Abstract
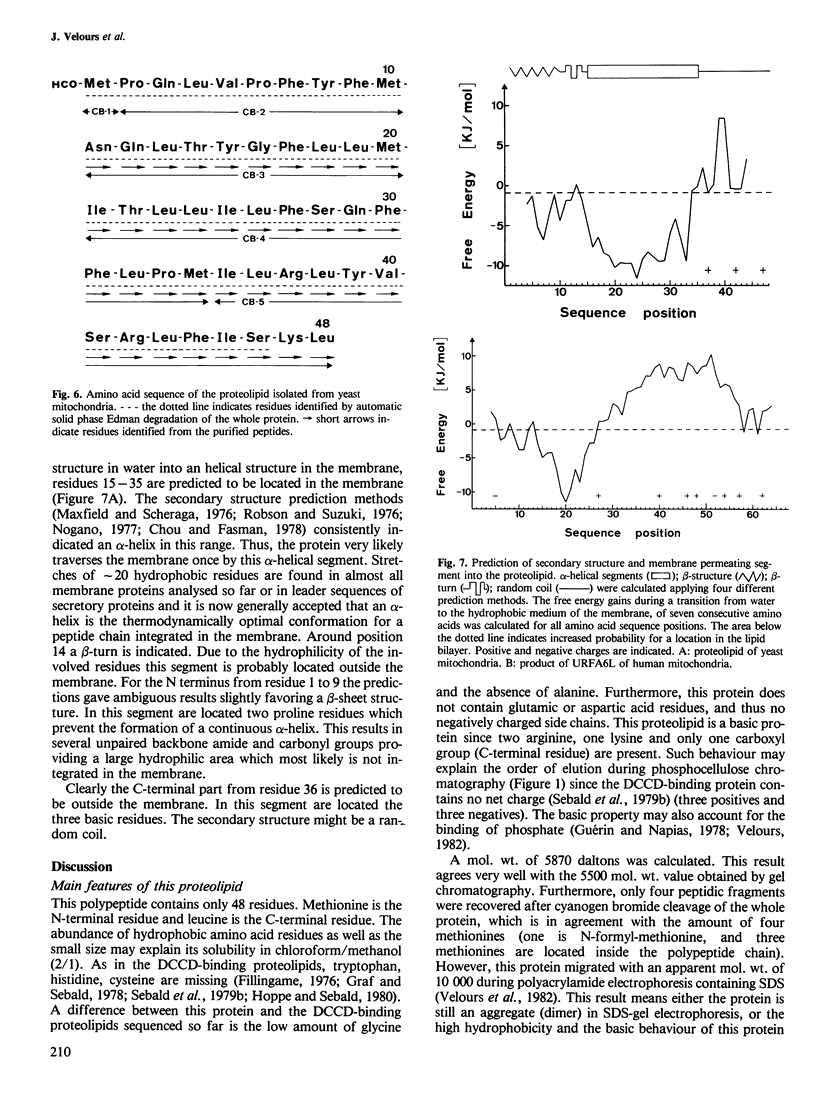
The purification and the amino acid sequence of a proteolipid translated on ribosomes in yeast mitochondria is reported. This protein, which is a subunit of the ATP synthase, was purified by extraction with chloroform/methanol (2/1) and subsequent chromatography on phosphocellulose and reverse phase h.p.l.c. A mol. wt. of 5500 was estimated by chromatography on Bio-Gel P-30 in 80% formic acid. The complete amino acid sequence of this protein was determined by automated solid phase Edman degradation of the whole protein and of fragments obtained after cleavage with cyanogen bromide. The sequence analysis indicates a length of 48 amino acid residues. The calculated mol. wt. of 5870 corresponds to the value found by gel chromatography. This polypeptide contains three basic residues and no negatively charged side chain. The three basic residues are clustered at the C terminus. The primary structure of this protein is in full agreement with the predicted amino acid sequence of the putative polypeptide encoded by the mitochondrial aap1 gene recently discovered in Saccharomyces cerevisiae. Moreover, this protein shows 50% homology with the amino acid sequence of a putative polypeptide encoded by an unidentified reading frame also discovered near the mitochondrial ATPase subunit 6 gene in Aspergillus nidulans.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Anderson S., de Bruijn M. H., Coulson A. R., Eperon I. C., Sanger F., Young I. G. Complete sequence of bovine mitochondrial DNA. Conserved features of the mammalian mitochondrial genome. J Mol Biol. 1982 Apr 25;156(4):683–717. doi: 10.1016/0022-2836(82)90137-1. [DOI] [PubMed] [Google Scholar]
- Beyreuther K., Raufuss H., Schrecker O., Hengstenberg W. The phosphoenolpyruvate-dependent phosphotransferase system of Staphylococcus aureus. 1. Amino-acid sequence of the phosphocarrier protein HPr. Eur J Biochem. 1977 May 2;75(1):275–286. doi: 10.1111/j.1432-1033.1977.tb11527.x. [DOI] [PubMed] [Google Scholar]
- Bibb M. J., Van Etten R. A., Wright C. T., Walberg M. W., Clayton D. A. Sequence and gene organization of mouse mitochondrial DNA. Cell. 1981 Oct;26(2 Pt 2):167–180. doi: 10.1016/0092-8674(81)90300-7. [DOI] [PubMed] [Google Scholar]
- Blondin G. A. Resolution of the mitochondrial N,N'-dicylclohexylcarbodiimide binding proteolipid fraction into three similar sized proteins. Biochem Biophys Res Commun. 1979 Apr 27;87(4):1087–1094. doi: 10.1016/s0006-291x(79)80019-4. [DOI] [PubMed] [Google Scholar]
- Cattell K. J., Lindop C. R., Knight I. G., Beechey R. B. The identification of the site of action of NN'-dicyclohexylcarbodi-imide as a proteolipid in mitochondrial membranes. Biochem J. 1971 Nov;125(1):169–177. doi: 10.1042/bj1250169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chateaubodeau G. A., Guérin M., Guérin B. Perméabilité de la membrane interne des mitochondries de levure: étude des relations entre structure et activité. Biochimie. 1976;58(5):601–610. doi: 10.1016/s0300-9084(76)80230-1. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Esparza M., Velours J., Guerin B. Existence of two different species of mitochondrially translated proteolipids in ATPase complex of yeast mitochondria. FEBS Lett. 1981 Nov 2;134(1):63–66. doi: 10.1016/0014-5793(81)80551-0. [DOI] [PubMed] [Google Scholar]
- Fillingame R. H. Purification of the carbodiimide-reactive protein component of the ATP energy-transducing system of Escherichia coli. J Biol Chem. 1976 Nov 10;251(21):6630–6637. [PubMed] [Google Scholar]
- Fillingame R. H. The proton-translocating pumps of oxidative phosphorylation. Annu Rev Biochem. 1980;49:1079–1113. doi: 10.1146/annurev.bi.49.070180.005243. [DOI] [PubMed] [Google Scholar]
- Friedl P., Schairer H. U. The isolated F0 of Escherichia coli aTP-synthase is reconstitutively active in H+-conduction and ATP-dependent energy-transduction. FEBS Lett. 1981 Jun 15;128(2):261–264. doi: 10.1016/0014-5793(81)80094-4. [DOI] [PubMed] [Google Scholar]
- Gay N. J., Walker J. E. The atp operon: nucleotide sequence of the promoter and the genes for the membrane proteins, and the delta subunit of Escherichia coli ATP-synthase. Nucleic Acids Res. 1981 Aug 25;9(16):3919–3926. doi: 10.1093/nar/9.16.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf T., Sebald W. The dicyclohexylcarbodiimide-binding protein of the mitochondrial ATPase complex from beef heart. Isolation and amino acid composition. FEBS Lett. 1978 Oct 15;94(2):218–222. doi: 10.1016/0014-5793(78)80941-7. [DOI] [PubMed] [Google Scholar]
- Grisi E., Brown T. A., Waring R. B., Scazzocchio C., Davies R. W. Nucleotide sequence of a region of the mitochondrial genome of Aspergillus nidulans including the gene for ATPase subunit 6. Nucleic Acids Res. 1982 Jun 11;10(11):3531–3539. doi: 10.1093/nar/10.11.3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerin M., Napias C. Phosphate transport in yeast mitochondria: purification and characterization of a mitoribosomal synthesis dependent proteolipid showing a high affinity for phosphate. Biochemistry. 1978 Jun 27;17(13):2510–2516. doi: 10.1021/bi00606a009. [DOI] [PubMed] [Google Scholar]
- Hensgens L. A., Grivell L. A., Borst P., Bos J. L. Nucleotide sequence of the mitochondrial structural gene for subunit 9 of yeast ATPase complex. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1663–1667. doi: 10.1073/pnas.76.4.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppe J., Sebald W. Amino acid sequence of the proteolipid subunit of the proton-translocating ATPase complex from the thermophilic bacterium PS-3. Eur J Biochem. 1980;107(1):57–65. doi: 10.1111/j.1432-1033.1980.tb04624.x. [DOI] [PubMed] [Google Scholar]
- Horn M. J., Laursen R. A. Solid-phase edman degradation: attachment of carboxyl-terminal homoserine peptides to an insoluble resin. FEBS Lett. 1973 Nov 1;36(3):285–288. doi: 10.1016/0014-5793(73)80392-8. [DOI] [PubMed] [Google Scholar]
- Kanazawa H., Mabuchi K., Kayano T., Noumi T., Sekiya T., Futai M. Nucleotide sequence of the genes for F0 components of the proton-translocating ATPase from Escherichia coli: prediction of the primary structure of F0 subunits. Biochem Biophys Res Commun. 1981 Nov 30;103(2):613–620. doi: 10.1016/0006-291x(81)90495-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laursen R. A. Coupling techniques in solid-phase sequencing. Methods Enzymol. 1977;47:277–288. doi: 10.1016/0076-6879(77)47032-0. [DOI] [PubMed] [Google Scholar]
- Lottspeich F. Identification of the phenylthiohydantoin derivatives of amino acids by high pressure liquid chromatography, using a ternary, isocratic solvent system. Hoppe Seylers Z Physiol Chem. 1980 Dec;361(12):1829–1834. doi: 10.1515/bchm2.1980.361.2.1829. [DOI] [PubMed] [Google Scholar]
- Macino G., Tzagoloff A. Assembly of the mitochondrial membrane system. The DNA sequence of a mitochondrial ATPase gene in Saccharomyces cerevisiae. J Biol Chem. 1979 Jun 10;254(11):4617–4623. [PubMed] [Google Scholar]
- Macino G., Tzagoloff A. Assembly of the mitochondrial membrane system: sequence analysis of a yeast mitochondrial ATPase gene containing the oli-2 and oli-4 loci. Cell. 1980 Jun;20(2):507–517. doi: 10.1016/0092-8674(80)90637-6. [DOI] [PubMed] [Google Scholar]
- Macreadie I. G., Novitski C. E., Maxwell R. J., John U., Ooi B. G., McMullen G. L., Lukins H. B., Linnane A. W., Nagley P. Biogenesis of mitochondria: the mitochondrial gene (aap1) coding for mitochondrial ATPase subunit 8 in Saccharomyces cerevisiae. Nucleic Acids Res. 1983 Jul 11;11(13):4435–4451. doi: 10.1093/nar/11.13.4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxfield F. R., Scheraga H. A. Status of empirical methods for the prediction of protein backbone topography. Biochemistry. 1976 Nov 16;15(23):5138–5153. doi: 10.1021/bi00668a030. [DOI] [PubMed] [Google Scholar]
- Murphy M., Roberts H., Choo W. M., Macreadie I., Marzuki S., Lukins H. B., Linnane A. W. Biogenesis of mitochondria. oli2 Mutations affecting the coupling of oxidation to phosphorylation in Saccharomyces cerevisiae. Biochim Biophys Acta. 1980 Oct 3;592(3):431–444. doi: 10.1016/0005-2728(80)90090-0. [DOI] [PubMed] [Google Scholar]
- Nagano K. Triplet information in helix prediction applied to the analysis of super-secondary structures. J Mol Biol. 1977 Jan 15;109(2):251–274. doi: 10.1016/s0022-2836(77)80033-8. [DOI] [PubMed] [Google Scholar]
- Negrin R. S., Foster D. L., Fillingame R. H. Energy-transducing H+-ATPase of Escherichia coli. Reconstitution of proton translocation activity of the intrinsic membrane sector. J Biol Chem. 1980 Jun 25;255(12):5643–5648. [PubMed] [Google Scholar]
- Nielsen J., Hansen F. G., Hoppe J., Friedl P., von Meyenburg K. The nucleotide sequence of the atp genes coding for the F0 subunits a, b, c and the F1 subunit delta of the membrane bound ATP synthase of Escherichia coli. Mol Gen Genet. 1981;184(1):33–39. doi: 10.1007/BF00271191. [DOI] [PubMed] [Google Scholar]
- Orian J. M., Murphy M., Marzuki S. Mitochondrially synthesized protein subunits of the yeast mitochondrial adenosine triphosphatase. A reassessment. Biochim Biophys Acta. 1981 Jan 29;652(1):234–239. doi: 10.1016/0005-2787(81)90227-6. [DOI] [PubMed] [Google Scholar]
- Robson B., Suzuki E. Conformational properties of amino acid residues in globular proteins. J Mol Biol. 1976 Nov 5;107(3):327–356. doi: 10.1016/s0022-2836(76)80008-3. [DOI] [PubMed] [Google Scholar]
- Sebald W., Graf T., Lukins H. B. The dicyclohexylcarbodiimide-binding protein of the mitochondrial ATPase complex from Neurospora crassa and Saccharomyces cerevisiae. Identification and isolation. Eur J Biochem. 1979 Feb 1;93(3):587–599. doi: 10.1111/j.1432-1033.1979.tb12859.x. [DOI] [PubMed] [Google Scholar]
- Sierra M. F., Tzagoloff A. Assembly of the mitochondrial system. Purification of a mitochondrial product of the ATPase. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3155–3159. doi: 10.1073/pnas.70.11.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swank R. T., Munkres K. D. Molecular weight analysis of oligopeptides by electrophoresis in polyacrylamide gel with sodium dodecyl sulfate. Anal Biochem. 1971 Feb;39(2):462–477. doi: 10.1016/0003-2697(71)90436-2. [DOI] [PubMed] [Google Scholar]
- Velours J., Esparza M., Guerin B. Amino acid composition of a new mitochondrially translated proteolipid isolated from yeast mitochondria and from the OSATPase complex. Biochem Biophys Res Commun. 1982 Dec 31;109(4):1192–1199. doi: 10.1016/0006-291x(82)91903-9. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Membrane proteins: the amino acid composition of membrane-penetrating segments. Eur J Biochem. 1981 Nov;120(2):275–278. doi: 10.1111/j.1432-1033.1981.tb05700.x. [DOI] [PubMed] [Google Scholar]