Abstract
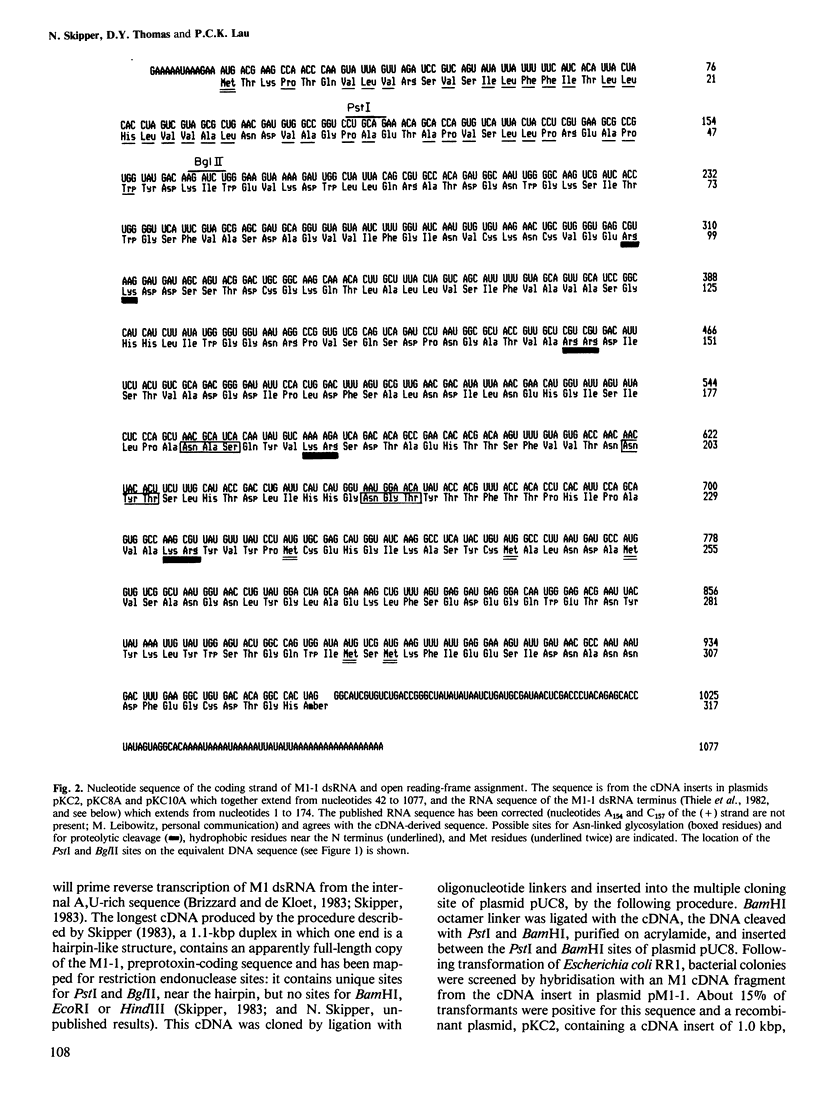
Complementary DNA (cDNA) copies of the M1-1, toxin-coding region of the yeast M1 double-stranded RNA (dsRNA) have been cloned and sequenced. These sequences, in combination with the known terminal sequence of M1-1 dsRNA, identify a translation reading frame for a 316 amino acid protein of 34.7 kd, similar in size to the preprotoxin produced from M1 dsRNA by in vitro translation. Potential glycosylation sites in the preprotoxin peptide are identified. Based on its methionine content the extracellular yeast toxin appears to be contained within the C-terminal region of the precursor.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aubert J. P., Biserte G., Loucheux-Lefebvre M. H. Carbohydrate-peptide linkage in glycoproteins. Arch Biochem Biophys. 1976 Aug;175(2):410–418. doi: 10.1016/0003-9861(76)90528-2. [DOI] [PubMed] [Google Scholar]
- Beeley J. G. Peptide chain conformation and the glycosylation of glycoproteins. Biochem Biophys Res Commun. 1977 Jun 20;76(4):1051–1055. doi: 10.1016/0006-291x(77)90962-7. [DOI] [PubMed] [Google Scholar]
- Bennetzen J. L., Hall B. D. Codon selection in yeast. J Biol Chem. 1982 Mar 25;257(6):3026–3031. [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Betlach M. C., Boyer H. W. Construction and characterization of new cloning vehicles. I. Ampicillin-resistant derivatives of the plasmid pMB9. Gene. 1977;2(2):75–93. doi: 10.1016/0378-1119(77)90074-9. [DOI] [PubMed] [Google Scholar]
- Bostian K. A., Hopper J. E., Rogers D. T., Tipper D. J. Translational analysis of the killer-associated virus-like particle dsRNA genome of S. cerevisiae: M dsRNA encodes toxin. Cell. 1980 Feb;19(2):403–414. doi: 10.1016/0092-8674(80)90514-0. [DOI] [PubMed] [Google Scholar]
- Bostian K. A., Jayachandran S., Tipper D. J. A glycosylated protoxin in killer yeast: models for its structure and maturation. Cell. 1983 Jan;32(1):169–180. doi: 10.1016/0092-8674(83)90507-x. [DOI] [PubMed] [Google Scholar]
- Both G. W., Bellamy A. R., Street J. E., Siegman L. J. A general strategy for cloning double-stranded RNA: nucleotide sequence of the Simian-11 rotavirus gene 8. Nucleic Acids Res. 1982 Nov 25;10(22):7075–7088. doi: 10.1093/nar/10.22.7075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brizzard B. L., De Kloet S. R. Reverse transcription of yeast double-stranded RNA and ribosomal RNA using synthetic oligonucleotide primers. Biochim Biophys Acta. 1983 Jan 20;739(1):122–131. doi: 10.1016/0167-4781(83)90052-0. [DOI] [PubMed] [Google Scholar]
- Bussey H. Physiology of killer factor in yeast. Adv Microb Physiol. 1981;22:93–122. doi: 10.1016/s0065-2911(08)60326-4. [DOI] [PubMed] [Google Scholar]
- Bussey H., Sacks W., Galley D., Saville D. Yeast killer plasmid mutations affecting toxin secretion and activity and toxin immunity function. Mol Cell Biol. 1982 Apr;2(4):346–354. doi: 10.1128/mcb.2.4.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashdollar L. W., Esparza J., Hudson G. R., Chmelo R., Lee P. W., Joklik W. K. Cloning the double-stranded RNA genes of reovirus: sequence of the cloned S2 gene. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7644–7648. doi: 10.1073/pnas.79.24.7644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Nakanishi S., Inoue A., Kita T., Nakamura M., Numa S. Studies of cloned DNA encoding the structure for the bovine corticotropin-beta-lipotropin precursor protein. Ann N Y Acad Sci. 1980;343:415–424. doi: 10.1111/j.1749-6632.1980.tb47270.x. [DOI] [PubMed] [Google Scholar]
- Comb M., Seeburg P. H., Adelman J., Eiden L., Herbert E. Primary structure of the human Met- and Leu-enkephalin precursor and its mRNA. Nature. 1982 Feb 25;295(5851):663–666. doi: 10.1038/295663a0. [DOI] [PubMed] [Google Scholar]
- Dyall-Smith M. L., Elleman T. C., Hoyne P. A., Holmes I. H., Azad A. A. Cloning and sequence of UK bovine rotavirus gene segment 7: marked sequence homology with simian rotavirus gene segment 8. Nucleic Acids Res. 1983 May 25;11(10):3351–3362. doi: 10.1093/nar/11.10.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghibelli L., Usala S. J., Mukhopadhyay R., Haselkorn R. Polyadenylation and reverse transcription of bacteriophage phi 6 double-stranded RNA. Virology. 1982 Jul 30;120(2):318–328. doi: 10.1016/0042-6822(82)90033-2. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard S. C., Ivatt R. J. Synthesis and processing of asparagine-linked oligosaccharides. Annu Rev Biochem. 1981;50:555–583. doi: 10.1146/annurev.bi.50.070181.003011. [DOI] [PubMed] [Google Scholar]
- Imai M., Richardson M. A., Ikegami N., Shatkin A. J., Furuichi Y. Molecular cloning of double-stranded RNA virus genomes. Proc Natl Acad Sci U S A. 1983 Jan;80(2):373–377. doi: 10.1073/pnas.80.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau P. C., Spencer J. H. An efficient synthetic primer for the M13 cloning dideoxy sequencing system. Biosci Rep. 1982 Sep;2(9):687–696. doi: 10.1007/BF01114830. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi S., Inoue A., Kita T., Nakamura M., Chang A. C., Cohen S. N., Numa S. Nucleotide sequence of cloned cDNA for bovine corticotropin-beta-lipotropin precursor. Nature. 1979 Mar 29;278(5703):423–427. doi: 10.1038/278423a0. [DOI] [PubMed] [Google Scholar]
- Palfree R. G., Bussey H. Yeast killer toxin: purification and characterisation of the protein toxin from Saccharomyces cerevisiae. Eur J Biochem. 1979 Feb 1;93(3):487–493. doi: 10.1111/j.1432-1033.1979.tb12847.x. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skipper N. Synthesis of a double-stranded cDNA transcript of the killer toxin-coding region of the yeast M1 double-stranded RNA. Biochem Biophys Res Commun. 1983 Jul 29;114(2):518–525. doi: 10.1016/0006-291x(83)90811-2. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., Quinn P. S., Chan S. J., Marsh J., Tager H. S. Processing mechanisms in the biosynthesis of proteins. Ann N Y Acad Sci. 1980;343:1–16. doi: 10.1111/j.1749-6632.1980.tb47238.x. [DOI] [PubMed] [Google Scholar]
- Stern A. S., Jones B. N., Shively J. E., Stein S., Undenfriend S. Two adrenal opioid polypeptides: proposed intermediates in the processing of proenkephalin. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1962–1966. doi: 10.1073/pnas.78.3.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele D. J., Wang R. W., Leibowitz M. J. Separation and sequence of the 3' termini of M double-stranded RNA from killer yeast. Nucleic Acids Res. 1982 Mar 11;10(5):1661–1678. doi: 10.1093/nar/10.5.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. Y., Dubuc G., Narang S. Escherichia coli plasmid vectors containing synthetic translational initiation sequences and ribosome binding sites fused with the lacZ gene. Gene. 1982 Sep;19(2):211–219. doi: 10.1016/0378-1119(82)90008-7. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Welsh J. D., Leibowitz M. J. Localization of genes for the double-stranded RNA killer virus of yeast. Proc Natl Acad Sci U S A. 1982 Feb;79(3):786–789. doi: 10.1073/pnas.79.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B. Genetic control of replication of the double-stranded RNA segments of the killer systems in Saccharomyces cerevisiae. Arch Biochem Biophys. 1983 Apr 1;222(1):1–11. doi: 10.1016/0003-9861(83)90496-4. [DOI] [PubMed] [Google Scholar]


