Abstract
Evidence is given that tetrameric sorbitol dehydrogenase from sheep liver contains one zinc atom per subunit, most probably located at the active site, and no other specifically bound zinc or iron atom. In alcohol dehydrogenases that are structurally related to sorbitol dehydrogenase, more than one zinc atom per subunit can complicate investigations of zinc atom function. Therefore, sorbitol dehydrogenase will be particularly valuable for defining the precise roles of zinc in alcohol and polyol dehydrogenases, and for establishing correlations of structure and function with other important zinc-containing proteins.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
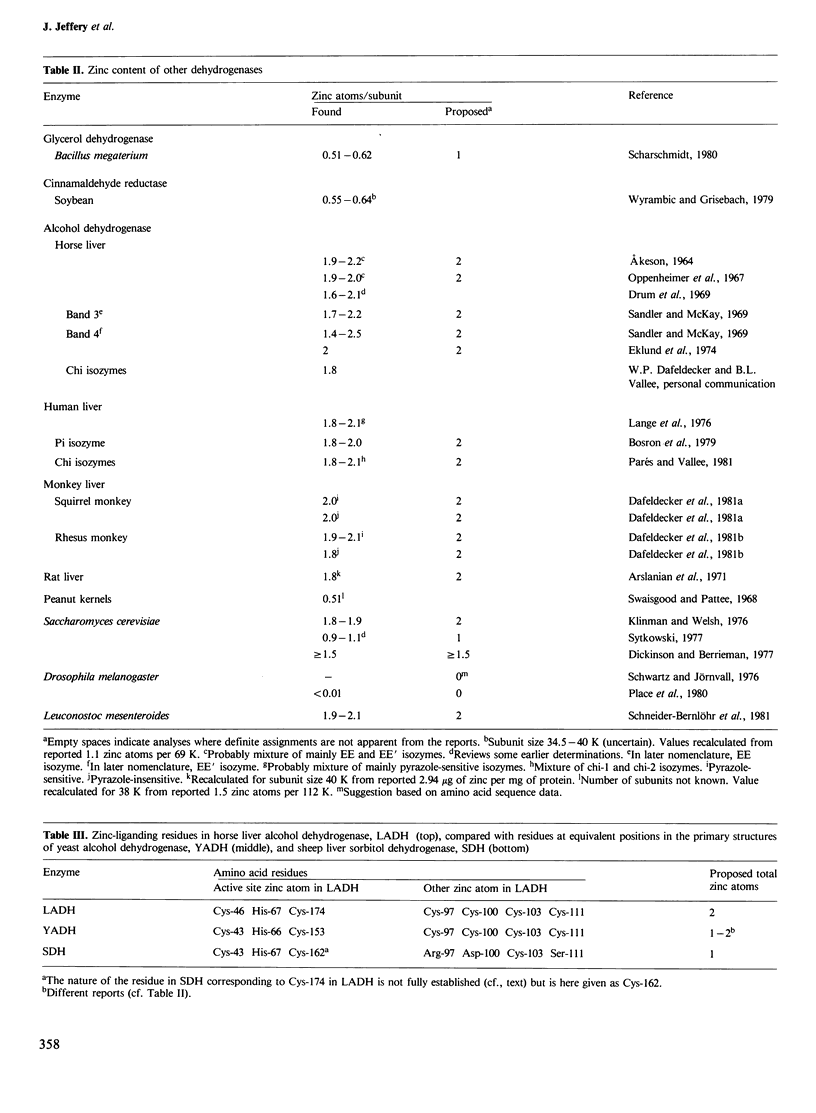
- Akeson A. On the zinc content of horse liver alcohol dehydrogenase. Biochem Biophys Res Commun. 1964 Oct 14;17(3):211–214. doi: 10.1016/0006-291x(64)90385-7. [DOI] [PubMed] [Google Scholar]
- Arslanian M. J., Pascoe E., Reinhold J. G. Rat liver alcohol dehydrogenase. Purification and properties. Biochem J. 1971 Dec;125(4):1039–1047. doi: 10.1042/bj1251039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkman D. E., Sandstead H. H., Park J. H. An examination of the zinc requirement for the catalytic activity of 3-phosphoglyceraldehyde dehydrogenase. J Biol Chem. 1970 Mar 10;245(5):1036–1040. [PubMed] [Google Scholar]
- Barton J. K., Basile L. A., Paranawithana S. R. The presence of zinc in the restriction enzyme Eco RI. J Biol Chem. 1982 Jul 25;257(14):7911–7914. [PubMed] [Google Scholar]
- Bennetzen J. L., Hall B. D. The primary structure of the Saccharomyces cerevisiae gene for alcohol dehydrogenase. J Biol Chem. 1982 Mar 25;257(6):3018–3025. [PubMed] [Google Scholar]
- Bosron W. F., Li T. K., Dafeldecker W. P., Vallee B. L. Human liver pi-alcohol dehydrogenase: kinetic and molecular properties. Biochemistry. 1979 Mar 20;18(6):1101–1105. doi: 10.1021/bi00573a026. [DOI] [PubMed] [Google Scholar]
- Branlant G., Biellmann J. F. Purification and some properties of aldehyde reductases from pig liver. Eur J Biochem. 1980 Apr;105(3):611–621. doi: 10.1111/j.1432-1033.1980.tb04539.x. [DOI] [PubMed] [Google Scholar]
- Cedergren-Zeppezauer E., Samama J. P., Eklund H. Crystal structure determinations of coenzyme analogue and substrate complexes of liver alcohol dehydrogenase: binding of 1,4,5,6-tetrahydronicotinamide adenine dinucleotide and trans-4-(N,N-dimethylamino)cinnamaldehyde to the enzyme. Biochemistry. 1982 Sep 28;21(20):4895–4908. doi: 10.1021/bi00263a011. [DOI] [PubMed] [Google Scholar]
- Dafeldecker W. P., Meadow P. E., Parés X., Vallee B. L. Simian liver alcohol dehydrogenase: isolation and characterization of isoenzymes from Macaca mulatta. Biochemistry. 1981 Nov 10;20(23):6729–6734. doi: 10.1021/bi00526a031. [DOI] [PubMed] [Google Scholar]
- Dafeldecker W. P., Parés X., Vallee B. L., Bosron W. F., Li T. K. Simian liver alcohol dehydrogenase: isolation and characterization of isoenzymes from Saimiri sciureus. Biochemistry. 1981 Feb 17;20(4):856–861. doi: 10.1021/bi00507a031. [DOI] [PubMed] [Google Scholar]
- Dickinson F. M., Berrieman S. The reactions of 1,10-phenanthroline with yeast alcohol dehydrogenase. Biochem J. 1977 Oct 1;167(1):237–244. doi: 10.1042/bj1670237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich H., MacGibbon A. K., Dunn M. F., Zeppezauer M. Investigation of the pH dependencies of coenzyme binding to liver alcohol dehydrogenase lacking zinc ion at the active sites. Biochemistry. 1983 Jul 5;22(14):3432–3438. doi: 10.1021/bi00283a020. [DOI] [PubMed] [Google Scholar]
- Dietrich H., Zeppezauer M. Spectral evidence for three metal-linked ionization equilibria in the interaction of cobalt(II) horse liver alcohol dehydrogenase with coenzyme and substrate. J Inorg Biochem. 1982 Nov;17(3):227–235. doi: 10.1016/s0162-0134(00)80101-4. [DOI] [PubMed] [Google Scholar]
- Drum D. E., Li T. K., Vallee B. L. Considerations in evaluating the zinc content of horse liver alcohol dehydrogenase preparations. Biochemistry. 1969 Sep;8(9):3783–3791. doi: 10.1021/bi00837a044. [DOI] [PubMed] [Google Scholar]
- Drum D. E., Vallee B. L. Optical properties of catalytically active cobalt and cadmium liver alcohol dehydrogenases. Biochem Biophys Res Commun. 1970 Oct 9;41(1):33–39. doi: 10.1016/0006-291x(70)90464-x. [DOI] [PubMed] [Google Scholar]
- Dunn M. F., Dietrich H., MacGibbon A. K., Koerber S. C., Zeppezauer M. Investigation of intermediates and transition states in the catalytic mechanisms of active site substituted cobalt(II), nickel(II), zinc(II), and cadmium(II) horse liver alcohol dehydrogenase. Biochemistry. 1982 Jan 19;21(2):354–363. doi: 10.1021/bi00531a024. [DOI] [PubMed] [Google Scholar]
- Eklund H., Brändén C. I., Jörnvall H. Structural comparisons of mammalian, yeast and bacillar alcohol dehydrogenases. J Mol Biol. 1976 Mar 25;102(1):61–73. doi: 10.1016/0022-2836(76)90073-5. [DOI] [PubMed] [Google Scholar]
- Eklund H., Nordström B., Zeppezauer E., Söderlund G., Ohlsson I., Boiwe T., Brändén C. I. The structure of horse liver alcohol dehydrogenase. FEBS Lett. 1974 Aug 25;44(2):200–204. doi: 10.1016/0014-5793(74)80725-8. [DOI] [PubMed] [Google Scholar]
- Eklund H., Plapp B. V., Samama J. P., Brändén C. I. Binding of substrate in a ternary complex of horse liver alcohol dehydrogenase. J Biol Chem. 1982 Dec 10;257(23):14349–14358. [PubMed] [Google Scholar]
- Eklund H., Samama J. P., Wallén L. Pyrazole binding in crystalline binary and ternary complexes with liver alcohol dehydrogenase. Biochemistry. 1982 Sep 28;21(20):4858–4866. doi: 10.1021/bi00263a005. [DOI] [PubMed] [Google Scholar]
- Eklund H., Samma J. P., Wallén L., Brändén C. I., Akeson A., Jones T. A. Structure of a triclinic ternary complex of horse liver alcohol dehydrogenase at 2.9 A resolution. J Mol Biol. 1981 Mar 15;146(4):561–587. doi: 10.1016/0022-2836(81)90047-4. [DOI] [PubMed] [Google Scholar]
- Jeffery J., Cummins L., Carlquist M., Jörnvall H. Properties of sorbitol dehydrogenase and characterization of a reactive cysteine residue reveal unexpected similarities to alcohol dehydrogenases. Eur J Biochem. 1981 Nov;120(2):229–234. doi: 10.1111/j.1432-1033.1981.tb05693.x. [DOI] [PubMed] [Google Scholar]
- Jörnvall H., Eklund H., Brändén C. I. Subunit conformation of yeast alcohol dehydrogenase. J Biol Chem. 1978 Dec 10;253(23):8414–8419. [PubMed] [Google Scholar]
- Jörnvall H., Persson M., Jeffery J. Alcohol and polyol dehydrogenases are both divided into two protein types, and structural properties cross-relate the different enzyme activities within each type. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4226–4230. doi: 10.1073/pnas.78.7.4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jörnvall H. The primary structure of yeast alcohol dehydrogenase. Eur J Biochem. 1977 Feb;72(3):425–442. doi: 10.1111/j.1432-1033.1977.tb11267.x. [DOI] [PubMed] [Google Scholar]
- Kisselev L. L., Favorova O. O., Nurbekov M. K., Dmitriyenko S. G., Engelhardt W. A. Bovine tryptophanyl-tRNA synthetase. A zinc metalloenzyme. Eur J Biochem. 1981 Dec;120(3):511–517. doi: 10.1111/j.1432-1033.1981.tb05729.x. [DOI] [PubMed] [Google Scholar]
- Klinman J. P., Welsh K. The zinc content of yeast alcohol dehydrogenase. Biochem Biophys Res Commun. 1976 Jun 7;70(3):878–884. doi: 10.1016/0006-291x(76)90673-2. [DOI] [PubMed] [Google Scholar]
- Kvassman J., Larsson A., Pettersson G. Substituent effects on the ionization step regulating desorption and catalytic oxidation of alcohols bound to liver alcohol dehydrogenase. Eur J Biochem. 1981 Mar;114(3):555–563. doi: 10.1111/j.1432-1033.1981.tb05180.x. [DOI] [PubMed] [Google Scholar]
- Lange L. G., Sytkowski A. J., Vallee B. L. Human liver alcohol dehydrogenase: purification, composition, and catalytic features. Biochemistry. 1976 Oct 19;15(21):4687–4693. doi: 10.1021/bi00666a023. [DOI] [PubMed] [Google Scholar]
- Makinen M. W., Maret W., Yim M. B. Neutral metal-bound water is the base catalyst in liver alcohol dehydrogenase. Proc Natl Acad Sci U S A. 1983 May;80(9):2584–2588. doi: 10.1073/pnas.80.9.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maret W., Andersson I., Dietrich H., Schneider-Bernlöhr H., Einarsson R., Zeppezauer M. Site-specific substituted cobalt(II) horse liver alcohol dehydrogenases. Preparation and characterization in solution, crystalline and immobilized state. Eur J Biochem. 1979 Aug 1;98(2):501–512. doi: 10.1111/j.1432-1033.1979.tb13211.x. [DOI] [PubMed] [Google Scholar]
- Oppenheimer H. L., Green R. W., McKay R. H. Function of zinc in horse liver alcohol dehydrogenase. Arch Biochem Biophys. 1967 Mar;119(1):552–559. doi: 10.1016/0003-9861(67)90490-0. [DOI] [PubMed] [Google Scholar]
- Parés X., Vallee B. L. New human liver alcohol dehydrogenase forms with unique kinetic characteristics. Biochem Biophys Res Commun. 1981 Jan 15;98(1):122–130. doi: 10.1016/0006-291x(81)91878-7. [DOI] [PubMed] [Google Scholar]
- Plapp B. V., Eklund H., Jones T. A., Brändén C. I. Three-dimensional structure of isonicotinimidylated liver alcohol dehydrogenase. J Biol Chem. 1983 May 10;258(9):5537–5547. [PubMed] [Google Scholar]
- Russell D. W., Smith M., Williamson V. M., Young E. T. Nucleotide sequence of the yeast alcohol dehydrogenase II gene. J Biol Chem. 1983 Feb 25;258(4):2674–2682. [PubMed] [Google Scholar]
- Russell P. R., Hall B. D. The primary structure of the alcohol dehydrogenase gene from the fission yeast Schizosaccharomyces pombe. J Biol Chem. 1983 Jan 10;258(1):143–149. [PubMed] [Google Scholar]
- Sandler N., McKay R. H. Zinc content and specific activity of horse liver alcohol dehydrogenase isoenzymes. Biochem Biophys Res Commun. 1969 Apr 10;35(1):151–155. doi: 10.1016/0006-291x(69)90497-5. [DOI] [PubMed] [Google Scholar]
- Schneider-Bernlöhr H., Fiedler H., Gerber M., Weber C., Zeppezauer M. Alcohol dehydrogenase from Leuconostoc mesenteroides: molecular properties in comparison with the yeast and horse liver enzyme. Int J Biochem. 1981;13(12):1215–1224. doi: 10.1016/0020-711x(81)90067-7. [DOI] [PubMed] [Google Scholar]
- Schneider G., Eklund H., Cedergren-Zeppezauer E., Zeppezauer M. Structure of the complex of active site metal-depleted horse liver alcohol dehydrogenase and NADH. EMBO J. 1983;2(5):685–689. doi: 10.1002/j.1460-2075.1983.tb01485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M. F., Jörnvall H. Structural analyses of mutant and wild-type alcohol dehydrogenases from drosophila melanogaster. Eur J Biochem. 1976 Sep;68(1):159–168. doi: 10.1111/j.1432-1033.1976.tb10774.x. [DOI] [PubMed] [Google Scholar]
- Scopes R. K. An iron-activated alcohol dehydrogenase. FEBS Lett. 1983 Jun 13;156(2):303–306. doi: 10.1016/0014-5793(83)80517-1. [DOI] [PubMed] [Google Scholar]
- Slater J. P., Mildvan A. S., Loeb L. A. Zinc in DNA polymerases. Biochem Biophys Res Commun. 1971 Jul 2;44(1):37–43. doi: 10.1016/s0006-291x(71)80155-9. [DOI] [PubMed] [Google Scholar]
- Wills C., Jörnvall H. Amino acid substitutions in two functional mutants of yeast alcohol dehydrogenase. Nature. 1979 Jun 21;279(5715):734–736. doi: 10.1038/279734a0. [DOI] [PubMed] [Google Scholar]
- Wills C., Jörnvall H. The two major isozymes of yeast alcohol dehydrogenase. Eur J Biochem. 1979 Sep;99(2):323–331. doi: 10.1111/j.1432-1033.1979.tb13260.x. [DOI] [PubMed] [Google Scholar]
- Wyrambik D., Grisebach H. Enzymic synthesis of lignin precursors. Further studies on cinnamyl-alcohol dehydrogenase from soybean-cell-suspension cultures. Eur J Biochem. 1979 Jul;97(2):503–509. doi: 10.1111/j.1432-1033.1979.tb13138.x. [DOI] [PubMed] [Google Scholar]
- von Bahr-Lindström H., Andersson L., Mosbach K., Jörnvall H. Purification and characterization of chicken liver alcohol dehydrogenase. FEBS Lett. 1978 May 15;89(2):293–297. doi: 10.1016/0014-5793(78)80239-7. [DOI] [PubMed] [Google Scholar]


