Abstract
Cytoskeleton remodelling is a prerequisite step for the morphological transition from preadipocytes to mature adipocytes. Although microtubules play a pivotal role in organizing cellular structure, regulation of microtubule dynamics during adipogenesis remains unclear. In the present paper we show that acetylation of α-tubulin is up-regulated during adipogenesis, and adipocyte development is dependent on α-tubulin acetylation, as expression of an acetylation-resistant α-tubulin mutant significantly inhibits adipogenesis. Moreover, acetylation of α-tubulin is under the control of the acetyltransferase MEC-17 and deacetylases SIRT2 (Sirtuin 2) and HDAC6 (histone deacetylase 6). Adipocyte development is inhibited in MEC-17-knockdown cells, but enhanced in MEC-17-overexpressing cells. Finally, we show that katanin, a microtubule-severing protein with enhanced activity on acetylated α-tubulin, is actively involved in adipogenesis. We propose that co-ordinated up-regulation of α-tubulin acetylation initiates cytoskeleton remodelling by promoting α-tubulin severing by katanin which, in turn, allows expansion of lipid droplets and accelerates the morphological transition toward mature adipocytes.
Keywords: acetylation, cytoskeleton, histone deacetylase 6 (HDAC6), Sirtuin 2 (SIRT2)
INTRODUCTION
Obesity, diabetes and other metabolic diseases have become a major health burden in almost all of the developed countries and many developing nations. Elucidation of the underlying mechanism of fat cell development is pivotal for developing clinical strategies to treat these diseases. In mammals, a large amount of energy is stored in adipose tissue, which consists of highly specialized adipocytes. Under excessive caloric intake, energy is stored in the form of triacylglycerol by the process of lipogenesis. Although fat can be temporarily stored in the liver, long-term storage of fat is predominantly done in adipocytes under normal physiological conditions. New adipocytes arise through adipogenesis, a complex process involving differentiation of naturally occurring preadipocytes into mature adipocytes [1–3]. At the molecular level, groups of transcription factors are activated in temporal sequence. Well-studied early factors include members of the CCAAT enhancer family C/EBP (CCAAT/enhancer-binding protein) β and C/EBPδ [1, 3], which sequentially activate the later transcription factors such as PPARγ (peroxisome-proliferator-activated receptor γ), a peroxisome-proliferator-activated receptor residing in the nucleus and C/EBPα, another CCAAT family member. Individual factors, e.g. PPARγ and C/EBPα, may interact with each other to maintain the adipogenic phenotype [4]. Expression of adipogenic regulators is controlled and influenced at multiple levels, including chromatin remodelling by post-translational modifications, such as methylation and acetylation of histones [5–7].
In the course of adipogenesis, fibroblast-like preadipocytes are transformed into lipid-filled morphologically distinct spherical mature adipocytes. Mature adipocytes are usually occupied by one big LD (lipid droplet) with all of the other organelles located at the periphery of the cell body. It was suggested nearly 20 years ago that the transition from fibroblasts to mature adipocytes involves significant reorganization of the cytoskeleton or cytoskeleton remodelling [8]. Indeed, disruption of MTs (microtubules), the main components of the cytoskeleton, leads to increased intracellular triacylglycerol accumulation [9] and significantly enhanced adipogenesis [10]. Other cytoskeletal components have also been linked to adipogenesis, for example, the intermediate filament vimentin forms a cage structure surrounding nascent LDs [11], whereas F-actin (filamentous actin) associates with caveolin during adipogenesis to form a unique caveolin–actin structure in mature adipocytes [12]. These studies suggest that cytoskeleton remodelling, including disruption of MTs, is a limiting step in the morphological transition during adipogenesis. However, it is not clear how MT-based cytoskeleton remodelling is regulated and initiated during adipogenesis.
MTs are composed of highly dynamic polymers of α- and β-tubulin heterodimers. In eukaryotic cells, tubulin subunits undergo various post-translational modifications that may influence the properties of MTs. For example, tubulin acetylation was shown to be critical for oligodendrocyte differentiation [13]. Acetylation of α-tubulin is controlled by the relative abundance and enzymatic activities of acetyltransferases and deacetylases. Although tubulin acetylation was first detected on the MT axonemes of Chlamydomonas motile cilia nearly 30 years ago [14], the identities of the enzymes regulating the acetylation process emerged much later, with the identification of the tubulin deacetylases HDAC6 (histone deacetylase 6) and SIRT2 (Sirtuin 2) [15–17]. The identification of MEC-17, or αTAT1, as the tubulin acetyltransferase responsible for adding an acetyl moiety to the ε-amino group of Lys40 on α-tubulin was discovered only recently in comparison [18, 19]. Another protein, the elongator complex subunit ELP3 (elongation protein 3), was proposed to be a tubulin acetyltransferase to control the levels of α-tubulin acetylation in neurons [20]. However, partially purified ELP3 displayed only weak acetyltransferase activity [20], and MTs remain highly acetylated in neurons from Caenorhabditis elegans or Drosophila ELP3-deletion mutant and cerebrum of familial dysautonomia, indicating that ELP3 may not be involved in α-tubulin acetylation [21–23]. Although α-tubulin acetylation has been shown to be critical for oligodendrocyte differentiation, it remains to be determined whether it plays a role in other physiological processes requiring cytoskeleton remodelling, such as adipogenesis.
Acetylation of α-tubulin has been shown to exhibit increased sensitivity for MT severing by katanin [24]. Katanin, an AAA (ATPase associated with various cellular activities) ATPase-domain-containing protein, is widely expressed and critical for neuronal development through its activity in mediating MT severing and reorganizing MTs in mature neurons [25]. Considering that MT-based cytoskeleton remodelling is also involved in the morphological transformation from preadipocytes to mature adipocytes, it is tempting to hypothesize that a similar mechanism operates in adipocyte development, in particular during the morphological transition in adipogenesis.
In the present study, we investigated whether acetylation of α-tubulin played a role during adipogenesis, and examined its regulation by the tubulin acetyltransferase MEC-17. We also studied whether the MT-severing protein katanin was involved in adipocyte development.
MATERIALS AND METHODS
Animal welfare
10-week-old C57BL/6 mice were fed on a HFD (high-fat diet) or LFD (low-fat diet) for 8 weeks. Gonadal adipose tissues were collected for protein extraction and immunoblotting. All experiments involving animals were reviewed and approved by the Institutional Animal Care and Use Committee of Agency for Science Technology and Research (A*STAR).
Cell culture
3T3-L1 cells were cultured in CS medium [high-glucose DMEM (Dulbecco’s modified Eagle’s medium; Gibco), 10 % FBS (fetal bovine serum; Gibco) and 1 % penicillin/streptomycin (Gibco)]. Confluent 3T3-L1 fibroblast cells were then subjected to MDI induction medium 2 days post-confluence. The components of the MDI medium were as follows: 1 µM dexamethasone, 0.5 mM IBMX (isobutylmethylxanthine), and 100 nM insulin in high-glucose DMEM containing 10 % FBS. At 2 days after exposure to the MDI induction medium, the medium was replaced with high-glucose DMEM supplemented with 10 % FBS and 100 nM insulin. Thereafter, medium was replaced with high-glucose DMEM plus 10 % FBS every 2 days. 293TN cells (System Biosciences) were grown in high-glucose DMEM supplemented with 10 % FBS and 1 % penicillin/streptomycin. All cells were maintained at 37 °C ina 5 % CO2 humidified incubator.
DNA constructs
Short hairpin oligonucleotides targeting murine SIRT2, HDAC6, MEC-17 and katanin were subcloned into lentiviral vector L309 to generate respective lentiviral KD (knockdown) constructs (Supplementary Table S1 at http://www.biochemj.org/bj/449/bj4490605add.htm). The murine Atat (encoding MEC-17) cDNA was released from pCMV-SPORT6-MEC-17 and cloned into the L309 vector to generate a MEC-17-overexpressing lentiviral plasmid. The coding sequence of EGFP [enhanced GFP (green fluorescent protein)]-tagged p60-katanin [26] and EGFP–tubulin [WT (wild-type) or K40R mutant] was inserted into the lentiviral vector L304 by blunt ligation to generate L304-EGFP-p60-katanin and L304-EGFP-tubulin respectively. L304 and L309 vectors were gifts from Dr Zhiping Pang (Stanford University, Stanford, CA, U.S.A.), pCMV-SPORT6-MEC-17 was from Dr Jacek Gaertig (University of Georgia, Athens, GA, U.S.A.) and the tubulin constructs were from Dr Tso-Pang Yao (Duke University, Durham, NC, U.S.A.) [27–29]. All of the DNA constructs were verified by sequencing.
Production of pseudovirus particles
Along with three packaging plasmids carrying the genes for gag, pol and env proteins, the KD and overexpression lentiviral vectors were transfected into 293TN cells. At 2 days after transfection, the culture medium laden with the pseudoviral particles was harvested and filtered.
Real-time qPCR (quantitative PCR)
Total RNA was extracted from cells using TRIzol® reagent (Invitrogen), followed by DNase treatment and reverse transcription with the Revertaid™ H minus First-strand cDNA Synthesis kit (Fermentas). The resulting cDNAs were used for quantification of mRNA expression levels with the SYBR® green method (ABI Biosystems). The expression level of Tbp (TATA-box binding protein) was used as an internal control. The primer sequences for qPCR reactions are listed in Supplementary Table S2 (at http://www.biochemj.org/bj/449/bj4490605add.htm).
Western blot analysis
Adipose tissue or cells were homogenized in a Tris-based lysis buffer on ice. Protein concentrations of the resulting lysates were quantified using the DC Protein Assay (Bio-Rad Laboratories). Samples containing 50 µg of protein were loaded into each well, resolved on a polyacrylamide gel, and blotted on to a nitrocellulose membrane using iBlot™ (Invitrogen). Membranes were then probed with antibodies and visualized with ECL (enhanced chemiluminescence) or ECLPlus reagents. Antibodies used in the present study were: anti-α-tubulin (Sigma, dilution 1:5000), anti-(acetylated α-tubulin) (Sigma, dilution 1:2000), anti-MEC-17 (Sigma, dilution 1:1000), anti-SIRT2 (Cell Signaling Technology, dilution 1:1000), anti-HDAC6 (Abcam, dilution 1:1000) and anti-β-actin (Santa Cruz Biotechnology, dilution 1:5000).
Oleic acid uptake
3T3-L1 cells were plated in 24-well plates on growth medium. The cells were treated with 400 µM oleic acid complexed to 0.4 % BSA for 18 h before fixation with 4 % formaldehyde. LDs were stained with LipidTOX (Invitrogen) according to the manufacturer’s instructions and visualized under a confocal microscope (Nikon).
Oil-Red O staining and propan-2-ol extraction
Oil-Red O staining and propan-2-ol extraction were performed as described previously [30, 31]. The stained cells were imaged on Nikon SMZ1500 and TS100 microscopes before they were air-dried. Stained lipids were extracted by using propan-2-ol for lipid content measurements. The absorbance of the extracted solution was measured at 500 nm wavelength as a measure of the relative cellular lipid content.
Immunostaining
3T3-L1 cells were seeded on to coverslips and fixed in 4 % paraformaldehyde. The cells were then permeabilized with 0.1 % Triton X-100, blocked with 10 % normal goat serum and probed with antibodies diluted in blocking buffer. Coverslips were mounted on to slides in DAPI (4′,6-diamidino-2-phenylindole) mounting medium (Invitrogen) and then imaged with A1R confocal microscopy (Nikon).
Statistical analysis
Comparisons of data were made by using a two-tailed unpaired t test with equal variance. Statistical significance was displayed as *P < 0.05, **P < 0.01 or ***P < 0.001, or as indicated in Figures.
RESULTS
Microtubule-based cytoskeleton remodelling during adipogenesis
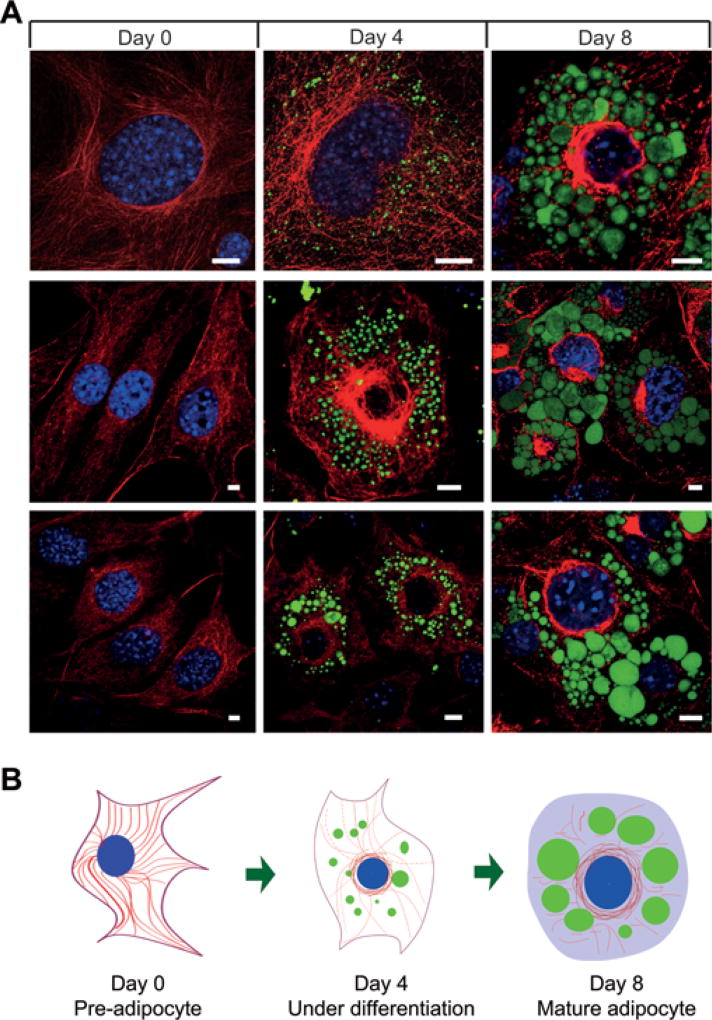
Previous studies have linked α-tubulin acetylation with oligodendrocyte differentiation [13], but it is not clear how tubulin acetylation contributes to cell differentiation. Since these cells undergo dramatic morphology changes during cell differentiation and maturation, it is possible that α-tubulin acetylation may initiate such changes by promoting cytoskeleton remodelling. Like oligodendrocytes, adipocytes also undergo significant changes during adipogenesis. To evaluate the dynamics of the endogenous MT network, we stained MTs at different stages of adipocyte differentiation. In undifferentiated pre-adipocytes (day 0), MTs were well organized in a regular array (Figure 1). When cells were under differentiation after adipogenesis was initiated (day 4), MTs appeared to be shorter and were arranged into a cage-like structure, which might be necessary to accommodate development of nascent LDs (Figure 1). As the cells further matured (day 8), MTs were mostly disrupted and disappeared at the regions around LDs, although they remained around the nucleus (Figure 1). To better understand the dynamic changes of the MTs, we measured the lengths of the MTs at selected time points [32]. The MT lengths showed significant reduction in cells at days 4 and 8 when compared with day 0 (35.1 ± 2.3 µm, 15.6 ± 1.0 µm and 8.3 ± 0.6 µm for days 0, 4 and 8 respectively; 50 MTs from five cells for each time point, P < 0.01 for days 4 and 8 compared with day 0). As well as the dynamics of MTs, we also examined LD development in relation to the MT network. Consistent with the notion that the MT network may be remodelled to allow LDs to emerge and expand, LDs were found inside the lattice-like structure of MTs when the cells were under differentiation (Figure 1 and Supplementary Movies S1 and S2 at http://www.biochemj.org/bj/449/bj4490605add.htm), and MTs were mostly disrupted or absent at the region near the growing LDs when the cells became mature (Figure 1 and Supplementary Movies S3 and S4 at http://www.biochemj.org/bj/449/bj4490605add.htm). To test whether MT disassembly promotes adipogenesis, we treated 3T3-L1 cells with nocodazole, which interferes with MT polymerization and promotes MT disassembly [15]. As measured on day 7 after adipogenic cocktail treatment, lipid accumulation (Supplementary Figure S1A at http://www.biochemj.org/bj/449/bj4490605add.htm) and total lipid content (Supplementary Figure S1B) were increased with increasing nocodazole concentrations. It is worth noting that the cells showed round morphology with an enlarged size at higher concentrations of nocodazole (Supplementary Figure S1A), consistent with previous reports that disassembly of cytoskeleton promoted adipogenesis in ES (embryonic stem) cells and bovine intramuscular preadipose cells [9, 10]. These results show that the MT network undergoes significant changes during adipogenesis, and suggest that MT-associated cytoskeletal remodelling may play an active role in adipogenesis by regulating LD expansion and morphological transition during adipocyte development.
Figure 1. MT-based cytoskeletal remodelling during adipocyte differentiation.
(A) 3T3-L1 cells at days 0, 4 and 8 after adipogenic cocktail treatment were fixed, co-stained with the anti-tubulin antibody (red) and LipidTOX reagent (green), and imaged on a confocal microscope for visualizing MTs and LDs. Scale bars = 10 µm. (B) A schematic drawing depicting morphological changes of the MT network during adipocyte differentiation. LDs, MTs and nuclei are indicated by green circles, red lines and blue circles respectively.
Up-regulation of α-tubulin acetylation during adipogenesis
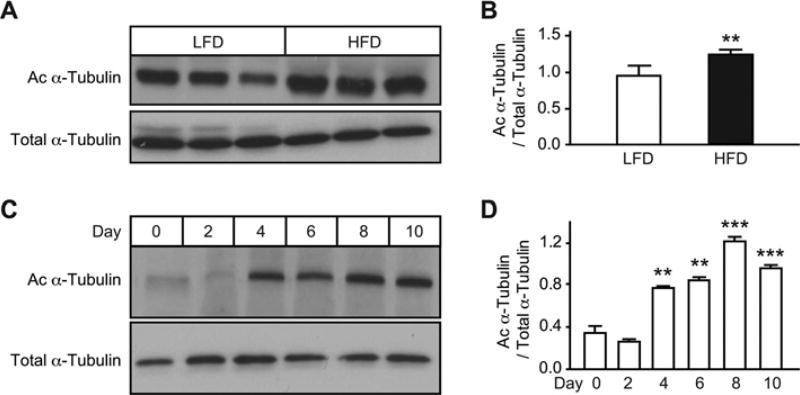
To test whether α-tubulin acetylation participates in the adipogenic process, we first examined the extent of α-tubulin acetylation in adipose tissue of mice fed on a HFD or LFD. Acetylation of α-tubulin was significantly higher in the adipose tissue of HFD-fed mice when compared with LFD-fed mice, suggesting that α-tubulin acetylation is associated with fat accumulation in vivo (Figures 2A and 2B). We next examined changes of α-tubulin acetylation during adipocyte differentiation, and found a general increase in acetylated α-tubulin levels in normal differentiating 3T3-L1 cells (Figures 2C and 2D). These data support a potential role for α-tubulin acetylation in adipogenesis.
Figure 2. Acetylation of α-tubulin is up-regulated during adipogenesis.
(A) Increased levels of acetylated α-tubulin (Ac α-Tubulin) in white adipose tissue of HFD-fed mice. C57BL/6 male mice were fed on a HFD or LFD for 8 weeks (n = 3 mice per group). Levels of acetylated α-tubulin and total α-tubulin were detected by Western blot analysis. The amount of acetylated α-tubulin was normalized to that of total α-tubulin (B). **P < 0.01. (C) Acetylation of α-tubulin was up-regulated during adipogenesis in 3T3-L1 cells. (D) The amount of acetylated α-tubulin was quantified and normalized to total α-tubulin. **P < 0.01 and ***P < 0.001.
To understand the regulation of α-tubulin acetylation during adipogenesis, we measured the expression profile and relative levels of tubulin acetyltransferase and deacetylases. Expression of the tubulin acetyltransferase MEC-17 showed a biphasic pattern: its expression decreased during the early stage of differentiation, followed by an increase to a steady level at the late phase of adipogenesis (Supplementary Figures S2A and S2B at http://www.biochemj.org/bj/449/bj4490605add.htm). In contrast, expression of the tubulin deacetylases SIRT2 and HDAC6 reduced upon adipogenic induction, and remained low throughout the adipogenic process (Supplementary Figure S3 at http://www.biochemj.org/bj/449/bj4490605add.htm). As tubulin acetylation is determined by the relative abundance of the acetyltransferase and deacetylases, the observed increase in acetylated α-tubulin levels during adipogenesis (Figures 2C and 2D) could be accounted for by the combined effect of up-regulation of MEC-17 and down-regulation of SIRT2 and HDAC6. Moreover, other lysine acetyltransferases, such as TAT2, may contribute to tubulin acetylation [19]. In summary, these results suggest that α-tubulin acetylation is regulated by co-ordinated activities of the acetyltransferase MEC-17 and the deacetylases SIRT2 and HDAC6.
Adipogenesis is dependent on α-tubulin acetylation
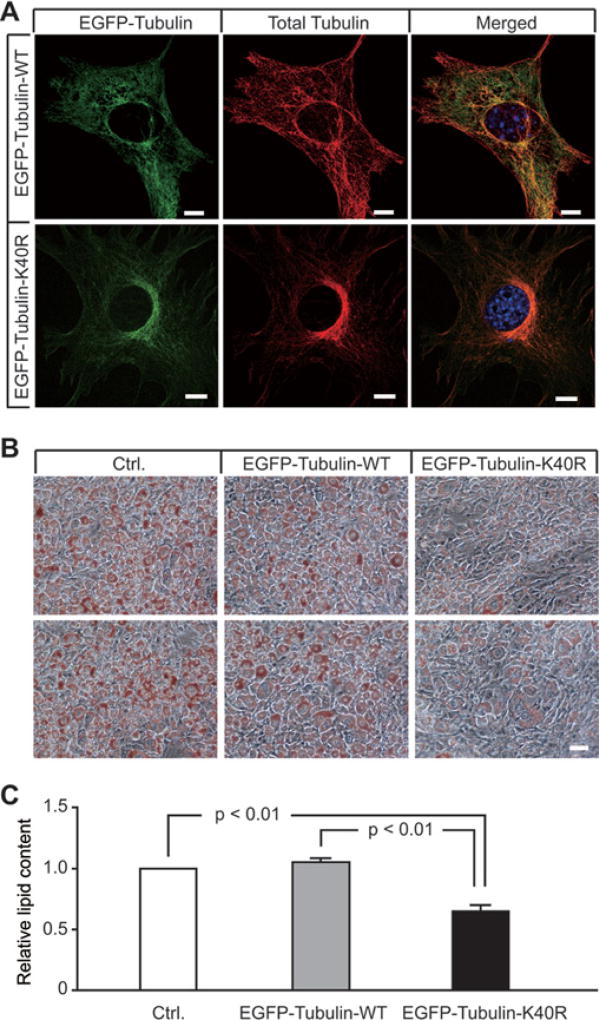
To test whether α-tubulin acetylation contributes to adipogenesis, we generated stable 3T3-L1 cell lines expressing EGFP-fused WT α-tubulin (EGFP–tubulin-WT) or an acetylation-resistant α-tubulin mutant (EGFP–tubulin-K40R), and examined lipid accumulation and total lipid content in these cells after differentiation. EGFP–tubulin-WT and EGFP–tubulin-K40R were expressed at comparable levels (Supplementary Figure S4A at http://www.biochemj.org/bj/449/bj4490605add.htm) and were able to incorporate into the MT network, indistinguishable from endogenous tubulin (Figure 3A). At an early stage of differentiation, both cell lines showed a clear and intact MT network (Supplementary Figure S4B). However, at a late stage of differentiation, cells expressing EGFP–tubulin-WT showed diffuse tubulin staining, indicative of a disrupted MT network, whereas the majority of cells expressing EGFP–tubulin-K40R retained a relatively intact MT network and fibroblast-like morphology (Supplementary Figure S4B). Compared with EGFP–tubulin-WT cells, EGFP–tubulin-K40R cells exhibited dramatically reduced lipid accumulation (Figure 3B) and total lipid content (Figure 3C), indicating that acetylation-resistant α-tubulin inhibits the morphological transition from preadipocytes to mature adipocytes by preventing cytoskeleton remodelling. These results suggest that acetylation of α-tubulin at Lys40 promotes adipogenesis, possibly by initiating MT severing and the morphological transition from preadipocytes to mature adipocytes.
Figure 3. An acetylation-resistant α-tubulin mutant inhibits lipid accumulation during adipocyte development.
(A) Stable 3T3-L1 cells expressing EGFP–α-tubulin (EGFP-Tubulin-WT) or EGFP–α-tubulin-K40R (EGFP-Tubulin-K40R) were fixed, stained with the anti-tubulin antibody and imaged on a confocal microscope. Note that EGFP-tagged α-tubulin was well incorporated into the MT network. Scale bars = 10 µm. (B) Stable 3T3-L1 cells expressing empty vector (Ctrl.), EGFP–α-tubulin-WT or EGFP–α-tubulin-K40R were stained with Oil-Red O and assessed by microscopy (20×). Two different fields are shown for each group to illustrate reproducibility. Scale bars = 50 µm. (C) Propan-2-ol extracts of Oil-Red O from the cells in (B) were used to determine relative total lipid content. Values are means ± S.E.M. (n = 3–4 independent experiments). P values are indicated on the histogram.
MEC-17 participates in the regulation of lipid accumulation during adipogenesis
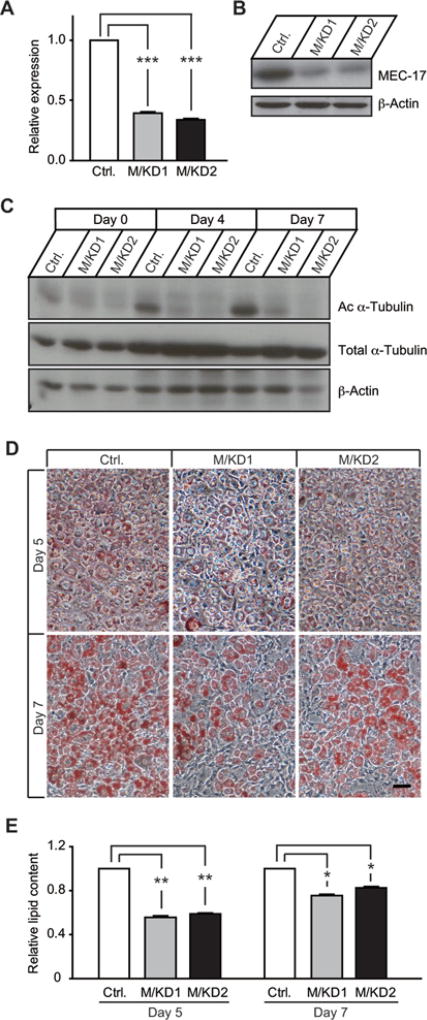
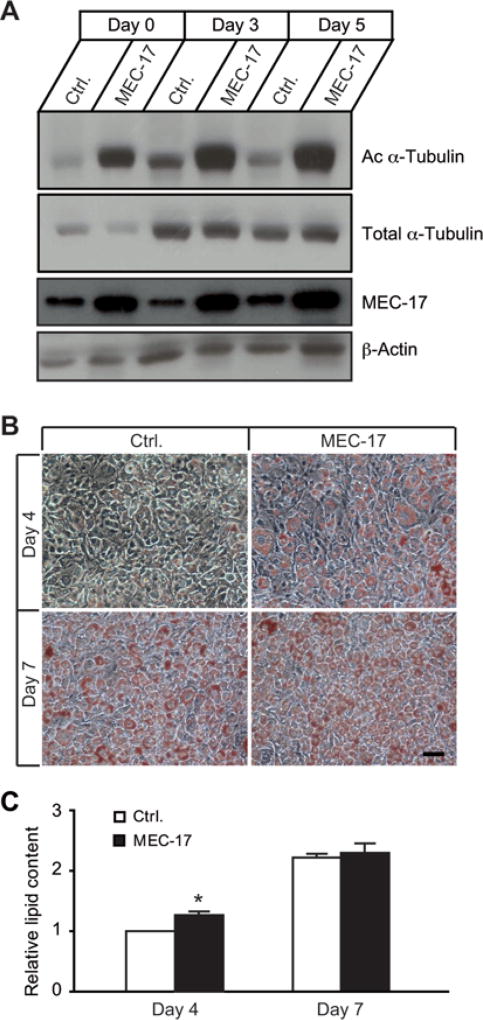
To examine whether MEC-17, an α-tubulin acetyltransferase, was involved in adipogenesis, we first generated stable MEC-17-KD 3T3-L1 cells with lentivirus-mediated shRNA (short hairpin RNA). Two stable cell lines with more than 60 % KD of MEC-17 expression were selected for characterization of adipocyte differentiation and lipid accumulation (Figures 4A and 4B, Supplementary Table S1). Consistent with the significant reduction in MEC-17 expression, the levels of acetylated α-tubulin were dramatically decreased at selected time points during adipogenesis (Figure 4C). This demonstrates that MEC-17 is a key regulator of α-tubulin acetylation. The extent of adipogenesis and lipid accumulation was significantly reduced in MEC-17-KD cells (Figures 4D and 4E), indicating that adipogenesis is dependent on α-tubulin acetylation, and that α-tubulin acetylation plays an active role in the adipogenic programme. Next, we measured lipid accumulation in 3T3-L1 cells with stable overexpression of MEC-17. As expected, MEC-17 overexpression led to significantly enhanced α-tubulin acetylation (Figure 5A), and increased lipid production and accumulation (Figures 5B and 5C). The effect on increased adipogenesis was more evident at day 4, but less so at day 7, after adipogenic induction, suggesting that a higher level of α-tubulin acetylation accelerates the adipogenic process without affecting the extent of adipogenesis at maturity. Taken together, these results provide evidence that MEC-17 participates in the regulation of lipid accumulation during adipogenesis by increasing α-tubulin acetylation.
Figure 4. KD of MEC-17 leads to impaired lipid accumulation during adipocyte development.
(A) MEC-17 was stably knocked down in 3T3-L1 cells by using lentivirus-mediated shRNA targeting murine MEC-17. Two stable cell lines (M/KD1 and M/KD2) with efficient down-regulation of MEC-17 were selected on the basis of mRNA levels by qPCR compared with the control group (scrambled shRNA) (A) or protein levels by Western blots analysis (B). (C) KD of MEC-17 caused significant down-regulation of α-tubulin acetylation. Acetylated α-tubulin (Ac α-Tubulin) and total α-tubulin were detected at different time points after adipogenic cocktail treatment. (D and E) Reduced lipid accumulation and total lipid content in MEC-17-KD cells at days 5 and 7 after adipogenic cocktail treatment. Intracellular lipids were stained with Oil-Red O and imaged at 20× magnification. Values are means ± S.E.M. (n = 3–4 independent experiments). *P < 0.05, **P < 0.01 and ***P < 0.001. Scale bar = 50 µm.
Figure 5. Overexpression of MEC-17 promotes α-tubulin acetylation and accelerates lipid accumulation during adipocyte development.
(A) The levels of acetylated α-tubulin (Ac α-Tubulin) were apparently increased in 3T3-L1 cells with stable overexpression of MEC-17 compared with the control group (Ctrl.) which expressed empty vector at days 0, 3 and 5 after adipogenic cocktail treatment. (B) 3T3-L1 cells with stable overexpression of MEC-17 were fixed and stained with Oil-Red O at days 4 and 7 after adipogenic cocktail treatment. Oil-Red O staining was apparently more intense in MEC-17-overexpressing cells at day 4, but no obvious difference was observed at day 7. (C) Propan-2-ol extracts of Oil-Red O from the cells in (C) were used to determine relative total lipid content. Values are means ± S.E.M. (n = 3–4 independent experiments). *P < 0.05. Scale bar = 50 µm.
As the extent of α-tubulin acetylation directly regulates the adipogenic process, we tested whether SIRT2 and HDAC6 also regulate adipogenesis by affecting the levels of α-tubulin acetylation. We generated stable 3T3-L1 cells with KD of SIRT2 or HDAC6 (Supplementary Figure S5A at http://www.biochemj.org/bj/449/bj4490605add.htm). Although the KD efficiency for SIRT2 and HDAC6 was different, KD of SIRT2 or HDAC6 led to similar levels of enhanced α-tubulin acetylation (Supplementary Figures S5B and S5C). Furthermore, we observed increased lipid accumulation in SIRT2- or HDAC6-KD cells (Supplementary Figures S5D and S5E). It is worth noting that KD of HDAC6 did not affect lipogenesis at day 7 after adipogenic induction, whereas increased lipid accumulation was observed in SIRT2-KD cells at the same time point. Similar observations of increased lipid accumulation at early and late stages of adipocyte differentiation was shown in SIRT2-KD cells, and was attributed to increased FoxO1 (forkhead box O1) acetylation and PPARγ activation [33]. These results support the notion that α-tubulin acetylation accelerates the adipogenic process during the morphological transition, but does not affect the extent of lipogenesis at the late stage of adipogenesis.
Katanin participates in acetylation-mediated cytoskeleton remodelling during adipogenesis
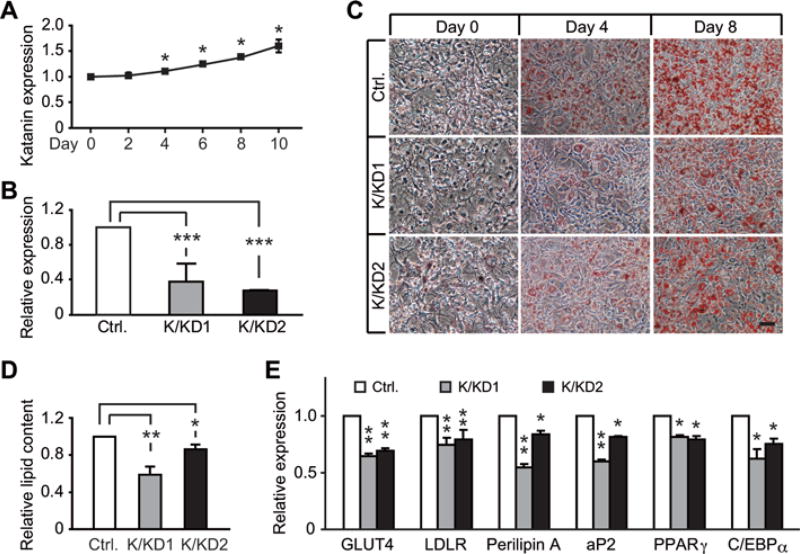
As acetylation of α-tubulin was shown to have increased sensitivity to katanin severing [24], we first examined the developmental changes of katanin expression during adipogenesis. We found that expression levels of katanin exhibited a gradual increase starting from day 4 post-adipogenic induction, and reached ~150 % of baseline level in mature adipocytes (Figure 6A). We next tested whether the distribution of katanin supported its role as a regulator of MTs and the cytoskeletal network during adipogenesis. In preadipocytes (day 0), a low degree of overlap was seen between katanin and tubulin, but the overlap was much increased at days 3 and 5 after adipogenic stimulation (Supplementary Figure S6A at http://www.biochemj.org/bj/449/bj4490605add.htm). Pearson’s correlation coefficients for katanin and tubulin co-localization were significantly increased during adipogenesis (Supplementary Figure S6B), suggesting an increased katanin–tubulin association in the course of adipogenesis. These findings suggest that the dynamics of MTs is intimately related to the distribution of katanin during adipocyte differentiation. To test whether katanin was involved in regulating adipogenesis, we transduced 3T3-L1 cells with lentivirus carrying shRNA sequences that target murine Katn (katanin), and selected two stable cell lines with efficient gene suppression (Figure 6B). KD of katanin expression led to reduced lipid accumulation (Figure 6C) and total lipid content (Figure 6D) when compared with the control cells transduced with virus carrying scrambled shRNA sequence. As expected, katanin-KD cells showed significantly reduced expression of adipocyte markers, such as GLUT4 (glucose transporter 4), LDLR (low-density lipoprotein receptor), perilipin and aP2 (adipocyte P2), and mild reduction of two master transcription factors, PPARγ and C/EBPα (Figure 6E). These findings suggest that katanin is essential for normal adipocyte differentiation, and support our model that katanin is actively involved in cytoskeleton remodelling by severing acetylated α-tubulin which, in turn, promotes the morphological transition towards mature adipocytes, and accommodates LD development and expansion.
Figure 6. KD of katanin inhibits adipogenesis in 3T3-L1 cells.
(A) Expression levels of katanin were assessed by qPCR at various time points during adipocyte differentiation. Values are means ± S.E.M. n = 3 independent experiments, each measured in triplicate. (B) Two stable cell lines (K/KD1 and K/KD2) with efficient down-regulation of katanin compared with the control (Ctrl.) group (scrambled shRNA) were selected on the basis of mRNA levels by qPCR. (C) Stable katanin (K/KD1 and K/KD2)-KD 3T3-L1 cells were stained with Oil-Red O at days 0, 4 and 8 after adipogenic cocktail treatment. Scale bar = 50 µm. (D) Propan-2-ol extracts of Oil-Red O were used to assess relative total lipid content in katanin-KD cells at day 8 post-adipogenic cocktail treatment. Values are means ± S.E.M. n = 3–4 independent experiments. (E) Expression levels of adipocyte markers and adipogenic regulators were determined by qPCR in katanin-KD cells at day 8 post-adipogenic cocktail treatment. Values are means ± S.E.M. n = 3 independent experiments, each measured in triplicate. *P < 0.05, **P < 0.01 and ***P < 0.001. aP2, adipocyte P2; GLUT4, glucose transporter 4; LDLR, low-density lipoprotein receptor.
DISCUSSION
Cytoskeleton remodelling is an essential step for the morphological transition from fibroblast-like preadipocytes to lipid-filled unilocular mature adipocytes during adipocyte development. In the present study, we show that α-tubulin acetylation plays an active role in adipogenesis, and that α-tubulin acetylation is under the control of the tubulin acetyltransferase MEC-17 and deacetylases SIRT2 and HDAC6. Furthermore, acetylation of α-tubulin may increase its sensitivity to katanin-mediated severing and the ensuing cytoskeleton remodelling.
Tubulin acetylation has been shown to be enriched during neuronal commitment [34] to regulate oligodendrocyte differentiation [13]. Differentiation of oligodendrocytes is accompanied by extensive morphological changes. Similarly, adipocyte development requires cytoskeleton remodelling to accommodate LD development and expansion, and to allow morphological transition from fibroblast-like preadipocytes to round adipocytes. The finding in the present study that α-tubulin acetylation plays an active role in adipocyte differentiation suggests that the specific post-translational modification in α-tubulin may be a key regulating step for the differentiation of various cell types. We further suggest and demonstrate katanin as the link between increased α-tubulin acetylation and cytoskeleton remodelling, as acetylation increases the sensitivity of α-tubulin to katanin severing [24] and acetylation-resistant α-tubulin inhibits adipogenesis (Figure 3 and Supplementary Figure S4).
The acetylation of α-tubulin is regulated by the relative levels of acetyltransferase and deacetylases. We found that MEC-17 or αTAT1 directly controls the acetylation level of α-tubulin in loss- and gain-of-function experiments (Figures 4 and 5), supporting its proposed role as an α-tubulin acetyltransferase [18, 19]. Furthermore, we confirmed that HDAC6 and SIRT2 are involved in the regulation of α-tubulin acetylation [15–17]. HDAC6, a class II HDAC, is mostly co-localized with MTs in the cytoplasm and functions to deacetylate α-tubulin both in vitro and in vivo [15]. SIRT2, a second α-tubulin deacetylase, has been shown to interact with HDAC6, but function independently in deacetylating α-tubulin [16]. Besides α-tubulin, SIRT2 also deacetylates and activates the transcription factor FoxO1, which inhibits adipogenesis by binding to PPARγ [33]. We found that MEC-17- and HDAC6-mediated α-tubulin regulation accelerates the adipogenic process, probably by promoting cytoskeleton remodelling, without affecting the extent of lipid accumulation (Figures 4 and 5, and Supplementary Figure S5). In contrast, in addition to accelerating the adipogenic process, KD of SIRT2 leads to increased lipid accumulation (Supplementary Figure S5), an effect that may be accounted for by the regulation of the FoxO1/PPARγ pathway by SIRT2 [33].
The expression level of katanin is correlated with its enhanced MT-severing activity [25, 26], thus overexpression of katanin should promote adipogenesis by accelerating cytoskeleton remodelling. Consistent with our model that acetylation serves as a signal to katanin action, katanin levels only increased from day 4 post-adipogenic induction, coinciding with the increased acetylation levels of α-tubulin. Further studies are needed to understand the mechanism underlying the co-ordinated regulation of acetylation-modifying enzymes and katanin.
Another unresolved issue is how katanin-mediated MT severing reorganizes the cytoskeleton network. Our model that acetylation of α-tubulin serves as a signal to induce katanin-mediated MT severing and consequent cytoskeleton remodelling suggests that α-tubulin acetylation determines the timing, whereas the action of katanin determines the subcellular sites of initiation of cytoskeleton remodelling. As the acetylation site Lys40 is at the luminal side and buried inside the protein, it is possible that the modification may perturb the lattice of the polymer to allow katanin access, and thus break the MTs [24]. This further illustrates the importance for the co-ordinated regulation of acetylation and katanin severing in neuronal development as previously reported [24], and in adipogenesis (the present study).
In summary, in the present paper we report that acetylation of α-tubulin is essential for adipocyte development, which is controlled by the acetyltransferase MEC-17 and two deacetylases SIRT2 and HDAC6. We also provide evidence that katanin, which preferentially severs acetylated α-tubulin, may participate in the adipogenic process by promoting cytoskeleton remodelling. We propose that co-ordinated up-regulation of α-tubulin acetylation initiates cytoskeleton remodelling by promoting α-tubulin severing by katanin which, in turn, accommodates the formation and growth of LDs, and accelerates the morphological transition towards mature adipocytes.
Supplementary Material
Acknowledgments
We thank Dr Cai Li and Dr E-Shyong Tai for critical reading of the paper before submission and helpful comments, and members of the Laboratory of Metabolic Medicine for discussions. We also thank SBIC-Nikon Imaging Centre for support with microscopic imaging.
FUNDING
This work was supported by an intramural funding from the A*STAR Biomedical Medical Research Council (to W.H.) and the National Science Foundation (NSF) [grant number 0841245 (to P.W.B.)].
Abbreviations used
- C/EBP
CCAAT/enhancer-binding protein
- DMEM
Dulbecco’s modified Eagle’s medium
- EGFP
enhanced green fluorescent protein
- ELP3
elongation protein 3
- FBS
fetal bovine serum
- FoxO1
forkhead box O1
- HDAC6
histone deacetylase 6
- HFD
high-fat diet
- KD
knockdown
- LD
lipid droplet
- LFD
low-fat diet
- MT
microtubule
- PPARγ
peroxisome-proliferator-activated receptor γ
- qPCR
quantitative PCR
- shRNA
short hairpin RNA
- SIRT2
Sirtuin 2
- WT
wild-type
Footnotes
AUTHOR CONTRIBUTION
Wulin Yang, Xiangxiang Guo and Shermaine Thein performed experiments and analysed the data. Weiping Han conceptualized the study. Wulin Yang, Feng Xu, Shigeki Sugii, Peter Baas, George Radda and Weiping Han designed the research. Wulin Yang and Weiping Han wrote the paper.
References
- 1.Gregoire FM, Smas CM, Sul HS. Understanding adipocyte differentiation. Physiol. Rev. 1998;78:783–809. doi: 10.1152/physrev.1998.78.3.783. [DOI] [PubMed] [Google Scholar]
- 2.Rosen ED, Spiegelman BM. Molecular regulation of adipogenesis. Annu. Rev. Cell Dev. Biol. 2000;16:145–171. doi: 10.1146/annurev.cellbio.16.1.145. [DOI] [PubMed] [Google Scholar]
- 3.Tontonoz P, Spiegelman BM. Fat and beyond: the diverse biology of PPARγ. Annu. Rev. Biochem. 2008;77:289–312. doi: 10.1146/annurev.biochem.77.061307.091829. [DOI] [PubMed] [Google Scholar]
- 4.Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: tracking obesity to its source. Cell. 2007;131:242–256. doi: 10.1016/j.cell.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Martin C, Zhang Y. The diverse functions of histone lysine methylation. Nat. Rev. Mol. Cell Biol. 2005;6:838–849. doi: 10.1038/nrm1761. [DOI] [PubMed] [Google Scholar]
- 6.Narlikar GJ, Fan HY, Kingston RE. Cooperation between complexes that regulate chromatin structure and transcription. Cell. 2002;108:475–487. doi: 10.1016/s0092-8674(02)00654-2. [DOI] [PubMed] [Google Scholar]
- 7.Guo W, Zhang KM, Tu K, Li YX, Zhu L, Xiao HS, Yang Y, Wu JR. Adipogenesis licensing and execution are disparately linked to cell proliferation. Cell Res. 2009;19:216–223. doi: 10.1038/cr.2008.319. [DOI] [PubMed] [Google Scholar]
- 8.Spiegelman BM, Farmer SR. Decreases in tubulin and actin gene expression prior to morphological differentiation of 3T3 adipocytes. Cell. 1982;29:53–60. doi: 10.1016/0092-8674(82)90089-7. [DOI] [PubMed] [Google Scholar]
- 9.Takenouchi T, Miyashita N, Ozutsumi K, Rose MT, Aso H. Role of caveolin-1 and cytoskeletal proteins, actin and vimentin, in adipogenesis of bovine intramuscular preadipocyte cells. Cell Biol. Int. 2004;28:615–623. doi: 10.1016/j.cellbi.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Feng T, Szabo E, Dziak E, Opas M. Cytoskeletal disassembly and cell rounding promotes adipogenesis from ES cells. Stem Cell Rev. 2010;6:74–85. doi: 10.1007/s12015-010-9115-8. [DOI] [PubMed] [Google Scholar]
- 11.Franke WW, Hergt M, Grund C. Rearrangement of the vimentin cytoskeleton during adipose conversion: formation of an intermediate filament cage around lipid globules. Cell. 1987;49:131–141. doi: 10.1016/0092-8674(87)90763-x. [DOI] [PubMed] [Google Scholar]
- 12.Kanzaki M, Pessin JE. Caveolin-associated filamentous actin (Cav-actin) defines a novel F-actin structure in adipocytes. J. Biol. Chem. 2002;277:25867–25869. doi: 10.1074/jbc.C200292200. [DOI] [PubMed] [Google Scholar]
- 13.Li W, Zhang B, Tang J, Cao Q, Wu Y, Wu C, Guo J, Ling EA, Liang F. Sirtuin 2, a mammalian homolog of yeast silent information regulator-2 longevity regulator, is an oligodendroglial protein that decelerates cell differentiation through deacetylating α-tubulin. J. Neurosci. 2007;27:2606–2616. doi: 10.1523/JNEUROSCI.4181-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.L’Hernault SW, Rosenbaum JL. Chlamydomonas α-tubulin is posttranslationally modified by acetylation on the epsilon-amino group of a lysine. Biochemistry. 1985;24:473–478. doi: 10.1021/bi00323a034. [DOI] [PubMed] [Google Scholar]
- 15.Hubbert C, Guardiola A, Shao R, Kawaguchi Y, Ito A, Nixon A, Yoshida M, Wang XF, Yao TP. HDAC6 is a microtubule-associated deacetylase. Nature. 2002;417:455–458. doi: 10.1038/417455a. [DOI] [PubMed] [Google Scholar]
- 16.North BJ, Marshall BL, Borra MT, Denu JM, Verdin E. The human Sir2 ortholog, SIRT2, is an NAD+ -dependent tubulin deacetylase. Mol. Cell. 2003;11:437–444. doi: 10.1016/s1097-2765(03)00038-8. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, Li N, Caron C, Matthias G, Hess D, Khochbin S, Matthias P. HDAC-6 interacts with and deacetylates tubulin and microtubules in vivo. EMBO J. 2003;22:1168–1179. doi: 10.1093/emboj/cdg115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akella JS, Wloga D, Kim J, Starostina NG, Lyons-Abbott S, Morrissette NS, Dougan ST, Kipreos ET, Gaertig J. MEC-17 is an α-tubulin acetyltransferase. Nature. 2010;467:218–222. doi: 10.1038/nature09324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shida T, Cueva JG, Xu Z, Goodman MB, Nachury MV. The major α-tubulin K40 acetyltransferase α TAT1 promotes rapid ciliogenesis and efficient mechanosensation. Proc. Natl. Acad. Sci. U.S.A. 2010;107:21517–21522. doi: 10.1073/pnas.1013728107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Creppe C, Malinouskaya L, Volvert ML, Gillard M, Close P, Malaise O, Laguesse S, Cornez I, Rahmouni S, Ormenese S, et al. Elongator controls the migration and differentiation of cortical neurons through acetylation of α-tubulin. Cell. 2009;136:551–564. doi: 10.1016/j.cell.2008.11.043. [DOI] [PubMed] [Google Scholar]
- 21.Chen C, Tuck S, Bystrom AS. Defects in tRNA modification associated with neurological and developmental dysfunctions in Caenorhabditis elegans elongator mutants. PLoS Genet. 2009;5:e1000561. doi: 10.1371/journal.pgen.1000561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miskiewicz K, Jose LE, Bento-Abreu A, Fislage M, Taes I, Kasprowicz J, Swerts J, Sigrist S, Versees W, Robberecht W, Verstreken P. ELP3 controls active zone morphology by acetylating the ELKS family member Bruchpilot. Neuron. 2011;72:776–788. doi: 10.1016/j.neuron.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 23.Cheishvili D, Maayan C, Cohen-Kupiec R, Lefler S, Weil M, Ast G, Razin A. IKAP/Elp1 involvement in cytoskeleton regulation and implication for familial dysautonomia. Hum. Mol. Genet. 2011;20:1585–1594. doi: 10.1093/hmg/ddr036. [DOI] [PubMed] [Google Scholar]
- 24.Sudo H, Baas PW. Acetylation of microtubules influences their sensitivity to severing by katanin in neurons and fibroblasts. J. Neurosci. 2010;30:7215–7226. doi: 10.1523/JNEUROSCI.0048-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu W, Solowska JM, Qiang L, Karabay A, Baird D, Baas PW. Regulation of microtubule severing by katanin subunits during neuronal development. J. Neurosci. 2005;25:5573–5583. doi: 10.1523/JNEUROSCI.0834-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu W, Qiang L, Solowska JM, Karabay A, Korulu S, Baas PW. The microtubule-severing proteins spastin and katanin participate differently in the formation of axonal branches. Mol. Biol. Cell. 2008;19:1485–1498. doi: 10.1091/mbc.E07-09-0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dent EW, Callaway JL, Szebenyi G, Baas PW, Kalil K. Reorganization and movement of microtubules in axonal growth cones and developing interstitial branches. J. Neurosci. 1999;19:8894–8908. doi: 10.1523/JNEUROSCI.19-20-08894.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao YS, Hubbert CC, Yao TP. The microtubule-associated histone deacetylase 6 (HDAC6) regulates epidermal growth factor receptor (EGFR) endocytic trafficking and degradation. J. Biol. Chem. 2010;285:11219–11226. doi: 10.1074/jbc.M109.042754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pang ZP, Cao P, Xu W, Sudhof TC. Calmodulin controls synaptic strength via presynaptic activation of calmodulin kinase II. J. Neurosci. 2010;30:4132–4142. doi: 10.1523/JNEUROSCI.3129-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Payne VA, Grimsey N, Tuthill A, Virtue S, Gray SL, Dalla Nora E, Semple RK, O’Rahilly S, Rochford JJ. The human lipodystrophy gene BSCL2/seipin may be essential for normal adipocyte differentiation. Diabetes. 2008;57:2055–2060. doi: 10.2337/db08-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Janderova L, McNeil M, Murrell AN, Mynatt RL, Smith SR. Human mesenchymal stem cells as an in vitro model for human adipogenesis. Obes. Res. 2003;11:65–74. doi: 10.1038/oby.2003.11. [DOI] [PubMed] [Google Scholar]
- 32.Fletcher LM, Welsh GI, Oatey PB, Tavare JM. Role for the microtubule cytoskeleton in GLUT4 vesicle trafficking and in the regulation of insulin-stimulated glucose uptake. Biochem. J. 2000;352:267–276. [PMC free article] [PubMed] [Google Scholar]
- 33.Jing E, Gesta S, Kahn CR. SIRT2 regulates adipocyte differentiation through FoxO1 acetylation/deacetylation. Cell Metab. 2007;6:105–114. doi: 10.1016/j.cmet.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Falconer MM, Vielkind U, Brown DL. Establishment of a stable, acetylated microtubule bundle during neuronal commitment. Cell Motil. Cytoskeleton. 1989;12:169–180. doi: 10.1002/cm.970120306. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.