays hydroxyproline content in the lungs after acid hydrolysis. Scale bars, 50 μm. Data from three independent experiments are shown. Error bars, S.D. *, p < 0.05; ***, p < 0.001.
Gab1/2 function in the distinct signal responsiveness to IL-4 activation
Next, we explored the mechanisms underpinning the defective M2 polarization in Gab1/2-deficient macrophages. Binding of the Th2 cytokine IL-4 signal with IL-4Rα subsequently recruits a downstream signaling complex to activate tyrosine phosphorylation events, which are essential for M2-polarized macrophages. It has been demonstrated that following IL-4R activation, cytoplasmic STAT6 and AKT are simultaneously phosphorylated to diverge IL-4 responsiveness (6, 17, 47, 48). STAT6 is a well-defined transcription factor downstream of IL-4R activation that regulates M2-related genes, including Arg1, FIZZ1, Ym1, and CD206. As indicated in Fig. 5 (A–D), STAT6 phosphorylation was measured in the Gab1/2-deleted BMDMs in response to IL-4, and our results showed that loss of Gab1 had no effect on IL-4-activated STAT6 signaling (Fig. 5A), whereas loss of Gab2 caused a significant attenuation of STAT6 phosphorylation (Fig. 5B). Intriguingly, we then found diminished AKT phosphorylation in Gab1MyKO BMDMs (Fig. 5C) but only a slight decrease in AKT phosphorylation in Gab2−/− BMDMs (Fig. 5D). In addition, in vitro Gab1/2 were inactivated using shRNA-mediated deletion in RAW264.7 cells as a further assessment of IL-4R responsiveness. As illustrated in supplemental Fig. 5, Gab1 knockdown resulted in a marked reduction in AKT phosphorylation but not in STAT6 (supplemental Fig. 5A); however, Gab2 knockdown led to decreased phosphorylation of STAT6, but AKT remained intact (supplemental Fig. 5B). Our preliminary findings link reduced AKT activation rather than STAT6 activation to impaired M2 polarization in Gab1MyKO mice. To address the AKT-dependent effects, we took a genetic approach to rescue AKT signaling in Gab1MyKO BMDMs by adenovirus-mediated myristoylated AKT (myr-AKT; constitutively active AKT) transduction. As illustrated in Fig. 5E, immunoblotting analysis revealed increased levels of basal phospho-AKT in Gab1MyKO BMDMs after adenoviral transduction of myr-AKT. qPCR analysis indicated that overexpression of active AKT increased the induction of M2 genes (Arg1, FIZZ1, and Ym1) following IL-4 stimulation (Fig. 5F). Finally, we determined whether this signal preference mediated by Gab1/2 is phenotypically correlated with BLM-induced M2 activation. To probe this possibility, we harvested alveolar macrophages from BLM-treated Gab1MyKO and Gab2−/− mice (Fig. 5, G and H). We observed a considerable reduction in phosphorylated AKT in Gab1MyKO mice but not in Gab2−/− mice (Fig. 5G); moreover, the activation of STAT6 was more evidently attenuated in Gab2−/− than in Gab1MyKO mice (Fig. 5H). Although this trend was readily apparent, it depends to a large extent on macrophages and their particular circumstances. Overall, our findings suggest that Gab1/2 function in the distinct signal responsiveness to IL-4 activation, supporting the notion that the docking proteins confer IL-4 signal preference in M2-polarized macrophages.
Figure 5.
Distinctive roles of Gab1/2 in IL-4-mediated signaling. BMs from Gab1f/f, Gab1MyKO, Gab2+/−, and Gab2−/− mice were cultured with M-CSF for 7 days and then stimulated with IL-4 (20 ng/ml) for 0, 5, 30, 60, and 120 min. A and B, left, whole-cell lysates of BMDMs from Gab1f/f and Gab1MyKO (A) and Gab2+/− and Gab2−/− (B) mice were analyzed by immunoblotting analysis with phosphorylated and total STAT6 antibodies. Right, densitometry analysis of the indicated band intensities from all experiments. C and D, left, whole-cell lysates of BMDMs from Gab1f/f and Gab1MyKO (C) and Gab2+/− and Gab2−/− (D) mice were analyzed by immunoblotting analysis with phosphorylated and total AKT antibodies. Right, densitometry analysis of the indicated band intensities from all experiments. E, immunoblotting analysis of Gab1MyKO BMDMs transduced with myr-AKT or EV. F, myr-AKT Gab1MyKO BMDMs and EV Gab1MyKO BMDMs were stimulated with IL-4 followed 24 h later by analysis of M2 marker expression. G and H, left, whole-cell lysates of alveolar macrophages from BLM-treated Gab1f/f and Gab1MyKO (G) and Gab2+/− and Gab2−/− (H) mice were analyzed by immunoblotting analysis with the indicated antibodies. Each sample represents four mice. Right, densitometry analysis of the indicated band intensities from all experiments. Data from three independent experiments are shown. Error bars, S.D. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Gab1/2 selectively favors the formation of the signal complex driven by IL-4
To examine the mechanism by which Gab1/2 regulate the IL-4 signal preference, we conducted co-immunoprecipitation assays to compare the signal interactions modulated by Gab1/2 in response to IL-4. We observed that Gab1 but not Gab2 selectively interacted with the p85 subunit of PI3K upon IL-4 stimulation, which may account for a stronger decline in AKT activation in Gab1 deficiency than that in Gab2 deficiency (Fig. 6A). It has been demonstrated that IL-4R enables JAK1 recruitment and then sequentially mediates STAT6 activation, thereby regulating a subset of M2-associated genes (6). As expected, we found that loss of Gab2 in BMDMs suppressed the phosphorylation of JAK1 (Fig. 6B). In addition, IL-4 stimulation promoted Gab2 interaction with IL-4Rα (Fig. 6C). Further experiments revealed that the interaction of IL-4Rα with JAK1 induced by IL-4 was substantially abrogated in Gab2−/− BMDMs (Fig. 6D). Together, it is likely that Gab1/2 confer the IL-4 signal preference through selective association with either PI3K/AKT or JAK1/STAT6, respectively. We concluded that this preferred formation of a signal complex is responsible for the IL-4 signal preference observed in Gab1/2-deficient macrophages.
Figure 6.
Gab1 binds to p85 and Gab2 promotes the association of JAK1 with IL-4Rα. A, RAW264.7 cells were stimulated with IL-4 (20 ng/ml) for 30 min, and total cell lysates were subjected to immunoprecipitation (IP) with anti-Gab1 antibody (left) or anti-p85 antibody (right) followed by immunoblotting analysis with anti-p85 antibody, anti-Gab1 antibody, or anti-Gab2 antibody. B, BMDMs from Gab2+/− and Gab2−/− mice were stimulated with IL-4 (20 ng/ml) for the indicated periods, and whole-cell lysates were measured by immunoblotting analysis using anti-phospho-JAK1 and JAK1 antibodies. C, RAW264.7 cells were treated with IL-4 (20 ng/ml) for 30 min. Total cell lysates were prepared and subjected to immunoprecipitation with anti-Gab2 antibody (left) or anti-IL-4Rα antibody (right) followed by immunoblotting analysis with anti-IL-4Rα antibody or anti-Gab2 antibody. D, BMDMs isolated from Gab2+/− and Gab2−/− mice were stimulated with IL-4 (20 ng/ml) for 30 min. Whole-cell lysates were immunoprecipitated with anti-JAK1 antibody. Immunoblotting analysis was performed with anti-IL-4Rα antibody. Data from three independent experiments are shown.
Discussion
It has been extensively reported that alternatively activated (M2) macrophages are involved in the progression of lung fibrosis; however, the precise mechanism underlying this process is still not clear (4, 9, 49, 50). In this study, we defined a novel role of the Gab docking protein family in regulating M2 polarization-mediated lung fibrosis. We found that an increased level of Gab1/2 in alveolar macrophages is coupled with M2 skewing in mice challenged with BLM. Gab1/2 deletion in macrophages suppressed M2 polarization, which was phenotypically correlated with the attenuated fibrosis observed in Gab1MyKO and Gab2−/− mice. Mechanistically, Gab1/2 are synergistically involved in IL-4-regulated Th2 responses but serve as a non-redundant control for IL-4 activation. Our results further demonstrated that Gab1 regulates AKT activation, whereas Gab2 mediates STAT6 enhancement in response to IL-4, which supports the notion that the Gab1/2 docking proteins confer the IL-4 signal preference in M2-associated lung fibrosis. These findings may help us to better understand the finely tuned role of docking proteins in the pathogenesis of idiopathic pulmonary fibrosis.
Macrophages are mononuclear phagocytes, crucial determinants in orchestrating almost all forms of wound healing and fibrotic repair (5, 51). In tissues, monocytes/macrophages respond to various environmental cues and are rapidly activated. This process is highly plastic and characterized by functional polarization into activated macrophages. Conceptually, polarized macrophages have been categorized as M1 (classically activated) and M2 (alternatively activated) (48, 52, 53). This M1/M2 paradigm has been questioned recently in terms of its accuracy and practicality, given the high heterogeneity of polarized macrophages under certain circumstances (54). Despite that, the functions of M2-polarized macrophage are widely accepted in this phenotypic shift toward fibrosis in the lungs. Emerging evidence has demonstrated that monocytes/macrophages could be skewed into M2 polarization by a broad array of pro-fibrotic cytokines, leading to the marked elevation of M2 biomarkers, including Arg1, Ym1, FIZZ1, and CD206, in the BLM-induced murine model (15, 55–57). Analysis of clinical samples from IPF patients has clearly shown the pathological relevance of these observations, implying a promising therapeutic potential (58). Undoubtedly, elucidating this mechanism is a high priority and may facilitate the development of macrophage-directed interventions for this currently untreatable disease.
In this work, we first observed an elevated level of Gab1/2 in M2-polarized macrophages harvested from the lungs of BLM-induced mice, suggesting the potential involvement of Gab1/2 in M2-driven fibrosis (Fig. 1). Further investigation demonstrated an important role of Gab1/2 in promoting M2 polarization in BMDMs from transgenic mice lacking either Gab1 or Gab2 (Fig. 2). Herein, we employed LysM–Cre–mediated excision to induce a selective deletion in monocytes/macrophages. LysM-driven Cre recombinases are also expressed to some degree in neuronal subsets, neutrophils, and certain other blood cells, although LysM–Cre–mediated deletion in macrophages is still highly efficient (59–61). However, there is currently no true macrophage-specific Cre deletion technique. Thus, testing the deletion efficiency by immunoblotting assays is essential, as stated by Peter Murray (60). Accordingly, the degree of Gab1 knockdown in purified alveolar macrophages was assessed by immunoblotting analysis. As indicated in supplemental Fig. 4C, Gab1 levels were efficiently lowered in purified alveolar macrophages. Chitin is a structural component of fungi and helminths, and the administration of chitin strongly induced macrophage M2 polarization, manifested by the up-regulated expression of M2 markers (44, 45). Herein, we used in vivo models of chitin to clarify the functional roles of Gab1/2 in M2 polarization (Fig. 3), suggesting a broad effect of Gab1/2 on M2-polarized macrophages. In this work, our data clearly define the positive regulation of Gab1/2 in M2-polarized macrophages, which contributes to pulmonary fibrosis. Future studies are needed to evaluate additional functions of Gab1/2 in tissue-specific macrophages and M2-associated disorders.
Gab proteins, which serve as docking proteins for integrating phosphorylation, comprise a family of adaptor proteins including mammalian Gab1, Gab2, and Gab3 (22). Gab1 and Gab2 are ubiquitously expressed with a widespread expression pattern (22, 25). Gab3 has limited expression in hematopoietic cells, and Gab3-deficient mice exhibit no obvious defects in normal development and hematopoiesis (27, 62). Thus, this work focused on dissecting the functions of Gab1 and Gab2 in macrophages. In fact, M2 polarization can be triggered by a broad array of cytokines and pathogenic agents, so we further investigated a possible mechanism by which Gab1/2 mediate M2 polarization in response to canonical IL-4 stimulation. Although the regulation of M2 by IL-4 is diverse, emerging evidence clearly indicates that upon stimulation, IL-4 binds IL-4Rα and then recruits IL-2Rγ (type I receptor), leading to the activation of the non-receptor tyrosine kinase JAK1, enabling the phosphorylation, dimerization, and translocation of STAT6 to the nucleus, where it binds to the promoters of IL-4-responsive genes (6, 17, 18, 63). In addition, the PI3K/AKT signaling pathway is also engaged by IL-4Rα in parallel to the JAK1/STAT6 pathway to regulate the expression of M2 markers (47, 64). Intriguingly, we found a distinctive role of Gab1/2 in IL-4-mediated downstream elements, although Gab1/2 deficiency in macrophages led to an apparently similar M2-associated fibrosis (Fig. 4). It is evident that in response to IL-4 stimulation, Gab1 preferentially activates PI3K/AKT signaling, whereas Gab2 favors JAK1/STAT6 activation (Fig. 5). Consistent with this, we noticed a preferential interaction of Gab1 with p85, whereas Gab2 facilitates the interaction of IL-4Rα with JAK1 (Fig. 6), attributable to the M2-associated phenotypic shift and fibrosis observed in the mice. Despite lacking catalytic activity, docking proteins confer signal specificity through their selective preference for protein interactions. In this work, we presented an illustrative example that the Gab1/2 docking proteins enable the IL-4 signal preference, commonly contributing to M2 polarization and the development of fibrosis. Future studies may provide additional information regarding the precise contribution of the IL-4 signal preference by Gab1/2 divergence in M2 subpopulations in fibrotic lungs. With such efforts, clarifying the signal convergence and divergence should greatly facilitate our understanding of the pathogenesis of this idiopathic pulmonary disorder.
In conclusion, our findings highlight a non-redundant role of the Gab1/2 docking proteins in M2 polarization, an important determinant of M2-associated pulmonary fibrosis. Interfering with the Gab1/2-mediated IL-4 signal preference could be a more effective and less toxic therapeutic option for macrophage-guided therapy.
Experimental procedures
Mice
Gab1flox/flox mice (41) were crossed with LysMcre/+ mice to generate conditional Gab1 KO mice (Gab1MyKO). Gab2−/− mice were bred with Gab2+/− mice to generate Gab2 KO animals (65). All animal protocols were approved by the animal care and use committee of the Zhejiang University School of Medicine.
Antibodies and reagents
Recombinant murine IL-4 and M-CSF were obtained from Peprotech (Rocky Hill, NJ). The following antibodies were used for immunoblotting and immunoprecipitation: rabbit polyclonal anti-Gab1 (3232S), rabbit monoclonal anti-Gab2 (3239S), rabbit polyclonal anti-AKT (catalog no. 9272), rabbit monoclonal anti-phospho-AKT (Thr-308) (catalog no. 2965), rabbit monoclonal anti-PI3K p85 (4257S), and rabbit polyclonal anti-STAT6 (9362S) (1:1000; Cell Signaling Technology, Beverly, MA); rabbit polyclonal anti-Arg1 (sc-20150), rabbit polyclonal anti-JAK1 (sc-277), and mouse monoclonal anti-fibronectin (sc-8422) (1:500, Santa Cruz Biotechnology, Inc.); rabbit polyclonal anti-α-SMA (ab5694), rabbit polyclonal anti-Col I (ab34710), and rabbit polyclonal anti-STAT6 (phospho-Tyr-641) (ab54461) (1:1000; Abcam, Cambridge, MA); and rabbit polyclonal anti-JAK1 (phospho-Tyr-1022) (1:1000; Bioworld). The antibodies used for flow cytometry were phycoerythrin anti-mouse CD206 (141705, BioLegend, San Diego, CA), allophycocyanin anti-mouse/human CD11b (101212, BioLegend), and F4/80-FITC (11–4801, eBioscience, San Diego, CA).
BLM-induced pulmonary fibrosis
Gab1f/f, Gab1MyKO, Gab2+/−, and Gab2−/− mice (8–10 weeks old) were intratracheally instilled with 2.5 units/kg BLM. Mice subjected to the same volume of SAL served as controls, and the mice were sacrificed by injection of pentobarbital 21 days after the challenge for the analysis of lung fibrosis. All experiments in this study were reviewed and approved by the animal care and use committee of Zhejiang University School of Medicine.
Histopathology and immunohistochemical analyses
For histopathology analysis, lungs were inflation-fixed with an intratracheal injection of 4% paraformaldehyde in PBS at 25 cm H2O and immersed in the same fixative to preserve the pulmonary architecture, followed by paraffin embedding. The sections (5 μm) were stained with Masson's trichrome for assessment of collagen deposition. For immunostaining, the slides were probed with antibodies against CD206 (1:50; BioLegend).
BALF analysis
After 21 days of BLM administration, mice were euthanized by pentobarbital injection. The left lungs were tied at the bronchus, and the right lungs were lavaged with 0.5 ml of cold sterile Ca2+- and Mg2+-free PBS. This procedure was repeated three times, and ∼1.5 ml of BALF was recovered from each animal. The samples were pelleted at 1000 rpm for 5 min at 4 °C, and supernatants were stored at −80 °C until analyzed. To isolate and purify alveolar macrophages, the pellets were resuspended in DMEM/F-12 medium (Hyclone, Logan, UT) supplemented with 10% heat-inactivated FBS (Hyclone), 50 units/ml penicillin, and streptomycin (Hyclone) and plated for at least 90 min. Non-adherent cells were discarded, and adherent cells were gathered for FACS analysis. Macrophages were enriched to about 90% after quick adhesion, as described previously (66, 67).
Hydroxyproline assays
Lung tissues were weighed and acid-hydrolyzed with 6 n HCl, and hydroxyproline content was determined as a measure of fibrosis with a colorimetric assay, as described previously (68).
BMDM culture
To generate BMDMs, bone marrow cells were isolated from the femurs of mice and differentiated with M-CSF (10 ng/ml, Peprotech) for 7 days as described previously (69). Cells were cultured in DMEM/F-12 medium with 10% heat-inactivated FBS, 50 units/ml penicillin, and streptomycin. For M2 polarization, BMDMs were treated with IL-4 (20 ng/ml; Peprotech).
myr-AKT transduction
For myr-AKT transduction, BMDMs from Gab1MyKO mice were plated at 5 × 105 cells/well in 12-well tissue culture dishes and cultured overnight. Viral supernatants (empty vector (EV) or myr-AKT (Applied Biological Materials, Richmond, Canada)) were then added, and after 10 h, the medium was replaced, and cells were allowed to grow until further analysis.
Macrophage depletion and adoptive transfer
For alveolar macrophage depletion, 100 μl of clodronate liposome (Liposoma, Amsterdam, The Netherlands) was intratracheally administrated 48 h before BLM challenge (8, 70, 71). For adoptive transfer, BMDMs from the indicated mice (Gab1f/f, Gab1MyKO, Gab2+/−, or Gab2−/−) were stimulated with IL-4 for 12 h, and 1 × 106 BMDMs in 20 μl of PBS were transferred intratracheally in the clodronate liposome-treated Gab1f/f or Gab2+/− mice at day 7 of BLM administration. The mice were sacrificed for analysis of pulmonary fibrosis after 14 days of adoptive transfer.
Lentivirus production and infection
To knock down Gab1/2 in RAW264.7 cells, shRNA-Gab1-PLKO.1, shRNA-Gab2-PLKO.1, and control scramble lentiviral vectors were obtained (Sigma-Aldrich) and were used to transfect 293T cells with pMD2.G and psPAX2 (catalog nos. 12259 and 12260; Addgene, Cambridge, MA) using Lipofectamine 3000 reagent (Invitrogen). After 48 h, viral supernatant was collected, filtered through a 0.45-μm filter (Millipore, Danvers, MA), and added to RAW264.7 cells with Polybrene (Santa Cruz Biotechnology, Inc.). 12 h later, the medium was replaced, and cells were allowed to grow until analysis.
Flow cytometry
Cells were washed with cold PBS three times and stained with fluorochrome-conjugated monoclonal antibodies against mouse CD206 (BioLegend) for 30 min at 4 °C. After incubation, cells were washed with cold PBS three times and subsequently subjected to FACS analysis using an AECA NovoCyte TM system (ACEA Biosciences, San Diego, CA). Data were analyzed using FlowJo software version 7.6.
Quantitative PCR analysis
To measure gene expression in macrophages, total RNA was isolated with TRIzol reagent (Invitrogen), and reverse transcription was performed using ReverTra Ace (Toyobo, Osaka, Japan) according to the manufacturer's instructions. Real-time qPCR was performed on a Light-Cycler Roche480 (Roche Molecular Systems) using the FastStart Universal SYBR Green Master Kit (Roche Diagnostics, Mannheim, Germany). The mRNA levels were calculated using the ΔΔCt method. All qPCR primers and their sequences were as follows: Arg1 (5′-CTCCAAGCCAAAGTCCTTAGAG-3′, 5′-AGGAGCTGTCATTAGGGACATC-3′), Ym1 (5′-TCTCTACTCCTCAGAACCGTCAGA-3′, 5′-ATGTTTGTCCTTAGGAGGGCTTC-3′), FIZZ1(5′-TACTTGCAACTGCCTGTGCTTACT-3′, 5′-TATCAAAGCTGGGTTCTCCACCTC-3′), and β-actin (5′-GGCTGTATTCCCCTCCATCG-3′, 5′-CCAGTTGGTAACAATGCCATGT-3′).
Immunoblotting and co-immunoprecipitation assays
Cells were washed with cold PBS and lysed in radioimmune precipitation lysis buffer (150 mm NaCl, 1% Triton X-100, 0.5% sodium deoxycholate, 50 mm Tris-HCl (pH 8.0), and 0.1% SDS) with EDTA-free protease inhibitor tablets and phosphatase inhibitors (Roche Diagnostics). Equal amounts of protein were separated by SDS-PAGE and transferred onto nitrocellulose membranes (Pall, Port Washington, NY) for immunoblotting with primary antibodies as indicated.
For the co-immunoprecipitation assay, cells were lysed with Nonidet P-40 lysis buffer (150 mm NaCl, 50 mm Tris-HCl (pH 7.4), 1% Nonidet P-40) containing protease and phosphatase inhibitors (Roche Diagnostics), and the lysates were immunoprecipitated overnight at 4 °C using antibodies against the following proteins: Gab1/2, p85, JAK1, and IL-4Rα coupled to protein A-agarose (Roche Diagnostics). Immunoprecipitates were washed six times with Nonidet P-40 lysis buffer and resuspended in 1× protein loading buffer. Protein levels were determined by immunoblotting as indicated above. Three independent experiments were performed.
Densitometry analysis
Shorter exposures of films were chosen for densitometry analysis to ensure that the intensities of bands were in the linear range of the film, and the integrated densities of indicated bands were calculated with National Institutes of Health ImageJ Software. The relative abundance of phosphorylated protein was calculated as a ratio of the density of the phosphorylated band divided by the density of the total protein band.
Chitin administration
Chitin (Sigma-Aldrich) was washed with PBS and sonicated for 30 min on ice. About 800 ng of chitin was injected intraperitoneally, and peritoneal macrophages were collected 2 days after administration.
Immunofluorescence
After stimulation with IL-4 for 48 h, BMDMs were washed with PBS, fixed in 4% paraformaldehyde solution for 20 min, and permeabilized with 0.1% Triton X-100 for 20 min. After serum blocking for 1 h at room temperature, BMDMs were then incubated with anti-Arg1 antibody overnight. The next day, BMDMs were washed with PBS, incubated with Alexa Fluor® 594 goat anti-rabbit IgG (1:400; Invitrogen) for 1 h at room temperature, washed with PBS, and immersed in DAPI to visualize the nuclei. Staining was imaged with an inverted confocal microscope (Carl Zeiss, Göttingen, Germany).
Statistical analysis
All results were expressed as the mean ± S.D. The p value was calculated using the GraphPad Prism version 5 statistical program and determined by two-tailed Student's t test. A value of p < 0.05 was considered statistically significant.
Author contributions
X. G., T. L., H. C., X. Z., and Y. K. designed the study; X. G., T. L., Y. X., X. X., and Z. Z. performed experiments, collected, and analyzed data; Y. Z., J. X., and K. X. provided advice on experimental design; X. G., X. Z., and Y. K. wrote the paper. All authors approved the final version of the manuscript.
Supplementary Material
Acknowledgments
We thank Peng Xiao (Sir Run Run Shaw Hospital School of Medicine, Zhejiang University) for assistance with flow cytometry analysis, Dr Gen-Sheng Feng (University of California) for the Gab1f/f and Gab2−/− mice, and Wei Yin (Zhejiang University School of Medicine) for assistance with confocal microscopy.
This work was supported by Key Project of the National Natural Science Foundation of China Grant 81530001 (to Y. K.), Chinese Ministry of Science and Technology Grant 2016YFA0501800 (to Y. K.), and National Natural Science Foundation of China Grant 81671426 (to K. X.). The authors declare that they have no conflicts of interest with the contents of this article.
This article contains supplemental Figs. 1–5.
- IPF
- idiopathic pulmonary fibrosis
- SAL
- saline
- BLM
- bleomycin
- BALF
- bronchoalveolar lavage fluid
- BMDM
- bone marrow-derived macrophage
- Gab
- Grb2-associated binder
- SMA
- smooth muscle actin
- Col I
- collagen I
- myr-AKT
- myristoylated AKT
- qPCR
- quantitative PCR
- IL-4R
- IL-4 receptor
- EV
- empty vector.
References
- 1. King T. E. Jr., Pardo A., and Selman M. (2011) Idiopathic pulmonary fibrosis. Lancet 378, 1949–1961 [DOI] [PubMed] [Google Scholar]
- 2. Noble P. W., Barkauskas C. E., and Jiang D. (2012) Pulmonary fibrosis: patterns and perpetrators. J. Clin. Invest. 122, 2756–2762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gross T. J., and Hunninghake G. W. (2001) Idiopathic pulmonary fibrosis. N. Engl. J. Med. 345, 517–525 [DOI] [PubMed] [Google Scholar]
- 4. Wynn T. A., and Barron L. (2010) Macrophages: master regulators of inflammation and fibrosis. Semin. Liver Dis. 30, 245–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wynn T. A., and Vannella K. M. (2016) Macrophages in tissue repair, regeneration, and fibrosis. Immunity 44, 450–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gordon S., and Martinez F. O. (2010) Alternative activation of macrophages: mechanism and functions. Immunity 32, 593–604 [DOI] [PubMed] [Google Scholar]
- 7. Gangadharan B., Hoeve M. A., Allen J. E., Ebrahimi B., Rhind S. M., Dutia B. M., and Nash A. A. (2008) Murine gammaherpesvirus-induced fibrosis is associated with the development of alternatively activated macrophages. J. Leukoc. Biol. 84, 50–58 [DOI] [PubMed] [Google Scholar]
- 8. Gibbons M. A., MacKinnon A. C., Ramachandran P., Dhaliwal K., Duffin R., Phythian-Adams A. T., van Rooijen N., Haslett C., Howie S. E., Simpson A. J., Hirani N., Gauldie J., Iredale J. P., Sethi T., and Forbes S. J. (2011) Ly6C(hi) monocytes direct alternatively activated profibrotic macrophage regulation of lung fibrosis. Am. J. Respir. Crit. Care Med. 184, 569–581 [DOI] [PubMed] [Google Scholar]
- 9. Wynn T. A. (2004) Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat. Rev. Immunol. 4, 583–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pesce J. T., Ramalingam T. R., Mentink-Kane M. M., Wilson M. S., El Kasmi K. C., Smith A. M., Thompson R. W., Cheever A. W., Murray P. J., and Wynn T. A. (2009) Arginase-1-expressing macrophages suppress Th2 cytokine-driven inflammation and fibrosis. PLoS Pathog. 5, e1000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chung M. J., Liu T., Ullenbruch M., and Phan S. H. (2007) Antiapoptotic effect of found in inflammatory zone (FIZZ)1 on mouse lung fibroblasts. J. Pathol. 212, 180–187 [DOI] [PubMed] [Google Scholar]
- 12. Liu T., Yu H., Ullenbruch M., Jin H., Ito T., Wu Z., Liu J., and Phan S. H. (2014) The in vivo fibrotic role of FIZZ1 in pulmonary fibrosis. PLoS One 9, e88362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Korthagen N. M., van Moorsel C. H., Barlo N. P., Ruven H. J., Kruit A., Heron M., van den Bosch J. M., and Grutters J. C. (2011) Serum and BALF YKL-40 levels are predictors of survival in idiopathic pulmonary fibrosis. Respir. Med. 105, 106–113 [DOI] [PubMed] [Google Scholar]
- 14. Pechkovsky D. V., Prasse A., Kollert F., Engel K. M., Dentler J., Luttmann W., Friedrich K., Müller-Quernheim J., and Zissel G. (2010) Alternatively activated alveolar macrophages in pulmonary fibrosis-mediator production and intracellular signal transduction. Clin. Immunol. 137, 89–101 [DOI] [PubMed] [Google Scholar]
- 15. Tao B., Jin W., Xu J., Liang Z., Yao J., Zhang Y., Wang K., Cheng H., Zhang X., and Ke Y. (2014) Myeloid-specific disruption of tyrosine phosphatase Shp2 promotes alternative activation of macrophages and predisposes mice to pulmonary fibrosis. J. Immunol. 193, 2801–2811 [DOI] [PubMed] [Google Scholar]
- 16. Yao Y., Wang Y., Zhang Z., He L., Zhu J., Zhang M., He X., Cheng Z., Ao Q., Cao Y., Yang P., Su Y., Zhao J., Zhang S., Yu Q., et al. (2016) Chop deficiency protects mice against bleomycin-induced pulmonary fibrosis by attenuating M2 macrophage production. Mol. Ther. 24, 915–925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wills-Karp M., and Finkelman F. D. (2008) Untangling the complex web of IL-4- and IL-13-mediated signaling pathways. Sci. Signal. 1, pe55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Van Dyken S. J., and Locksley R. M. (2013) Interleukin-4- and interleukin-13-mediated alternatively activated macrophages: roles in homeostasis and disease. Annu. Rev. Immunol. 31, 317–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mora A. L., Torres-González E., Rojas M., Corredor C., Ritzenthaler J., Xu J., Roman J., Brigham K., and Stecenko A. (2006) Activation of alveolar macrophages via the alternative pathway in herpesvirus-induced lung fibrosis. Am. J. Respir. Cell Mol. Biol. 35, 466–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gu H., and Neel B. G. (2003) The “Gab” in signal transduction. Trends Cell Biol. 13, 122–130 [DOI] [PubMed] [Google Scholar]
- 21. Wöhrle F. U., Daly R. J., and Brummer T. (2009) Function, regulation and pathological roles of the Gab/DOS docking proteins. Cell Commun. Signal. 7, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Holgado-Madruga M., Emlet D. R., Moscatello D. K., Godwin A. K., and Wong A. J. (1996) A Grb2-associated docking protein in EGF- and insulin-receptor signalling. Nature 379, 560–564 [DOI] [PubMed] [Google Scholar]
- 23. Herbst R., Carroll P. M., Allard J. D., Schilling J., Raabe T., and Simon M. A. (1996) Daughter of sevenless is a substrate of the phosphotyrosine phosphatase Corkscrew and functions during sevenless signaling. Cell 85, 899–909 [DOI] [PubMed] [Google Scholar]
- 24. Schutzman J. L., Borland C. Z., Newman J. C., Robinson M. K., Kokel M., and Stern M. J. (2001) The Caenorhabditis elegans EGL-15 signaling pathway implicates a DOS-like multisubstrate adaptor protein in fibroblast growth factor signal transduction. Mol. Cell. Biol. 21, 8104–8116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gu H., Pratt J. C., Burakoff S. J., and Neel B. G. (1998) Cloning of p97/Gab2, the major SHP2-binding protein in hematopoietic cells, reveals a novel pathway for cytokine-induced gene activation. Mol. Cell 2, 729–740 [DOI] [PubMed] [Google Scholar]
- 26. Holgado-Madruga M., Moscatello D. K., Emlet D. R., Dieterich R., and Wong A. J. (1997) Grb2-associated binder-1 mediates phosphatidylinositol 3-kinase activation and the promotion of cell survival by nerve growth factor. Proc. Natl. Acad. Sci. U.S.A. 94, 12419–12424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wolf I., Jenkins B. J., Liu Y., Seiffert M., Custodio J. M., Young P., and Rohrschneider L. R. (2002) Gab3, a new DOS/Gab family member, facilitates macrophage differentiation. Mol. Cell. Biol. 22, 231–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sármay G., Angyal A., Kertész A., Maus M., and Medgyesi D. (2006) The multiple function of Grb2 associated binder (Gab) adaptor/scaffolding protein in immune cell signaling. Immunol. Lett. 104, 76–82 [DOI] [PubMed] [Google Scholar]
- 29. Nakaoka Y., Nishida K., Narimatsu M., Kamiya A., Minami T., Sawa H., Okawa K., Fujio Y., Koyama T., Maeda M., Sone M., Yamasaki S., Arai Y., Koh G. Y., Kodama T., Hirota H., Otsu K., Hirano T., and Mochizuki N. (2007) Gab family proteins are essential for postnatal maintenance of cardiac function via neuregulin-1/ErbB signaling. J. Clin. Invest. 117, 1771–1781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wickrema A., Uddin S., Sharma A., Chen F., Alsayed Y., Ahmad S., Sawyer S. T., Krystal G., Yi T. L., Nishada K., Hibi M., Hirano T., and Platanias L. C. (1999) Engagement of Gab1 and Gab2 in erythropoietin signaling. J. Biol. Chem. 274, 24469–24474 [DOI] [PubMed] [Google Scholar]
- 31. Lock L. S., Maroun C. R., Naujokas M. A., and Park M. (2002) Distinct recruitment and function of Gab1 and Gab2 in Met receptor-mediated epithelial morphogenesis. Mol. Biol. Cell 13, 2132–2146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nishida K., and Hirano T. (2003) The role of Gab family scaffolding adapter proteins in the signal transduction of cytokine and growth factor receptors. Cancer Sci. 94, 1029–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Meng S., Chen Z., Munoz-Antonia T., and Wu J. (2005) Participation of both Gab1 and Gab2 in the activation of the ERK/MAPK pathway by epidermal growth factor. Biochem. J. 391, 143–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Itoh M., Yoshida Y., Nishida K., Narimatsu M., Hibi M., and Hirano T. (2000) Role of Gab1 in heart, placenta, and skin development and growth factor- and cytokine-induced extracellular signal-regulated kinase mitogen-activated protein kinase activation. Mol. Cell. Biol. 20, 3695–3704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sachs M., Brohmann H., Zechner D., Müller T., Hülsken J., Walther I., Schaeper U., Birchmeier C., and Birchmeier W. (2000) Essential role of Gab1 for signaling by the c-Met receptor in vivo. J. Cell Biol. 150, 1375–1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gu H., Saito K., Klaman L. D., Shen J., Fleming T., Wang Y., Pratt J. C., Lin G., Lim B., Kinet J. P., and Neel B. G. (2001) Essential role for Gab2 in the allergic response. Nature 412, 186–190 [DOI] [PubMed] [Google Scholar]
- 37. Nishida K., Wang L., Morii E., Park S. J., Narimatsu M., Itoh S., Yamasaki S., Fujishima M., Ishihara K., Hibi M., Kitamura Y., and Hirano T. (2002) Requirement of Gab2 for mast cell development and KitL/c-Kit signaling. Blood 99, 1866–1869 [DOI] [PubMed] [Google Scholar]
- 38. Wada T., Nakashima T., Oliveira-dos-Santos A. J., Gasser J., Hara H., Schett G., and Penninger J. M. (2005) The molecular scaffold Gab2 is a crucial component of RANK signaling and osteoclastogenesis. Nat. Med. 11, 394–399 [DOI] [PubMed] [Google Scholar]
- 39. Caron C., Spring K., Laramée M., Chabot C., Cloutier M., Gu H., and Royal I. (2009) Non-redundant roles of the Gab1 and Gab2 scaffolding adapters in VEGF-mediated signalling, migration, and survival of endothelial cells. Cell. Signal. 21, 943–953 [DOI] [PubMed] [Google Scholar]
- 40. Laramée M., Chabot C., Cloutier M., Stenne R., Holgado-Madruga M., Wong A. J., and Royal I. (2007) The scaffolding adapter Gab1 mediates vascular endothelial growth factor signaling and is required for endothelial cell migration and capillary formation. J. Biol. Chem. 282, 7758–7769 [DOI] [PubMed] [Google Scholar]
- 41. Zhang Y., Xu Y., Liu S., Guo X., Cen D., Xu J., Li H., Li K., Zeng C., Lu L., Zhou Y., Shen H., Cheng H., Zhang X., and Ke Y. (2016) Scaffolding protein Gab1 regulates myeloid dendritic cell migration in allergic asthma. Cell Res. 26, 1226–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang X., Zhang Y., Tao B., Wang D., Cheng H., Wang K., Zhou R., Xie Q., and Ke Y. (2012) Docking protein Gab2 regulates mucin expression and goblet cell hyperplasia through TYK2/STAT6 pathway. FASEB J. 26, 4603–4613 [DOI] [PubMed] [Google Scholar]
- 43. Moore B. B., and Hogaboam C. M. (2008) Murine models of pulmonary fibrosis. Am. J. Physiol. Lung Cell Mol. Physiol. 294, L152–L160 [DOI] [PubMed] [Google Scholar]
- 44. Satoh T., Takeuchi O., Vandenbon A., Yasuda K., Tanaka Y., Kumagai Y., Miyake T., Matsushita K., Okazaki T., Saitoh T., Honma K., Matsuyama T., Yui K., Tsujimura T., Standley D. M., et al. (2010) The Jmjd3-Irf4 axis regulates M2 macrophage polarization and host responses against helminth infection. Nat. Immunol. 11, 936–944 [DOI] [PubMed] [Google Scholar]
- 45. Reese T. A., Liang H. E., Tager A. M., Luster A. D., Van Rooijen N., Voehringer D., and Locksley R. M. (2007) Chitin induces accumulation in tissue of innate immune cells associated with allergy. Nature 447, 92–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ballinger M. N., Newstead M. W., Zeng X., Bhan U., Mo X. M., Kunkel S. L., Moore B. B., Flavell R., Christman J. W., and Standiford T. J. (2015) IRAK-M promotes alternative macrophage activation and fibroproliferation in bleomycin-induced lung injury. J. Immunol. 194, 1894–1904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wurster A. L., Withers D. J., Uchida T., White M. F., and Grusby M. J. (2002) Stat6 and IRS-2 cooperate in interleukin 4 (IL-4)-induced proliferation and differentiation but are dispensable for IL-4-dependent rescue from apoptosis. Mol. Cell. Biol. 22, 117–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sica A., and Mantovani A. (2012) Macrophage plasticity and polarization: in vivo veritas. J. Clin. Invest. 122, 787–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wynn T. A. (2011) Integrating mechanisms of pulmonary fibrosis. J. Exp. Med. 208, 1339–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mantovani A., Sozzani S., Locati M., Allavena P., and Sica A. (2002) Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 23, 549–555 [DOI] [PubMed] [Google Scholar]
- 51. Das A., Sinha M., Datta S., Abas M., Chaffee S., Sen C. K., and Roy S. (2015) Monocyte and macrophage plasticity in tissue repair and regeneration. Am. J. Pathol. 185, 2596–2606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ginhoux F., Schultze J. L., Murray P. J., Ochando J., and Biswas S. K. (2016) New insights into the multidimensional concept of macrophage ontogeny, activation and function. Nat. Immunol. 17, 34–40 [DOI] [PubMed] [Google Scholar]
- 53. Adams D. O., and Hamilton T. A. (1984) The cell biology of macrophage activation. Annu. Rev. Immunol. 2, 283–318 [DOI] [PubMed] [Google Scholar]
- 54. Murray P. J., Allen J. E., Biswas S. K., Fisher E. A., Gilroy D. W., Goerdt S., Gordon S., Hamilton J. A., Ivashkiv L. B., Lawrence T., Locati M., Mantovani A., Martinez F. O., Mege J. L., Mosser D. M., et al. (2014) Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 41, 14–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Misharin A. V., Morales-Nebreda L., Mutlu G. M., Budinger G. R., and Perlman H. (2013) Flow cytometric analysis of macrophages and dendritic cell subsets in the mouse lung. Am. J. Respir. Cell Mol. Biol. 49, 503–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Li D., Guabiraba R., Besnard A. G., Komai-Koma M., Jabir M. S., Zhang L., Graham G. J., Kurowska-Stolarska M., Liew F. Y., McSharry C., and Xu D. (2014) IL-33 promotes ST2-dependent lung fibrosis by the induction of alternatively activated macrophages and innate lymphoid cells in mice. J. Allergy Clin. Immunol. 134, 1422–1432.e11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gharib S. A., Johnston L. K., Huizar I., Birkland T. P., Hanson J., Wang Y., Parks W. C., and Manicone A. M. (2014) MMP28 promotes macrophage polarization toward M2 cells and augments pulmonary fibrosis. J. Leukoc. Biol. 95, 9–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Byrne A. J., Maher T. M., and Lloyd C. M. (2016) Pulmonary macrophages: a new therapeutic pathway in fibrosing lung disease? Trends Mol. Med. 22, 303–316 [DOI] [PubMed] [Google Scholar]
- 59. Clausen B. E., Burkhardt C., Reith W., Renkawitz R., and Förster I. (1999) Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 8, 265–277 [DOI] [PubMed] [Google Scholar]
- 60. Murray P. J. (2017) Macrophage polarization. Annu. Rev. Physiol. 79, 541–566 [DOI] [PubMed] [Google Scholar]
- 61. Orthgiess J., Gericke M., Immig K., Schulz A., Hirrlinger J., Bechmann I., and Eilers J. (2016) Neurons exhibit Lyz2 promoter activity in vivo: implications for using LysM-Cre mice in myeloid cell research. Eur. J. Immunol. 46, 1529–1532 [DOI] [PubMed] [Google Scholar]
- 62. Seiffert M., Custodio J. M., Wolf I., Harkey M., Liu Y., Blattman J. N., Greenberg P. D., and Rohrschneider L. R. (2003) Gab3-deficient mice exhibit normal development and hematopoiesis and are immunocompetent. Mol. Cell. Biol. 23, 2415–2424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Martinez F. O., Helming L., and Gordon S. (2009) Alternative activation of macrophages: an immunologic functional perspective. Annu. Rev. Immunol. 27, 451–483 [DOI] [PubMed] [Google Scholar]
- 64. Heller N. M., Qi X., Junttila I. S., Shirey K. A., Vogel S. N., Paul W. E., and Keegan A. D. (2008) Type I IL-4Rs selectively activate IRS-2 to induce target gene expression in macrophages. Sci. Signal. 1, ra17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ke Y., Wu D., Princen F., Nguyen T., Pang Y., Lesperance J., Muller W. J., Oshima R. G., and Feng G. S. (2007) Role of Gab2 in mammary tumorigenesis and metastasis. Oncogene 26, 4951–4960 [DOI] [PubMed] [Google Scholar]
- 66. Woodruff P. G., Koth L. L., Yang Y. H., Rodriguez M. W., Favoreto S., Dolganov G. M., Paquet A. C., and Erle D. J. (2005) A distinctive alveolar macrophage activation state induced by cigarette smoking. Am. J. Respir. Crit. Care Med. 172, 1383–1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Minutti C. M., Jackson-Jones L. H., García-Fojeda B., Knipper J. A., Sutherland T. E., Logan N., Rinqvist E., Guillamat-Prats R., Ferenbach D. A., Artigas A., Stamme C., Chroneos Z. C., Zaiss D. M., Casals C., and Allen J. E. (2017) Local amplifiers of IL-4Rα-mediated macrophage activation promote repair in lung and liver. Science 356, 1076–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gharaee-Kermani M., Hatano K., Nozaki Y., and Phan S. H. (2005) Gender-based differences in bleomycin-induced pulmonary fibrosis. Am. J. Pathol. 166, 1593–1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Herbert D. R., Hölscher C., Mohrs M., Arendse B., Schwegmann A., Radwanska M., Leeto M., Kirsch R., Hall P., Mossmann H., Claussen B., Förster I., and Brombacher F. (2004) Alternative macrophage activation is essential for survival during schistosomiasis and downmodulates T helper 1 responses and immunopathology. Immunity 20, 623–635 [DOI] [PubMed] [Google Scholar]
- 70. Dhaliwal K., Scholefield E., Ferenbach D., Gibbons M., Duffin R., Dorward D. A., Morris A. C., Humphries D., MacKinnon A., Wilkinson T. S., Wallace W. A., van Rooijen N., Mack M., Rossi A. G., Davidson D. J., Hirani N., Hughes J., Haslett C., and Simpson A. J. (2012) Monocytes control second-phase neutrophil emigration in established lipopolysaccharide-induced murine lung injury. Am. J. Respir. Crit. Care Med. 186, 514–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Van Rooijen N., and Sanders A. (1994) Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J. Immunol. Methods 174, 83–93 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.