Abstract
Ischemia reperfusion injury (IRI) evokes intra-graft inflammatory responses, which markedly augment alloimmune responses against the graft. Understanding the mechanisms underlying these responses is fundamental to develop therapeutic regimens to prevent/ameliorate organ IRI. Here, we demonstrate that IRI results in a marked increase in mitochondrial damage and autophagy in dendritic cells (DC). While autophagy is a survival mechanism for ischemic DC, it also augments their production of IL6. Allograft derived dendritic cells (ADDC) lacking autophagy related gene 5 (Atg5) showed higher death rates post-transplantation. Transplanted ischemic hearts from CD11cCre/Atg5 conditional knockout mice showed marked reduction in intra-graft expression of IL6 as compared to controls. To antagonize the effect of IL6 locally in the heart, we synthesized novel anti-IL6 nanoparticles with capacity for controlled release of anti-IL6 over time. As compared to systemic delivery of anti-IL6, localized delivery of anti-IL6 significantly reduced chronic rejection with a markedly lower amount administered. Despite improved allograft histology, there were no changes to splenic T cell populations, illustrating the importance of local IL6 in driving chronic rejection after IRI. These data carry potential clinical significance, by identifying an innovative, targeted strategy to manipulate organs prior to transplantation to diminish inflammation, leading to improved long term outcomes.
Introduction
Ischemia reperfusion injury (IRI) is inevitable in solid organ transplantation and is a primary cause of activation of innate immune responses (1). Incidence of IRI is increased due to the use of extended criteria donors and marginal donors (2-4), which often accumulate ischemic injuries more readily. Early intra-graft inflammation and activation of innate immunity leads to augmentation of alloimmunity, leading to increased rates of graft rejection and ultimately poorer transplant outcomes (5-8). Improved understanding of underlying mechanisms is critical to the development of targeted therapies to control IRI-induced alloimmunity.
In this study, an observation of early increase in intra-graft levels of IL6 in ischemia prompted us to examine the critical role of allograft derived dendritic cells (ADDC) as the potential source of IL6 responsible for CD4+ alloreactive T cell activity. We examined the importance of autophagy as a survival mechanism for ADDC under IRI. Induction of autophagy in DCs by starvation markedly increases the production of inflammatory cytokines (9, 10). However, the impact of IRI on augmenting autophagy in DCs and its implication in alloimmune responses is unknown. Specifically, we examined the importance of key Atg related protein 5 (Atg5) in the survival of DC under IRI and subsequent production of IL6. Finally, we developed the first controlled formulation of an anti-IL6 nanomedicine and an intra-graft delivery platform to target the early IL6 mediated inflammatory responses. We investigated the impact of perfusing organs with NP-anti-IL6 to directly target early innate inflammatory responses within the allograft. This strategy markedly reduced chronic heart allograft rejection, a major barrier to long-term allograft acceptance.
Materials and Methods
Mice
C57BL/6, B6.C-H2-Ab1bm12 (BM12) mice were purchased from Jackson Laboratory (Bar Harbor, ME, USA) and used at 6-10 weeks of age. CD11cCre/Atg5 conditional knockout mice, in which DCs lack Atg5 protein, were courtesy of Dr. Masaaki Komatsu (Tokyo Metropolitan Institute of Medical Science). All procedures were in compliance with the guidelines for the Care and Use of Laboratory Animals at Harvard University, Boston, MA. All protocols were approved by the institutional Animal Care and Use Committee.
Heterotopic cardiac transplantation
Heterotopic intra-abdominal cardiac transplantation was performed using microsurgical techniques as described previously (11). Ischemic group hearts were harvested and kept in UW solution for 8 hours prior to transplantation, whereas control group were transplanted within 30 minutes. Graft heartbeat was evaluated daily by palpation. Rejection was determined as complete cessation of cardiac contractility and was confirmed by direct visualization. For acute rejection model, donor hearts from C57BL/6 mice were transplanted across complete MHC mismatch into BALB/cJ mice. For chronic rejection, we transplanted hearts across one MHC mismatch using C56BL/6 and BM12 as recipients and donors (12).
In vivo treatment protocols
For systemic IL6 blockade, 100 μg anti-mouse IL6 antibody (Courtesy of Genentech) was injected intraperitoneally on days 0 to 3 and then on alternate days until sacrifice. For studies of locally delivered anti-IL6, donor hearts were perfused with 100 μg of anti-IL6 antibody and then transplanted immediately or after 8 hours of cold ischemia.
Lymphocyte extraction from transplanted heart grafts
Heart grafts were washed with PBS to remove any remaining clot, minced in RPMI-1640 medium containing % 0.1 collagenase and incubated for one hour at 37°C with 5% CO2 followed by addition of EDTA containing buffers. Cells were then filtered through 70μm strainer, counted and stained for flow cytometry.
Dendritic cells isolation and culture
Splenic DCs were isolated using CD11c magnetic beads (Miltenyi Biotec, Cambridge, MA). Bone marrow-derived DCs were generated as described previously (13). Briefly, femurs and tibiae of C57BL/6 mice were flushed with RPMI-1640 and washed twice with PBS. 3×106 cells were then plated in 100 mm ×20 mm petri dish in RPMI-1640 complete media (containing 10% FCS and 1% L-glutamine and penicillin-streptomycin) supplemented with 20 ng/ml of recombinant murine GMCSF (R&D Systems). Cells were cultured at 37 °C with 5% CO2, supplemented with 10 ng/ml rm-GMCSF on day 3 and harvested for experiments at day 6.
To simulate ischemia, DCs were harvested and cultured in the presence of different concentrations of H2O2 (Sigma Aldrich, Natick, MA). In some cultures, cells were pre-treated with 10 μM Spautin-1 (#SML0440, Sigma Aldrich, Natick, MA). Subsequently the cells were washed with complete medium and harvested for experiments.
Flow cytometry
Flow cytometric analysis of graft infiltrating cells, draining lymph node (DLN) and spleen was performed. For intracellular cytokine staining, cells were stimulated ex-vivo with PMA (50 ng/ml), Ionomycin (500 ng/ml) and GolgiStop for 4 hours, then permeabilized and stained with fluorochrome conjugated antibodies against IFNγ, IL17 and IL6. All antibodies were purchased from BD (Becton Dickinson Franklin Lakes, NJ). Cells were run on FACS Canto II (BD Biosciences, Franklin Lakes, NJ) instrument. Data were analyzed using FlowJo software.
Immunohistochemistry and Immunofluorescence
Heart grafts were harvested and preserved in Optima Cutting Temperature (OCT) compound (Tissue-Tek, Torrance, CA) and stored at -80 °C. Samples were cut into 5-μm sections, fixed, blocked and stained with co-responding antibodies. Anti-CD4, CD8, F4/80 and CD11c antibodies were purchased from BD Biosciences. Anti-LC3 and anti-fibronectin antibodies were purchased from Fischer Scientific and Abcam respectively. Sections were visualized using a Nikon E-1000 fluorescent microscope.
Lymphocyte infiltration and vascular scoring
Lymphocyte infiltration is graded 0 to 4 at 6 random fields of each heart H&E section blindly by two individual researchers (6 sections/heart, 3 mice per group). The grades are defined as follows: grade 0 (no lymphocyte infiltration), grade 1 (less than 25% lymphocyte infiltration), grade 2 (25% to 50% lymphocyte infiltration), grade 3 (50% to 75% lymphocyte infiltration) and grade 4 (more than 75% lymphocyte infiltration).
Vascular score is determined by a combination of vascular occlusion score and perivascular lymphocyte infiltration. Vascular occlusion is scored from grade 0 to 4 for every vessel (6 sections/heart, 3 mice per group). The grades are defined as follows: grade 0 (no occlusion), grade 1 (less than 50% occlusion), grade 2 (50% to 75% occlusion), grade 3 (75% to 95% occlusion) and grade 4 (more than 95% occlusion). The perivascular lymphocyte infiltration is scored as mentioned above and was then added to the vascular occlusion score.
Mitochondrial staining
Mitochondrial staining was done on splenic DCs isolated with CD11c magnetic beads (Miltenyi Biotec.) using 100 nM TMRE (TMRE-Mitochondrial Membrane Potential Assay Kit/Abcam) in phosphate buffered solution for 30 minutes. Cells were then washed, mounting media containing DAPI added, and slides prepared. Images were taken using Nikon E-1000 fluorescent microscope.
LC3-RFP lenti-viral transfection and live imaging
LC3-RFP viruses were generated by transfecting 293T cells. Media containing virus particles were collected at 48 and 72 h. For transduction of primary cultured BMDC, lentiviral particles were purified by ultracentrifugation of supernatants at 24,000 rpm in a Beckman SW40 Ti rotor. Live cell imaging experiments were carried out on a Nikon Eclipse Ti inverted microscope in a humidified chamber and 5% CO2.
Engineering and characterization of PLGA-based nanocarriers of anti-IL6
Poly(lactic-co-glycolic acid) (PLGA, 50:50 LA:GA (w:w)) nanoparticles loaded with anti-IL6 antibody were fabricated using a double emulsion-solvent evaporation method. First, 10 μg of PEG-PLGA (50:50) was dissolved in 1 mL of Ethyl Acetate. Then aqueous solution of anti-IL6 (1 mg/mL) was mixed with PEG-PLGA solution using probe sonication for 10 seconds over ice. Immediately, the first emulsion was transferred into 2 mL of poly(vinyl alcohol) solution (PVA, 0.5% w/v) and emulsified using probe sonication in three 10 seconds bursts (40% amplitude). The solution was allowed to cool in ice bath between each burst. The final emulsion was added into 50 mL DI water and stirred for two hours to evaporate the solvent and harden the nanoparticles. The anti-IL6 loaded PLGA nanoparticles (NP-anti-IL6) were collected and washed by centrifugation using Amicon Ultra-15 centrifugal filter units (MWCO 100 kDa) at 3,000 × g for 10 minutes. The size of the nanoparticles was then characterized using Dynamic Light Scattering (DLS) and Transmission Electron Microscopy. The concentration of the anti-IL6 loaded into PLGA particles was assessed by measuring absorbance of the supernatant after each washing step at λ=266 nm and comparing it to a calibration curve of the absorbance of various concentrations of anti-IL6 at 266 nm.
Quantitative RT-PCR
Harvested cardiac grafts were flash frozen in liquid nitrogen and used for RNA extraction. Tissues were homogenized and sonicated, and RNA was isolated using RNeasy mini kit (Qiagen, Valencia, CA). Harvested RNA was subsequently measured and used to prepare cDNA using iScript cDNA synthesis kit (Bio-Rad laboratories, Inc., Hercules, CA). Quantitative PCR was then performed for IL6, Atg5, Atg12, Beclin1, DEEP and MAP-L3B genes. Expression was assessed as compared to GAPDH expression. Relative gene expression was calculated using the 2ΔΔCT method.
Statistics
Kaplan-Meier survival graphs were constructed and log rank comparison of the groups was used to calculate p-values for survival comparisons between various groups. Data analysis was performed using GraphPad Prism (GraphPad Software, Inc., San Diego, CA). Differences between groups were evaluated by student's t-test or ANOVA to determine significance. *p<0.05 was considered a significant difference.
Results
Ischemia increases autophagy in DC
While increased autophagy due to stress (i.e. starvation) markedly increases DC production of inflammatory cytokines (9, 10), the impact of IRI on the metabolic programming and induction of autophagy in DCs remains to be explored. Oxidative stress has been shown to contribute to the decline in mitochondrial function and autophagy-related pathology in aging (14, 15).
We first examined the effect of oxidative stress on dendritic cell mitochondria in vitro using H2O2 exposure as a model of DC ischemia as described previously (13, 16-20). Control and oxidatively stressed DCs (OS-DCs) were stained with mitotracker red, which stains mitochondrial membrane potential. Immunofluorescent cell imaging revealed lower mitochondrial membrane potential in OS-DCs as compared to untreated DCs (Fig. 1A).
Figure 1. IRI increases autophagy in DCs and contributes to over production of IL6.
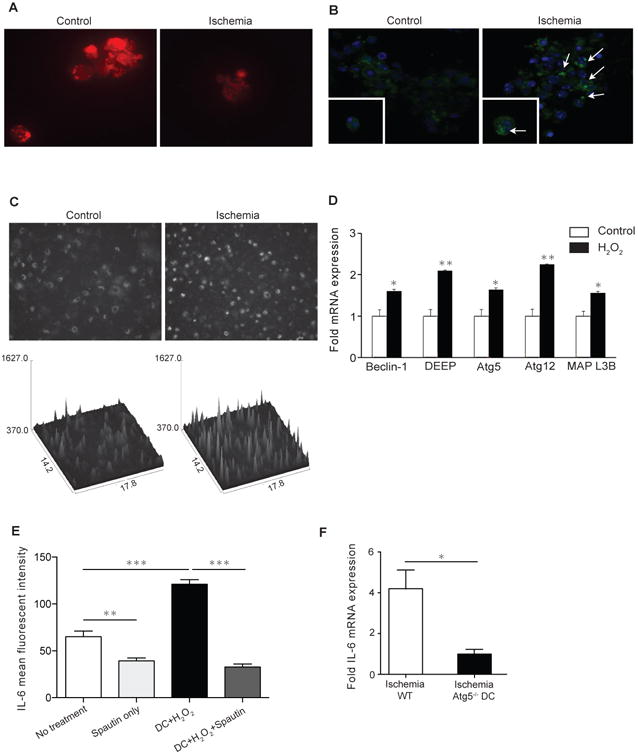
A) OS-DCs and control DCs were stained with mitotracker red to assess their mitochondrial membrane potential. OS-DCs show decreased membrane potential as compared to untreated controls. B) Increase in localized dense Atg5 staining was observed in the OS-DCs as compared to control DCs. C) DCs were transfected with LC3-RFP lenti-virus to localize formed autophagosomes. OS-DCs showed significant increase in autophagosome formation. Surface plot shows marked increase in intensity of red fluorescent protein in OS-DCs (mean intensity of 10 spots/field: 553.8 vs. 718.6, ***p<0.001 for baseline and ischemia, respectively.) D) OS-DCs showed significant up-regulation in gene expression of major autophagy makers: Atg5, Atg12, MAP-L3B, DEEP and Beclin-1 oxidative stress as compared to controls. E) Pre-treatment with autophagy inhibitor Spautin-1 reduced production of IL6 by OS-DCs in Luminex assay after 24 hrs. (***p<0.001). Although cell death subsequent to inhibition of autophagy has contributed to less IL-6 production (second column), however remainder of live cells did not increase IL-6 production in response to ischemic injury. F) Heart allografts from Ischemic WT and CD11cCre/Atg5 conditional knockouts were transplanted into BALB/cJ recipients. Hearts were harvested at day 1 post-transplant and studied for IL6 gene expression. Heart grafts with Atg5 deficient DC showed markedly lower expression of IL6 as compared to WT grafts (n=3 mice/group, *p<0.05).
We then examined the impact of oxidative stress on the induction of autophagy in DCs in vitro. Using immunofluorescence staining for Atg5, an ubiquitin ligase necessary for autophagosome elongation, our data demonstrated increased autophagosome formation in OS-DCs as compared to controls (Fig. 1B). OS-DCs and control DCs transfected with LC3-RFP lenti-virus were studied for autophagosome formation using immunofluorescence microscopy. We noted significant upregulation of autophagy in OS-DCs as shown by an increase in membranous form of LC3 protein, resulting in dense localized RFP with increased signal intensity (Fig. 1C).
We next examined the expression of major autophagy genes by qPCR on mRNA from splenic DCs. OS-DCs had significantly higher expression of autophagy related genes, including Atg5, Atg12, MAP L3B and DEEP, as compared to control DCs (n=3 samples/group, *p<0.05, **p<0.01) (Fig. 1D).
We previously have shown that IRI increases production of IL-6 by ischemic DC (13). We next studied the effect of autophagy on IL6 production from DCs. OS-DCs and DCs were treated with Spautin-1, an autophagy inhibitor. Increased levels of IL6 were present in culture media from OS-DCs compared to control, as measured by Luminex assay. However, blocking autophagy markedly reduced IL6 production by OS-DCs (Fig. 1E).
To study the role of DC Atg5 in increased IL6 production under IRI in vivo, ischemic hearts from WT C57BL/6 and CD11cCre/Atg5 conditional knockout donors were transplanted into BALB/cJ recipients. CD11cCre/Atg5 conditional knockout mice lack Atg5 protein exclusively in their DCs. Grafts were harvested at day 1 post-transplant, mRNA isolated and relative IL6 gene expression studied. Our data reveal markedly reduced IL6 expression in ischemic grafts with Atg5 deficient DCs as compared to WT grafts (n=3 mice/group, *p<0.05) (Fig. 1F).
Atg5 plays a key role in the survival of DC under IRI
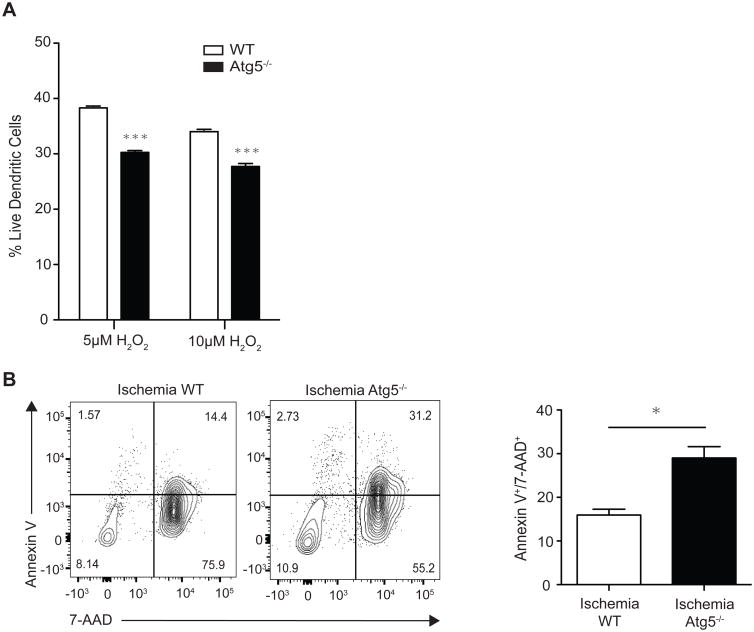
To assess the mechanism of decreased IL6 expression in ischemic grafts with Atg5-/- DCs, we isolated splenic DCs from WT C57BL/6 and CD11cCre/Atg5 conditional knockout mice and exposed them to H2O2 in vitro. DCs were then harvested and studied for cell death rate using flow cytometry. Our data demonstrate significantly lower survival rate in autophagy deficient DCs as compared to WT DCs under ischemic conditions (Fig. 2A).
Figure 2. Atg5 plays a key role in survival of DC under IRI.
A) Splenic DCs were isolated from WT and CD11cCre/Atg5 conditional knockout mice. DCs were stained for Annexin-V and 7-AAD after 20 hours of exposure to 5μM or 10μM of H2O2. Double negative cells were labeled as live. Atg5 deficient DCs showed significantly lower survival rates as compared to WT DCs. B) Ischemic WT or Atg5 conditional knockout hearts were transplanted into BALB/cJ recipients. Grafts were then harvested 1 day post-transplant. H2-kd negative (donor) DCs were studied for rate of death. Ischemic Atg5 deficient DCs showed significantly higher apoptosis as compared to WT donor DCs (Right upper quadrant) (n=3/group, *p<0.05).
We then studied the effect of Atg5 deficiency in ADDC on their survival in vivo. Ischemic hearts from either CD11cCre/Atg5 conditional knockout or WT C57BL/6 donors were transplanted into BALB/cJ recipients. Grafts were harvested on day 1 post-transplant. Graft-infiltrating cells were analyzed by flow cytometry to compare rate of death in CD11c+ DCs. ADDC deficient in Atg5 showed markedly increased apoptosis as compared to their WT controls (Fig. 2B). These results emphasize the role of autophagy in DC survival under ischemia and subsequent IL6 production.
IRI markedly induces DC autophagy and worsens chronic rejection
We then examined the impact of IRI on the development of chronic rejection, a major obstacle to long-term success in solid organ transplantation (21). Hearts from H2-Ab1bm12 (BM12) mice were transplanted into C57BL/6 mice, a single MHC class II mismatch model (22, 23). On day 14 post-transplant, ischemic grafts showed marked cell infiltration, increased myocyte injury, vascular occlusion and graft muscle necrosis as compared to controls (Fig. 3, A5 and A6 compared to A1 and A2). Fibronectin, a marker of tissue fibrosis, was increased in the ischemic grafts as compared to controls (Fig. 3, A7 compared to A3). There was a significant increase in F4/80+ macrophages in ischemic grafts as compared to controls (Fig. 3, A8 compared to A4).
Figure 3. Anti-IL6 therapy significantly decreases IRI induced autophagy and chronic allograft rejection.
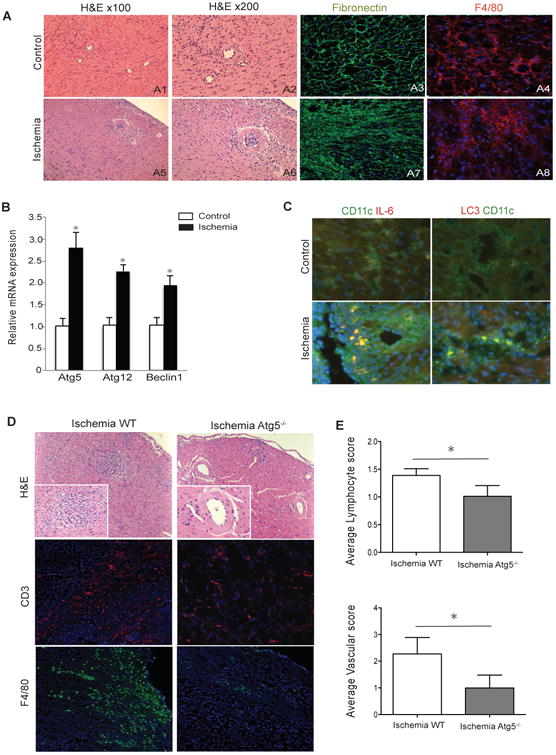
H2-Ab1bm12 (BM12) control and ischemic hearts were transplanted into C57BL/6 recipients. Grafts were harvested 14 days after transplant. A) Ischemic grafts showed significant increase in cell infiltration, more intense graft necrosis, vascular occlusion, tissue fibrosis (fibronectin) and macrophage infiltrates (A5-8) as compared to controls (A1-4). B) Ischemic heart grafts harvested at day 14 post-transplant showed significant increase in autophagy gene expression as compared to controls (n=3 to 4 mice/group, *p<0.05). C) Ischemic grafts showed higher expression of IL6 as well as autophagy markers co-localizing with CD11c+ DCs (CD11C+ in green, IL6 and LC3 in red). D) Ischemic CD11cCre/Atg5 conditional knockout hearts harvested from BM12 recipients 28 days post-transplantation (right) revealed less chronic rejection by means of vascular pathology and tissue infiltration compared to ischemic WT (left). E) Bar graphs showing significantly reduced lymphocyte and vascular score in ischemic CD11cCre/Atg5 conditional knockout donor hearts as compared to ischemic WT (n=3, *p<0.05 and *p<0.05, respectively)
We then examined the expression of autophagy genes in control and ischemic heart grafts harvested at day 14. We observed a marked increase in expression of autophagy related genes Atg5, Atg12 and Beclin-1 in the ischemic group as compared to controls (Fig. 3B). Immunofluorescence staining demonstrated more intense IL6 staining in ischemic grafts as compared to controls. Staining for CD11c showed strong co-localization of IL6 with DCs (Fig. 3C, left panel). Under ischemic conditions, we not only found increased LC3 expression, which is a marker of autophagy, but also its co-localization with CD11c+ DCs (Fig. 3C, right panel).
To study the effect of ADDC autophagy on chronic rejection, we transplanted ischemic hearts from either WT or CD11cCre/Atg5 conditional knockout mice into BM12 recipients. Hearts were harvested 28 days post-transplantation and examined for severity of chronic rejection. CD11cCre/Atg5 conditional knockout hearts revealed lower tissue lymphocyte infiltration, vasculopathy and less macrophage infiltration by histology (Figure 3D). Significantly less lymphocyte infiltration and vasculopathy were seen in CD11cCre/Atg5 conditional knockout grafts compared to control. (Fig. 3E, n=3/each, *p<0.05 and *p<0.05, respectively). At the same time, we did not observe differences in the percentage of T regs or effector cells (CD44high CD62llow) in the spleen or DLN of allograft recipients (Data not shown).
Intra-organ delivery of anti-IL6 therapy significantly decreases chronic rejection induced by IRI
As a potential therapeutic target, we then investigated the role of anti-IL6 in reducing IRI induced chronic rejection. C57BL/6 recipients of BM12 heart grafts with 8hr ischemia were treated with 100 μg systemic (intra-peritoneal) anti-IL6 antibody injections on days 0 to 3, followed by every other day injection until day 13 post transplant. Heart grafts were harvested on day 14 and examined for chronic rejection. As compared to untreated ischemic grafts, heart allografts from systemic anti-IL6 treated ischemic graft showed significantly less cell infiltration and vascular injury (Fig. 4, D1 and D2, compared to Fig3, A5 and A6). Systemic anti-IL6 treatment significantly suppressed macrophage infiltration in the ischemic grafts to a level comparable to non-ischemic controls (Fig. 4, D3 compared to Fig. 3, A4). Our findings suggest that systemic anti-IL6 therapy abrogates the effect of ischemia in the progression of chronic allograft rejection.
Figure 4. Intra-organ delivery of anti-IL6 therapy significantly decreases chronic rejection induced by IRI.
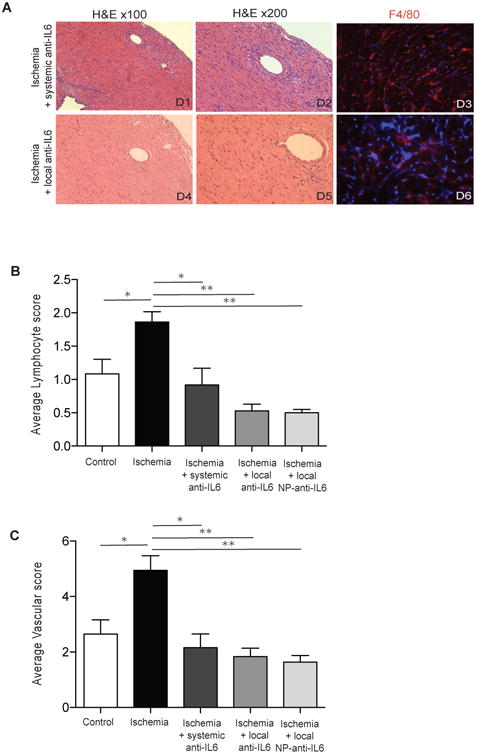
A) H2-Ab1bm12 (BM12) ischemic hearts were transplanted into C57BL/6 recipients with either systemic or local treatment with anti-IL6 antibody. Grafts were harvested 14 days after transplant. Both systemic (D1-3) and local anti-IL6 (D4-6) treatment significantly reduced cellular rejection, vascular injuries and macrophage infiltrates as compared to the untreated ischemia groups (Figure 3A, A5-A8). DAPI is in blue in immunofluorescent images. B) Bar graphs showing the quantification of cellular infiltrates and vascular injuries (n=3 to 4 mice/group, *p<0.05, **p<0.01).
Similar to other immunomodulatory agents, systemic anti-IL6 therapy is associated with risks (e.g., infection) (24). Given the importance of intra-graft IL6 expression in IRI-mediated rejection, we hypothesized that local delivery of anti-IL6 might be a useful alternative strategy. Heart grafts harvested from BM12 donors were perfused with 100 μg of anti-IL6 and kept in UW solution for 8 hours (Ischemia + local anti-IL6). Interestingly, a single dose of local intra-graft treatment (9 times lower than systemic dose) significantly protected ischemic grafts from chronic rejection as demonstrated by a reduction in inflammatory cell infiltrates, macrophage infiltration and vascular injury (Fig. 4, D4-6). Quantification of cell and vascular injuries in both groups are shown in Figure 4B and C. Our data indicate that intra-organ delivery of anti-IL6 confers significant protection against chronic rejection with significantly less systemic exposure to anti-IL6.
Double emulsion assembly and characterization of NP-anti-IL6
We then tested the hypothesis that perfusing the organ with anti-IL6 nanoparticles (NP-anti-IL6) allows for more effective control of intra-graft inflammatory responses with sustained release of anti-IL6. We also tested whether we could reduce anti-IL6 dosing using such platform. Moreover, direct delivery of NP-anti-IL6 to the organ prior to transplantation would reduce accumulation of NPs in non-targeted organs.
We encapsulated anti-IL6 in PLGA-NPs by dispersing the hydrophilic anti-IL6 in an immiscible PLGA solution using probe-sonication followed by second emulsification in an aqueous solution. This technique provides first NP-anti-IL6 with controlled size and narrow size distribution depending on the probe-sonication power and time. Figure 5A shows a transmission electron microscopy image of NP-anti-IL6, showing particles are spherical in shape, range in size from 50-200 nanometers (nm), majority of which are 100nm in diameter (Fig. 5B).
Figure 5. Effective suppression of IRI induced chronic rejection with anti-IL6 nanoparticles.
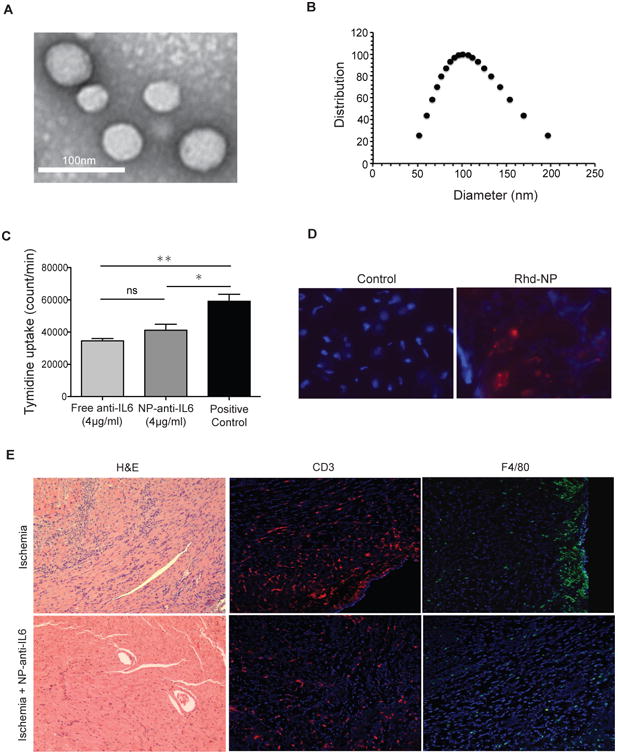
A) Representative TEM image of PLGA-NPs stained with 0.75% uranyl formate (scale bar 100 nm). B) Size distribution of anti-IL6 loaded PLGA-NPs measured by dynamic light scattering. C) NP-anti-IL6 significantly suppressed T cell proliferation in anti-CD3/CD28 T cell proliferation assay (*p<0.05 and **p<0.01). D) Fluorescent-labeled particles perfused through the hearts prior to transplantation were still detectable in grafts on day 7 post-transplant. E) NP-anti-IL6 treatment of ischemic heart prior to transplant significantly reduces chronic rejection as compare to ischemic control and less CD3 and F4/80. Original magnification: 100×. TEM: Transmission electron microscopy.
To test the efficiency of NP-anti-IL6 in retaining and release of anti-IL6 in vitro, NP-anti-IL6 and free anti-IL6 were added to a T cell proliferation assay. Significant suppression of T cell proliferation by both free anti-IL6 and NP-anti-IL6 was noted as compared to untreated stimulated T cells. However, there was no statistical difference between free anti-IL6 or particle-embedded anti-IL6 antibody in the level of inhibition of T cell proliferation in vitro (Fig. 5C).
Delivery of NP-anti-IL6 to the organ prior to transplantation reduces chronic rejection
To confirm tissue accumulation of NP, we perfused hearts prior to transplant with NP labeled with rhodamine and grafts were harvested at day 7. As compared to control unperfused hearts, rhodamine-labeled NPs (red) were detected in treated grafts (Figure 5D). We next sought to examine the efficacy of localized delivery of NP-anti-IL6 in suppressing chronic rejection. Ischemic heart grafts harvested from BM12 donors were perfused with NP-anti-IL6 (equivalent of 4 μg anti-IL6 antibody), transplanted into C57BL/6 recipients, and harvested on day 14. Ischemic grafts perfused with NP-anti-IL6 showed minimal cellular infiltration and almost no tissue necrosis, vascular infiltration or occlusion as compared to ischemia controls (Figure 5E left). We further phenotyped graft-infiltrating cells, which showed decreased frequency of CD3+ and F4/80+ infiltrating cells (Figure 5E middle and right). Interestingly, despite the marked histological differences, FACS analysis of spleen and DLN of recipient mice did not reveal discernable differences in the percentage of T regs or T effector population (CD44high CD62l low T cell) (data not shown).
Discussion
IRI is an inevitable complication of solid organ transplantation and a major source of intra-graft inflammation and innate immune activation and as such, targeting this process holds great potential to improve transplantation outcomes (25-28).
IL6 has been shown to promote allograft rejection in a number of studies (29-32). However, the role of IRI-induced autophagy in ADDC as the mechanism of induction of IL6 production by ADDC resulting in the augmentation of alloimmunity has not been previously explored.
ADDC are readily activated by danger molecules and reactive oxygen species produced within the graft in the setting of ischemia (13, 25). Autophagy is a cellular process involved in degradation of damaged intracellular organelles and has been shown to enhance DCs antigen presentation, maturity, production of inflammatory cytokines and CD4+ T cell priming (9, 33-36). Previous studies have shown elevated levels of autophagy in several cell types as a result of H2O2 exposure (37-41). While autophagy has been shown to play a role in T cell homeostasis and cytokine production, and therefore, also affects allograft survival (42, 43), its role in upregulating ADDC functions and augmenting alloimmune responses are unclear. We demonstrated higher autophagy levels in OS-DCs using quantitative PCR, immunofluorescence for Atg5, and following lenti-viral transfection of DCs with fluorescent-labeled LC3 gene in vitro. Interestingly, while OS-DCs showed increased IL6 production, blocking autophagy reduced the production of IL6. Mitochondrial damage in OS-DC has been reported to increase autophagy (44, 45). Damaged mitochondria can contribute to further injury and disruption of cell function via generation of more reactive oxygen species (37). Our data indicate that IRI also causes reduced mitochondrial membrane potential in DC. Using CD11cCre/Atg5 conditional knockout donors, we showed that lack of Atg5 in DC results in increased death rate following ischemia. Using hearts from CD11cCre/Atg5 conditional knockout donors reduced the severity of chronic rejection as compared to controls.
While animal studies have highlighted the importance of targeting IL6 to promote allograft survival (29, 30, 32, 46-50), the impact of anti-IL6 in reducing IRI induced alloimmunity has not been shown previously. Our data indicate that anti-IL6 treatment abrogates this effect of IRI. Immune manipulation of organs prior to transplant represents a highly innovative, significant and feasible approach in transplantation. Prior to transplantation, there is a unique opportunity to apply treatments directly to the organ. In addition to increasing the efficacy, local delivery also allows reduced systemic exposure to anti-IL6, which carries risks of infectious and other complications related to systemic immunosuppression (24).
Given the pleotropic functions of IL6 as an inflammatory and regulatory cytokine, the impact of anti-IL6 in IRI induced alloimmunity could be complex. IL6 has been shown to have both deleterious and beneficial effects in non-transplant IRI models (51-56). These differential results might be due to the differences in the timing of injury, type of IRI model and interventions applied. Nonetheless, numerous studies have highlighted the transplanted organ as the source of IL6 production, which supports the concept of targeting these pathways within the allograft. Moving forward, it would important to carry out kinetic studies of the release of anti-IL6 and examine the potential effects of anti-IL6 on various cardiac cell types including the metabolism of cardiomyocytes and their contractility over time post-transplant.
Targeted delivery of immune regulatory agents to the organ not only suppresses local alloreactive T cells involved in rejection but also prevents alloreactive T cell priming (57-60). Ischemic cardiac allografts perfused with free anti-IL6 antibody showed significantly suppressed chronic rejection. With increased control and characterization of materials at the nanoscale size, the field of nanotechnology has spurred a high level of interest in medicine, particularly in oncology (61) but has also increasingly received attention for application in transplantation (62-64). We therefore assessed whether nanodelivery, with sustained release capacity of anti-IL6 within the organ improves the efficacy of local delivery of anti-IL6. We used FDA approved biodegradable polymeric PLGA-NPs with controlled release capacity for anti-IL6. We engineered a NP carrier of anti-IL6 with excellent loading and release capacity for organ delivery. Using NP-anti-IL6, we were able to significantly reduce chronic rejection, with a dose that is more than 200 times lower than that of systemic anti-IL6 (total of 4 μg anti-IL6 in NP vs. 900 μg systemic). This is the first controlled formulation of an anti-IL6 nanomedicine, which holds the potential to shape future immunosuppressive therapies in transplantation. Interestingly we did not observe an effect on T regs or effector T cells in the periphery, emphasizing the lack of systemic immunosuppression. This also suggests that local IL6 production is a central contributor to the development of chronic rejection in a model of allograft ischemia. However given that we used clinically relevant polyclonal models, changes in the regulatory or effector T cell compartments may be less readily identified. Alloantigen specific transplant models may be useful to examine this in greater detail in future studies. Herein, our primary aim was to use clinically relevant models to generate translational data, focused on chronic rejection, which remains the single most important barrier to long-term graft survival. Our ultimate goal is to develop a translational combinatorial targeted-delivery technology for immunoregulatory molecules with broad clinical applicability.
In summary, our study indicates that IRI increases DC autophagy and production of IL6, enhancing chronic rejection. Delivering anti-IL6 to organs prior to transplantation via NPs represents a highly innovative strategy with significant clinical implications.
Acknowledgments
This work was supported in part by NIH grants RO1AI091930 and R56 AI123270 (R.A).
Abbreviations
- IRI
Ischemia reperfusion injury
- DC
Dendritic cell
- ADDC
Allograft derived dendritic cell
- NP
Nanoparticle
- DLN
Draining lymph node
- OS-DC
Oxidatively stressed DC
Footnotes
Author Contributions: Z.S., M.U., B.B., O.M. and T.I performed the experiments and analyzed data. W.X and M.Y. performed experiments. C.B. designed and performed experiments and also provided the LC3-RFP virus. R.A., Z.S., M.U., M.M., A.E. and S.T. designed the experiments and wrote the paper. All authors discussed the results and commented on the manuscript.
Disclosure: The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Fischbein MP, et al. CD40 signaling replaces CD4+ lymphocytes and its blocking prevents chronic rejection of heart transplants. J Immunol. 2000 Dec 15;165(12):7316–22. doi: 10.4049/jimmunol.165.12.7316. Epub 2000/12/20. eng. [DOI] [PubMed] [Google Scholar]
- 2.Heuer M, et al. Use of marginal organs in kidney transplantation for marginal recipients: too close to the margins of safety? European journal of medical research. Jan 29;15(1):31–4. doi: 10.1186/2047-783X-15-1-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang E, Segev DL, Rabb H. Kidney transplantation in the elderly. Seminars in nephrology. 2009 Nov;29(6):621–35. doi: 10.1016/j.semnephrol.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirth RA, Pan Q, Schaubel DE, Merion RM. Efficient utilization of the expanded criteria donor (ECD) deceased donor kidney pool: an analysis of the effect of labeling. Am J Transplant. Feb;10(2):304–9. doi: 10.1111/j.1600-6143.2009.02937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baldwin WM, 3rd, et al. Complement deposition in early cardiac transplant biopsies is associated with ischemic injury and subsequent rejection episodes. Transplantation. 1999 Sep 27;68(6):894–900. doi: 10.1097/00007890-199909270-00024. Epub 1999/10/09. eng. [DOI] [PubMed] [Google Scholar]
- 6.Wilhelm MJ, et al. Activation of the heart by donor brain death accelerates acute rejection after transplantation. Circulation. 2000;102(19):2426–33. doi: 10.1161/01.cir.102.19.2426. [DOI] [PubMed] [Google Scholar]
- 7.Solhjou Z, Athar H, Xu Q, Abdi R. Emerging therapies targeting intra-organ inflammation in transplantation. Am J Transplant. 2015 Feb;15(2):305–11. doi: 10.1111/ajt.13073. [DOI] [PubMed] [Google Scholar]
- 8.Methe H, Zimmer E, Grimm C, Nabauer M, Koglin J. Evidence for a role of toll-like receptor 4 in development of chronic allograft rejection after cardiac transplantation. Transplantation. 2004 Nov 15;78(9):1324–31. doi: 10.1097/01.tp.0000137930.40597.03. Epub 2004/11/19. eng. [DOI] [PubMed] [Google Scholar]
- 9.Morris S, et al. Autophagy-mediated dendritic cell activation is essential for innate cytokine production and APC function with respiratory syncytial virus responses. J Immunol. 2011 Oct 15;187(8):3953–61. doi: 10.4049/jimmunol.1100524. Epub 2011/09/14. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature. 2011 Jan 20;469(7330):323–35. doi: 10.1038/nature09782. Epub 2011/01/21. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corry RJ, Winn HJ, Russell PS. Primarily vascularized allografts of hearts in mice. The role of H-2D, H-2K, and non-H-2 antigens in rejection. Transplantation. 1973 Oct;16(4):343–50. doi: 10.1097/00007890-197310000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Azzi J, et al. Serine protease inhibitor 6 plays a critical role in protecting murine granzyme B-producing regulatory T cells. Journal of immunology. 2013 Sep 01;191(5):2319–27. doi: 10.4049/jimmunol.1300851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Batal I, et al. The mechanisms of up-regulation of dendritic cell activity by oxidative stress. Journal of leukocyte biology. 2014 Aug;96(2):283–93. doi: 10.1189/jlb.3A0113-033RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang CH, Wu SB, Wu YT, Wei YH. Oxidative stress response elicited by mitochondrial dysfunction: implication in the pathophysiology of aging. Experimental biology and medicine. 2013 May;238(5):450–60. doi: 10.1177/1535370213493069. [DOI] [PubMed] [Google Scholar]
- 15.Cui H, Kong Y, Zhang H. Oxidative stress, mitochondrial dysfunction, and aging. Journal of signal transduction. 2012;2012:646354. doi: 10.1155/2012/646354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fiorina P, et al. Characterization of Donor Dendritic Cells and Enhancement of Dendritic Cell Efflux With cc-Chemokine Ligand 21: A Novel Strategy to Prolong Islet Allograft Survival. Diabetes. 2007 Apr;56(4):912–20. doi: 10.2337/db06-1445. [DOI] [PubMed] [Google Scholar]
- 17.Jurewicz M, et al. Ischemic injury enhances dendritic cell immunogenicity via TLR4 and NF-kappa B activation. J Immunol. 2010 Mar 15;184(6):2939–48. doi: 10.4049/jimmunol.0901889. [DOI] [PubMed] [Google Scholar]
- 18.Colussi C, Albertini MC, Coppola S, Rovidati S, Galli F, Ghibelli L. H2O2-induced block of glycolysis as an active ADP-ribosylation reaction protecting cells from apoptosis. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2000 Nov;14(14):2266–76. doi: 10.1096/fj.00-0074com. [DOI] [PubMed] [Google Scholar]
- 19.Los M, Droge W, Stricker K, Baeuerle PA, Schulze-Osthoff K. Hydrogen peroxide as a potent activator of T lymphocyte functions. European journal of immunology. 1995 Jan;25(1):159–65. doi: 10.1002/eji.1830250127. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi A, et al. Preferential cell death of CD8+ effector memory (CCR7-CD45RA-) T cells by hydrogen peroxide-induced oxidative stress. J Immunol. 2005 May 15;174(10):6080–7. doi: 10.4049/jimmunol.174.10.6080. [DOI] [PubMed] [Google Scholar]
- 21.Schmauss D, Weis M. Cardiac allograft vasculopathy: recent developments. Circulation. 2008 Apr 22;117(16):2131–41. doi: 10.1161/CIRCULATIONAHA.107.711911. Epub 2008/04/23. eng. [DOI] [PubMed] [Google Scholar]
- 22.Schenk S, et al. Alloreactive T cell responses and acute rejection of single class II MHC-disparate heart allografts are under strict regulation by CD4+ CD25+ T cells. J Immunol. 2005 Mar 15;174(6):3741–8. doi: 10.4049/jimmunol.174.6.3741. [DOI] [PubMed] [Google Scholar]
- 23.Yang J, et al. The novel costimulatory programmed death ligand 1/B7.1 pathway is functional in inhibiting alloimmune responses in vivo. J Immunol. 2011 Aug 1;187(3):1113–9. doi: 10.4049/jimmunol.1100056. Epub 2011/06/24. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okuda Y. Review of tocilizumab in the treatment of rheumatoid arthritis. Biologics. 2008 Mar;2(1):75–82. doi: 10.2147/btt.s1828. Epub 2008/03/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murphy SP, Porrett PM, Turka LA. Innate immunity in transplant tolerance and rejection. Immunol Rev. 2011 May;241(1):39–48. doi: 10.1111/j.1600-065X.2011.01009.x. Epub 2011/04/15. eng. [DOI] [PubMed] [Google Scholar]
- 26.Kupiec-Weglinski JW. Tolerance induction. Current opinion in organ transplantation. 2008 Aug;13(4):331–2. doi: 10.1097/MOT.0b013e3283069d87. [DOI] [PubMed] [Google Scholar]
- 27.Land W. Innate alloimmunity: history and current knowledge. Exp Clin Transplant. 2007 Jun;5(1):575–84. [PubMed] [Google Scholar]
- 28.Land W, et al. The beneficial effect of human recombinant superoxide dismutase on acute and chronic rejection events in recipients of cadaveric renal transplants. Transplantation. 1994 Jan;57(2):211–7. doi: 10.1097/00007890-199401001-00010. [DOI] [PubMed] [Google Scholar]
- 29.Booth AJ, Grabauskiene S, Wood SC, Lu G, Burrell BE, Bishop DK. IL-6 promotes cardiac graft rejection mediated by CD4+ cells. Journal of immunology. 2011 Dec 1;187(11):5764–71. doi: 10.4049/jimmunol.1100766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liang Y, Christopher K, Finn PW, Colson YL, Perkins DL. Graft produced interleukin-6 functions as a danger signal and promotes rejection after transplantation. Transplantation. 2007 Sep 27;84(6):771–7. doi: 10.1097/01.tp.0000281384.24333.0b. [DOI] [PubMed] [Google Scholar]
- 31.Shen H, Goldstein DR. IL-6 and TNF-alpha synergistically inhibit allograft acceptance. Journal of the American Society of Nephrology : JASN. 2009 May;20(5):1032–40. doi: 10.1681/ASN.2008070778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao X, et al. Critical role of proinflammatory cytokine IL-6 in allograft rejection and tolerance. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2012 Jan;12(1):90–101. doi: 10.1111/j.1600-6143.2011.03770.x. [DOI] [PubMed] [Google Scholar]
- 33.Ireland JM, Unanue ER. Autophagy in antigen-presenting cells results in presentation of citrullinated peptides to CD4 T cells. J Exp Med. 2011 Dec 19;208(13):2625–32. doi: 10.1084/jem.20110640. Epub 2011/12/14. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cooney R, et al. NOD2 stimulation induces autophagy in dendritic cells influencing bacterial handling and antigen presentation. Nat Med. 2010 Jan;16(1):90–7. doi: 10.1038/nm.2069. Epub 2009/12/08. eng. [DOI] [PubMed] [Google Scholar]
- 35.Lee HK, et al. In vivo requirement for Atg5 in antigen presentation by dendritic cells. Immunity. 2010 Feb 26;32(2):227–39. doi: 10.1016/j.immuni.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reed M, Morris SH, Jang S, Mukherjee S, Yue Z, Lukacs NW. Autophagy-inducing protein beclin-1 in dendritic cells regulates CD4 T cell responses and disease severity during respiratory syncytial virus infection. J Immunol. 2013 Sep 1;191(5):2526–37. doi: 10.4049/jimmunol.1300477. Epub 2013/07/31. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hill BG, et al. Integration of cellular bioenergetics with mitochondrial quality control and autophagy. Biological chemistry. 2012 Dec;393(12):1485–512. doi: 10.1515/hsz-2012-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang H, et al. Oxidative stress induces parallel autophagy and mitochondria dysfunction in human glioma U251 cells. Toxicol Sci. 2009 Aug;110(2):376–88. doi: 10.1093/toxsci/kfp101. Epub 2009/05/20. eng. [DOI] [PubMed] [Google Scholar]
- 39.She C, Zhu LQ, Zhen YF, Wang XD, Dong QR. Activation of AMPK protects against hydrogen peroxide-induced osteoblast apoptosis through autophagy induction and NADPH maintenance: new implications for osteonecrosis treatment? Cell Signal. 2014 Jan;26(1):1–8. doi: 10.1016/j.cellsig.2013.08.046. Epub 2013/10/02. eng. [DOI] [PubMed] [Google Scholar]
- 40.Chen Y, McMillan-Ward E, Kong J, Israels SJ, Gibson SB. Oxidative stress induces autophagic cell death independent of apoptosis in transformed and cancer cells. Cell Death Differ. 2008 Jan;15(1):171–82. doi: 10.1038/sj.cdd.4402233. Epub 2007/10/06. eng. [DOI] [PubMed] [Google Scholar]
- 41.Li L, Chen Y, Gibson SB. Starvation-induced autophagy is regulated by mitochondrial reactive oxygen species leading to AMPK activation. Cell Signal. 2013 Jan;25(1):50–65. doi: 10.1016/j.cellsig.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 42.Hubbard VM, Valdor R, Patel B, Singh R, Cuervo AM, Macian F. Macroautophagy regulates energy metabolism during effector T cell activation. J Immunol. 2010 Dec 15;185(12):7349–57. doi: 10.4049/jimmunol.1000576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Verghese DA, Yadav A, Bizargity P, Murphy B, Heeger PS, Schroppel B. Costimulatory blockade-induced allograft survival requires Beclin1. Am J Transplant. 2014 Mar;14(3):545–53. doi: 10.1111/ajt.12610. [DOI] [PubMed] [Google Scholar]
- 44.Scherz-Shouval R, Shvets E, Fass E, Shorer H, Gil L, Elazar Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. The EMBO journal. 2007 Apr 4;26(7):1749–60. doi: 10.1038/sj.emboj.7601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen Y, Azad MB, Gibson SB. Superoxide is the major reactive oxygen species regulating autophagy. Cell Death Differ. 2009 Jul;16(7):1040–52. doi: 10.1038/cdd.2009.49. [DOI] [PubMed] [Google Scholar]
- 46.Booth AJ, Bishop DK. TGF-beta, IL-6, IL-17 and CTGF direct multiple pathologies of chronic cardiac allograft rejection. Immunotherapy. 2010 Jul;2(4):511–20. doi: 10.2217/imt.10.33. Epub 2010/07/20. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Diaz JA, Booth AJ, Lu G, Wood SC, Pinsky DJ, Bishop DK. Critical role for IL-6 in hypertrophy and fibrosis in chronic cardiac allograft rejection. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2009 Aug;9(8):1773–83. doi: 10.1111/j.1600-6143.2009.02706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fogal B, et al. Neutralizing IL-6 reduces human arterial allograft rejection by allowing emergence of CD161+ CD4+ regulatory T cells. Journal of immunology. 2011 Dec 15;187(12):6268–80. doi: 10.4049/jimmunol.1003774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Iida S, et al. Interleukin-6 receptor signaling disruption prevents cardiac allograft deterioration in mice. Exp Clin Transplant. 2012 Aug;10(4):375–85. doi: 10.6002/ect.2011.0159. Epub 2012/07/05. eng. [DOI] [PubMed] [Google Scholar]
- 50.Kimura N, et al. Interleukin-16 deficiency suppresses the development of chronic rejection in murine cardiac transplantation model. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2011 Dec;30(12):1409–17. doi: 10.1016/j.healun.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 51.Kielar ML, et al. Maladaptive role of IL-6 in ischemic acute renal failure. Journal of the American Society of Nephrology : JASN. 2005 Nov;16(11):3315–25. doi: 10.1681/ASN.2003090757. [DOI] [PubMed] [Google Scholar]
- 52.Jong WM, et al. Reduced acute myocardial ischemia-reperfusion injury in IL-6-deficient mice employing a closed-chest model. Inflammation research : official journal of the European Histamine Research Society [et al] 2016 Jun;65(6):489–99. doi: 10.1007/s00011-016-0931-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Patel NS, et al. Endogenous interleukin-6 enhances the renal injury, dysfunction, and inflammation caused by ischemia/reperfusion. The Journal of pharmacology and experimental therapeutics. 2005 Mar;312(3):1170–8. doi: 10.1124/jpet.104.078659. [DOI] [PubMed] [Google Scholar]
- 54.Nechemia-Arbely Y, et al. IL-6/IL-6R axis plays a critical role in acute kidney injury. Journal of the American Society of Nephrology : JASN. 2008 Jun;19(6):1106–15. doi: 10.1681/ASN.2007070744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de Vries DK, et al. Early renal ischemia-reperfusion injury in humans is dominated by IL-6 release from the allograft. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2009 Jul;9(7):1574–84. doi: 10.1111/j.1600-6143.2009.02675.x. [DOI] [PubMed] [Google Scholar]
- 56.Camargo CA, Jr, Madden JF, Gao W, Selvan RS, Clavien PA. Interleukin-6 protects liver against warm ischemia/reperfusion injury and promotes hepatocyte proliferation in the rodent. Hepatology. 1997 Dec;26(6):1513–20. doi: 10.1002/hep.510260619. [DOI] [PubMed] [Google Scholar]
- 57.Denton MD, et al. The role of the graft endothelium in transplant rejection: evidence that endothelial activation may serve as a clinical marker for the development of chronic rejection. Pediatr Transplant. 2000 Nov;4(4):252–60. doi: 10.1034/j.1399-3046.2000.00031.x. [DOI] [PubMed] [Google Scholar]
- 58.Baratin M, Bonin K, Daniel C. Frontline: Peripheral priming of alloreactive T cells by the direct pathway of allorecognition. European journal of immunology. 2004 Dec;34(12):3305–14. doi: 10.1002/eji.200425309. Epub 2004/10/16. eng. [DOI] [PubMed] [Google Scholar]
- 59.Marelli-Berg FM, Scott D, Bartok I, Peek E, Dyson J, Lechler RI. Activated murine endothelial cells have reduced immunogenicity for CD8+ T cells: a mechanism of immunoregulation? Journal of immunology. 2000 Oct 15;165(8):4182–9. doi: 10.4049/jimmunol.165.8.4182. [DOI] [PubMed] [Google Scholar]
- 60.Marelli-Berg FM, et al. Cognate recognition of the endothelium induces HY-specific CD8+ T-lymphocyte transendothelial migration (diapedesis) in vivo. Blood. 2004 Apr 15;103(8):3111–6. doi: 10.1182/blood-2003-08-2717. Epub 2004/04/09. eng. [DOI] [PubMed] [Google Scholar]
- 61.Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. 2015 Sep;33(9):941–51. doi: 10.1038/nbt.3330. Epub 2015/09/09. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Azzi J, et al. Polylactide-cyclosporin A nanoparticles for targeted immunosuppression. FASEB Journal. 2010 Oct;24(10):3927–38. doi: 10.1096/fj.10-154690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fisher JD, Acharya AP, Little SR. Micro and nanoparticle drug delivery systems for preventing allotransplant rejection. Clin Immunol. 2015 Sep;160(1):24–35. doi: 10.1016/j.clim.2015.04.013. Epub 2015/05/06. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shirali AC, et al. Nanoparticle delivery of mycophenolic acid upregulates PD-L1 on dendritic cells to prolong murine allograft survival. Am J Transplant. 2011 Dec;11(12):2582–92. doi: 10.1111/j.1600-6143.2011.03725.x. Epub 2011/09/03. eng. [DOI] [PubMed] [Google Scholar]