Abstract
Background
Contrast enhanced ultrasound (CEU) limb perfusion imaging is a promising approach for for evaluating peripheral artery disease (PAD). However, low signal enhancement in skeletal muscle has necessitated high-power intermittent imaging algorithms which are not clinically feasible. We hypothesized that CEU using a combination of intermediate power and a contrast agent resistant to inertial cavitation would allow real-time limb stress perfusion imaging.
Methods
In normal volunteers, CEU of the calf skeletal muscle was performed on separate days with Sonazoid, Optison, or Definity. Progressive reduction in the ultrasound pulsing interval (PI) was used to assess the balance between signal enhancement and agent destruction at escalating mechanical indices (MI 0.1 to 0.4). Real-time perfusion imaging at MI 0.1 to 0.4 using post-destructive replenishment kinetics was performed at rest and during 25 W plantar flexion contractile exercise.
Results
For Optison, limb perfusion imaging was unreliable at rest due to very low signal enhancement generated at all MIs, and was possible during exercise-induced hyperemia only at MI 0.1 due to agent destruction at higher MIs. For Definity, signal intensity progressively increased with MI, but was offset by microbubble destruction which resulted in modest signal enhancement during CEU perfusion imaging and distortion of replenishment curves at MI ≥0.2. For Sonazoid, there strong signal enhancement at MI ≥0.2 with little destruction detected only at MI 0.4. Accordingly, high signal intensity and non-distorted perfusion imaging was possible at MI 0.2–0.3 and detected an 8.0±5.7-fold flow reserve.
Conclusions
Rest-stress limb perfusion imaging in humans with real-time CEU, which requires only seconds to perform, is possible using microbubbles with viscoelastic properties that produce strong non-linear signal generation without destruction at intermediate acoustic pressures.
Keywords: Contrast-enhanced ultrasound, Microbubbles, Peripheral artery disease
There is increasing interest in applying stress-rest perfusion imaging protocols similar to those used in the heart to evaluate patients with peripheral artery disease (PAD). Limb blood flow assessment requires quantitative methods since regional heterogeneity cannot be used to diagnose PAD. Quantitative contrast enhanced ultrasound (CEU) perfusion imaging has been demonstrated to provide information on PAD severity beyond that provided by ankle-brachial index (ABI).1–3 The main challenge for CEU is the relatively low signal-to-noise produced during conventional low-power real-time CEU imaging, which is attributable to low skeletal muscle blood flow (0.05–0.3 ml/min/g).4,5 High acoustic power CEU imaging results in greater signal, however it also results in microbubble destruction through inertial cavitation, thereby requiring more time-consuming and technically difficult image acquisition protocols.
During low-power real-time CEU the signal produced by microbubble stable cavitation, defined as non-linear oscillation without destruction, increases with the acoustic power.6,7 However, there is an upper limit where stable cavitation converts to inertial cavitation. This limit is dependent largely on composition-related viscoelastic properties of microbubbles.7,8 It has been shown in small and large animal models that Sonazoid which is an acoustically durable lipid microbubble agents evidenced by a >2-fold higher bulk modulus than other lipid-shelled microbubbles, produces almost 5-fold higher signal during real-time CEU limb perfusion imaging when used in conjunction with intermediate power CEU.9 This advantage was due to this agent’s ability to undergo non-linear oscillation without destruction at mechanical indices (MI) of 0.3–0.4. In this study, we hypothesized that Sonazoid when used for limb perfusion imaging in humans would convey a benefit of higher signal-to-noise compared to more fragile microbubble agents that are destroyed by inertial cavitation during intermediate power CEU.
METHODS
Study Subjects
The study was approved by the Investigational Review Board at Oregon Health & Sciences University. The design was a prospective open-label non-randomized study performed in 14 healthy normal volunteers. Subjects ≥19 years of age without any major medical problems were recruited. Subjects were excluded for history or symptoms of coronary or peripheral artery disease, heart failure, muscle disease, vasculitis, known or suspected right-to-left shunt, pregnancy, or abnormalities in peripheral pulse examination. Subjects were also excluded for allergy to ultrasound contrast agents, eggs, or blood products.
Study Design
CEU limb perfusion imaging protocols were performed on two separate days separated by <14 days using Sonazoid (GE Healthcare) on one day and either Definity (Lantheus Medical Imaging) or Optison (GE Healthcare) (n=7 for each) on the other day. Subject vital signs and oxygen saturation were continuously measured during the protocol. Twelve subjects returned within 6 weeks to evaluate test-retest variability of both rest and stress CEU perfusion assessment with Sonazoid.
Microbubble Administration
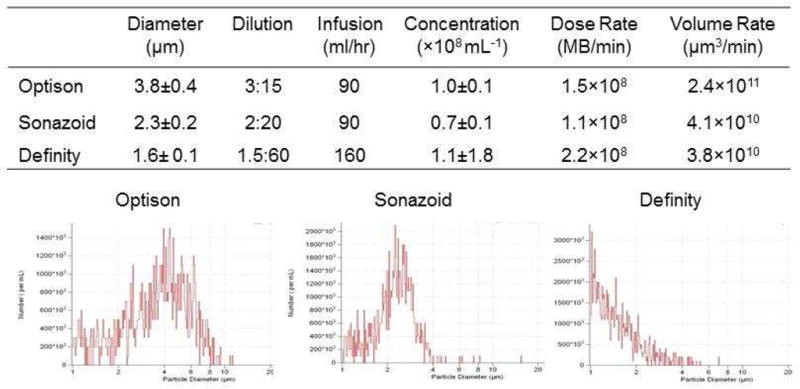
The use of Sonazoid was performed under an approved Investigational New Drug application from the United States Food and Drug Administration (IND 125975). Microbubble dilutions in 0.9% saline and intravenous infusion rates were varied for the three agents to produce similar dosing rate of microbubbles based on manufacturer information of microbubble concentration (see Figure 1). Manual agitation and rotation of the hand-held infusion pump was used to maintain a uniform microbubble suspension. Halfway through each imaging protocol, microbubble suspensions were sampled to measure actual microbubble concentration, diameter, and volume by electrozone sensing (Multisizer III, Beckman Coulter).
Figure 1.
Microbubble Characteristics and Dose Rates. The table contains data on microbubble (MB) mean size, dilution (v:v) in normal saline, infusion rate, and actual dosing rate based on a microbubble number and gas volume rate. Histograms of diameter distribution for each agent on elecrozone evaluation are illustrated at the bottom.
Contrast-enhanced Ultrasound Protocols
Transaxial imaging of the calf bilaterally one-third the distance from the popliteal fold to the ankle was performed using a phased-array transducer coupled to an ultrasound system (IE-33, Philips Ultrasound). A multi-pulse contrast-specific imaging algorithm using amplitude-modulation was performed at 1.8 MHz. Images were acquired at end-diastole by gating to the ECG. Gain settings at each MI and acoustic focus were optimized prior to contrast administration to levels below that which produced tissue speckle, and were kept constant between agents.
Protocol 1
CEU imaging was performed at a MI of 0.1, 0.2, 0.3 and 0.4 using pulsing intervals (PI) that were progressively shortened from every 10 to 1 cardiac cycles. With this protocol, the acoustic lability of microbubbles was defined by the progressive decline in signal enhancement with shorter PIs, whereas the maximal signal enhancement was measured at a PI of 10 which provides sufficient time for complete ultrasound sector volume replenishment.1,9
Protocol 2
To evaluate the influence of microbubble destruction and signal-to-noise during real-time quantitative CEU perfusion imaging, destruction-replenishment kinetics were measured. Microbubbles within the volume of tissue in the ultrasound sector were destroyed with a high-power (MI >0.9) 5-frame pulse sequence, after which real-time replenishment kinetics were assessed at a MI of 0.1, 0.2, 0.3, or 0.4. Frames were acquired at end-diastole gated to the ECG in order to minimize signal from intramuscular arteries,10 and in order to not expose microbubbles unnecessarily to potentially destructive ultrasound energy created by imaging frames that are not used for analysis. Background-subtracted intensity from a region-of-interest placed over the soleus was measured using the immediate post-destruction frame as background and time-intensity data were fit to the function:
where y is intensity at time t, A is the plateau intensity representing relative microvascular blood volume, and the rate constant β represents the microvascular flux rate.11,12
Exercise Perfusion
Unilateral calf muscle perfusion imaging at a MI of 0.1 and 0.3 was repeated during exercise. Subjects performed exercise with a 60° plantar-flexion arc on a calibrated pedal ergometer at 25 Watts of power. Plantar flexion was performed every 5 s for 1 minute. CEU acquisition was initiated within 2 seconds of exercise termination.
Microbubble Cavitation Response
In vivo cavitation of Sonazoid during ultrasound exposure at MIs of 0.1 to 0.4 was assessed by passive cavitation detection (PCD).13 A spherically focused broadband (10 KHz to 20 MHz) hydrophone (Y-107, Sonic Concepts, Inc., WA) with a focal depth of 20 mm and a focal width of 0.4 mm was confocally positioned with the ultrasound transducer on the calf. Received signals were digitized (100 MHz) and saved in a 4-channel oscilloscope (Waverunner, Teledyne LeCroy, Chestnut Ridge, NY) using sequence mode. Data analysis was performed with the Matlab (MathWorks, Natick, MA) using data averaged for 10 acquisitions.
Statistical Analysis
Statistical analysis was performed with Prism (V.6.02, GraphPad). For multiple comparisons, a one-way ANOVA with post-hoc Student’s t-test (paired for comparisons between agents and acoustic powers) and Bonferroni correction were used for normally distributed data based on the D’Agostino-Pearson omnibus test. For non-normally distributed data, a Kruskal-Wallis test was used for assessing changes in A or β according to MI or agent. When applicable, post-hoc Wilcoxon rank sum test (paired data) or Mann-Whitney test (for non-paired data) were used. Tests for differences in CEU data according to MI and post-hoc tests for linear trends were performed by repeated-measures ANOVA. Linear regression analysis was used for assessment of test-retest variability. For all tests, a p value of <0.05 was considered statistically significant.
RESULTS
Patient Characteristics and Microbubble Infusions
The median age of the subjects studied was 36±11 (range 22–49) years and the majority of the subjects were female (Table 1). Baseline vital signs and oxygen saturation were normal in all subjects and did not change during plantar flexion exercise. Microbubble infusions were well tolerated in all subjects and there was only one report of mild dyspnea 3–4 hours after study completion in a single subject receiving Definity. Microbubble size, size distribution, dosing information, and measured concentration after dilution are presented in Figure 1. Mean microbubble size and size distribution varied between agents. Despite efforts to produce equipoise in dosing using manufacturer information, the microbubble administration rates based on actual measurements after dilution differed. The highest microbubble dose rate was for Definity and the lowest for Sonazoid. Because of differences in microbubble size distribution, the microbubble gas volume rate was also calculated and was similar for Definity and Sonazoid, but higher for Optison.
Table 1.
Subject Demographics and Characteristics
| Age, yrs | 36±11 |
| Male/Female | 5/9 |
| Body mass index, kg/m2 | 24±3 |
| Heart rate, min−1 | |
| Sonazoid | 73±15 |
| Optison | 69±16 |
| Definity | 72±9 |
| Systolic BP, mm Hg | |
| Sonazoid | 127±18 |
| Optison | 126±16 |
| Definity | 116±9 |
| Diastolic BP, mm Hg | |
| Sonazoid | 76±10 |
| Optison | 76±11 |
| Definity | 67±10 |
BP, blood pressure
In vivo Microbubble Signal Intensity and Lability
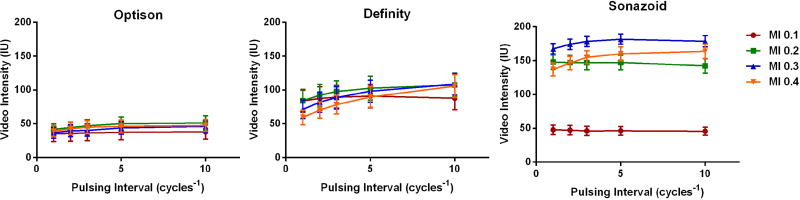
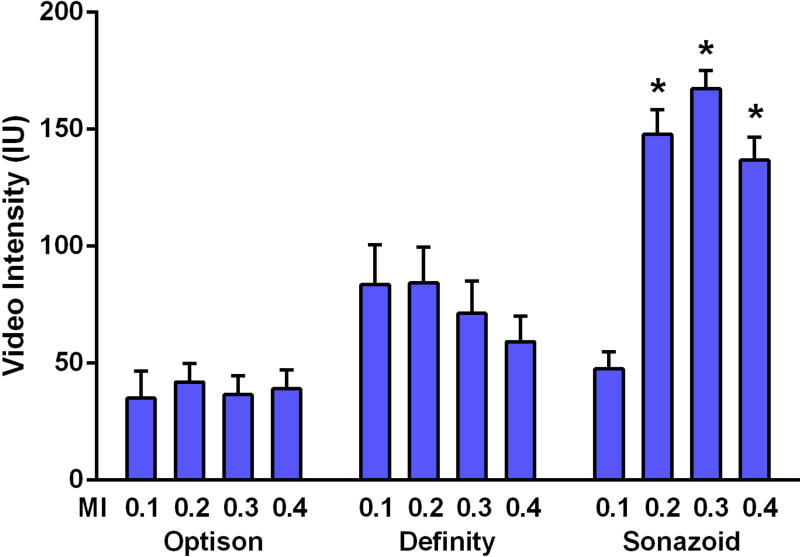
To evaluate the balance between CEU acoustic signal enhancement and microbubble destruction from inertial cavitation at each MI, the PI was gradually reduced from every 10 to 1 cardiac cycle (Figure 2). For Optison, there was no obvious change in signal intensity during shortening of the PI signal intensity at all MIs, although the signal was sufficiently low that destruction of microbubbles could not be excluded. Signal intensity was relatively greater for Definity, but a gradual reduction in intensity due to destruction occurred with PI shortening when imaging at MIs ≥0.2. For Sonazoid, signal enhancement was strong only at MIs ≥0.2, and only at MI of 0.4 was there evidence for a small amount of destruction. The mean signal enhancement at PI=1 which is the ideal ECG-gated interval for accurate mathematical fitting of post-destruction replenishment curves was highest for Sonazoid when imaged at intermediate MIs (Figure 3).
Figure 2.
Mean (±SEM) intensity from calf flexor muscles at rest while varying the mechanical index (MI) and pulsing interval (PI). The sequential reduction of intensity when shortening PI from every 10 to 1 cardiac cycles was used as an indicator for microbubble destruction from inertial cavitation.
Figure 3.
Mean (±SEM) video intensity values obtained at the ideal pulsing interval (every 1 cardiac cycle at diastole) for fitting post-destructive CEU perfusion imaging curves. *p<0.05 versus corresponding MI for other agents, and for Sonazoid at MI=0.1.
Rest and Stress Limb Perfusion
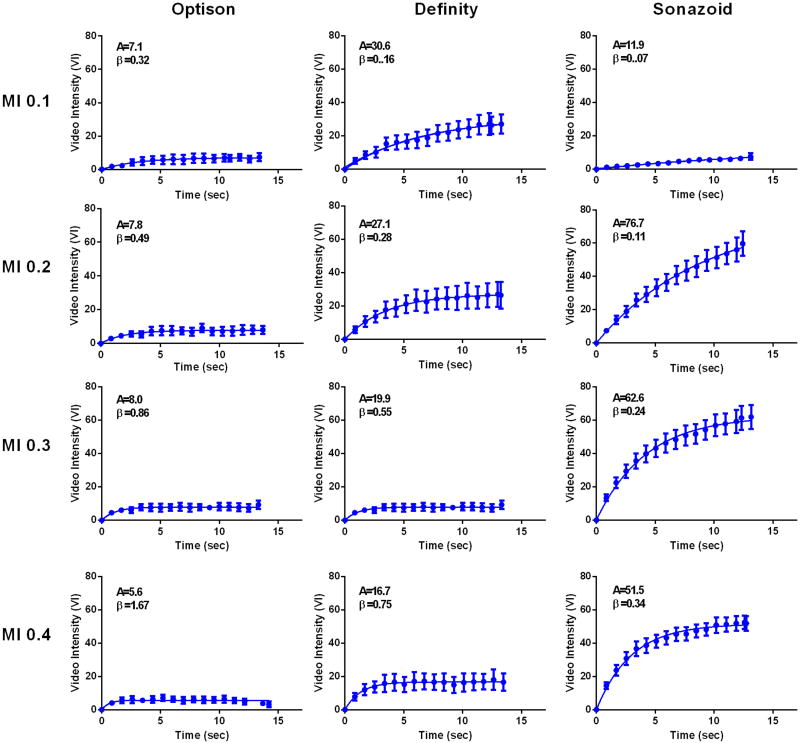
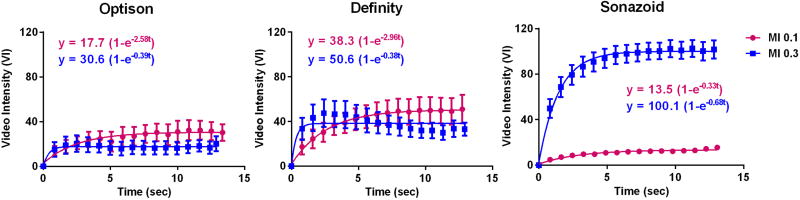
During CEU perfusion imaging at rest, the raw video intensity value for the first frame immediately after the destructive pulse sequence, which is used for background intensity, was similar for Optison, Definity, and Sonazoid; indicating equivalent clearance of contrast with each destructive pulse sequence (Table in On-line Supplement). Resting limb skeletal muscle CEU data for each agent at MI of 0.1 to 0.4 are presented in Figure 4 and 5. For Optison signal enhancement was low at all MIs, making comparisons between curves difficult. There were no statistically significant trends for changes in A-value or β with incremental increase in MI. For Definity, replenishment kinetics could easily be measured at all MIs. A progressive increase in the MI resulted in characteristic findings of microbubble destruction, manifest by reduction in the plateau intensity or A-value (ANOVA p<0.001, test for trend p=<0.001 for progressive reduction in A-value), and an increase in the calculated β-value (ANOVA p<0.001, test for trend p=<0.001 for progressive increase in β), the latter of which reflects in a shortening of the time to plateau intensity and has previously been shown to be an artifact of microbubble destruction during imaging.14 For Sonazoid, robust replenishment data was seen at an MI of 0.2 and higher, but with subtle evidence for destruction (reduction in plateau and increase in β) only at an MI of 0.4. There were statistically significant MI-related differences in the plateau intensities (A-values) for Sonazoid (ANOVA p<0.001), but this was attributable to lower A-value at an MI of 0.1 compared to other powers with no significant trend for decrease in A-value with MI.
Figure 4.
Mean (±SEM) video intensity values during CEU perfusion imaging with post-destructive replenishment curves from the resting calf muscle for each agent when varying the mechanical index (MI). The A- and β-values from these curves representing averaged data from all patients are provided for each graph.
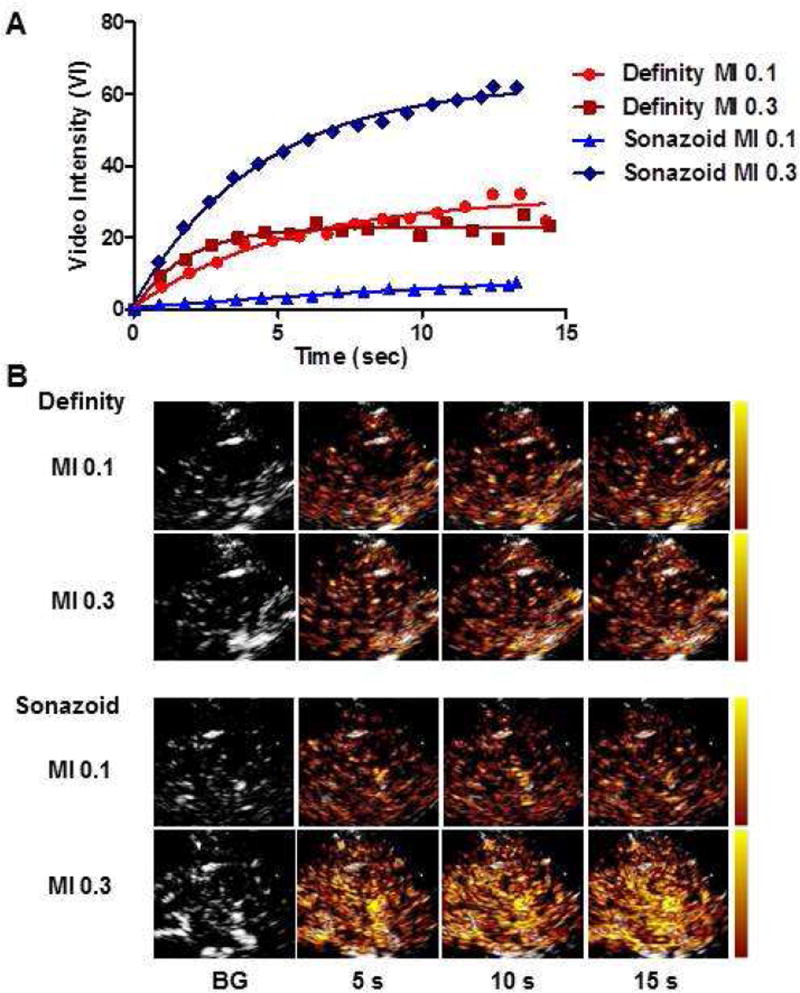
Figure 5.
Illustration of CEU Perfusion Imaging. (A) Time-intensity data and (B) corresponding background-subtracted CEU images (color-coding scale at left) at various time intervals after high-power destruction data from a single subject undergoing limb CEU at rest with Definity and Sonazoid. BG, background.
Limb skeletal muscle perfusion was assessed during contractile exercise at MIs of 0.1 and 0.3 (Figure 6). The increase in functional microvascular blood volume with exercise resulted in an ability to measure tissue perfusion with Optison but apparent agent destruction at MI 0.3 evidenced by curve distortion (lower plateau intensity and faster β-value than MI 0.1). Signal intensity for Definity at an MI of 0.1 was greater than for Optison but, again, there was evidence for curve distortion from destruction at MI 0.3. For Sonazoid little signal was seen at MI 0.1 whereas signal was much stronger at an MI 0.3, exceeding that seen for the other agents. Using rest and exercise stress data, Sonazoid imaged at an MI of 0.3 revealed an average flow reserve (Aβ stress:rest) of 8.0±5.7 and β reserve of 3.4±2.4; while Definity imaged at an MI of 0.1 (which produced non-distorted curves) revealed an average flow reserve of 5.9±5.4 and β reserve of 4.0±2.7.
Figure 6.
Mean (±SEM) video intensity values during CEU perfusion imaging with post-destructive replenishment curves from the calf muscle immediately after 60 s of contractile exercise for each agent at mechanical index (MI) of 0.1 or 0.3. Plateau intensity was greater (p<0.05) for Definity than Sonazoid at MI 0.1, and greater for Sonazoid than either Definity or Optison at MI 0.3 (corrected for multiple comparisons).
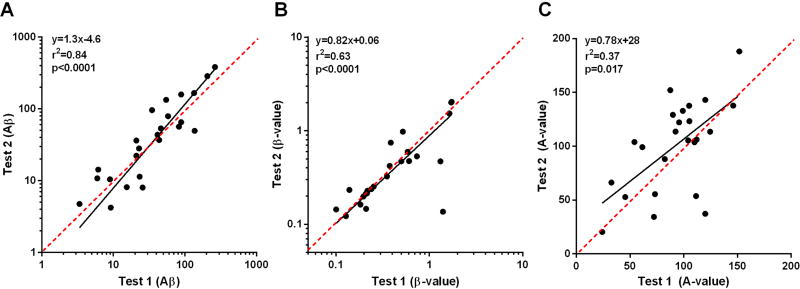
Twelve patients returned for test-retest variability of rest and stress CEU with Sonazoid at a MI of 0.3 (Figure 7). Despite known day to day variability in limb muscle perfusion,15 the degree of variability for both Aβ and β were low, whereas substantial variability was seen for the A-value.
Figure 7.
Correlations between CEU data on (A) Aβ, (B) β, and (C) A-value in the twelve subjects returning returning for test-retest variability for rest and stress perfusion with Sonazoid (MI 0.3). Red dashed lines represent lines of identity.
In Vivo Cavitation Detection
Passive cavitation detection was performed during limb imaging to characterize the acoustic responses for Sonazoid according to MI (Supplemental Figure). Frequency-amplitude spectra revealed increasing harmonic signal generation from stable cavitation with each incremental increase in MI and the appearance of only a small amount of broad-band signal from inertial cavitation at MI 0.3–0.4.
DISCUSSION
Currently, the lack of a widely-available method for limb muscle perfusion imaging is a major gap in the evaluation and management of patients with PAD. In this study we addressed a recognized limitation to applying real-time CEU to evaluate limb perfusion in PAD. When using conventional doses of microbubble contrast agents, real-time CEU signal intensity is quite low in human resting skeletal muscle due to very low blood flow (0.05 to 0.2 ml/min/g).4,5 In animal models, it has been possible to use CEU to assess limb muscle perfusion because of higher resting muscle blood flow and the ability to use large microbubble doses.16,17 To overcome low signal intensity in humans, high-MI intermittent-imaging schemes have been used to produce adequate time-intensity curves.1,18 These protocols are challenging to perform and image acquisition requires several minutes, thereby rendering perfusion imaging during exercise very difficult.
CEU perfusion imaging can also be performed with real-time low-MI imaging and has the advantage of being easier to perform and requires only seconds for image acquisition, thereby making exercise stress imaging possible. Signal generation during real-time imaging relies on the generation of strong signals produced by non-linear behavior of microbubbles during stable cavitation.6 The signal intensity during stable cavitation generally increases, albeit non-linearly,7 with the transmitted acoustic pressure which is usually represented on clinical scanners as the MI. There is a limit to how high the acoustic pressure can be increased since stable cavitation will convert to inertial cavitation and microbubble destruction which occurs from exaggerated oscillation and gas volume loss.19 When entering the inertial cavitation range, real-time imaging becomes problematic since microbubble destruction from each imaging frame produces strong signal but at the same time destroys a population of microbubbles resulting in reduced acoustic signal.9,14 The impact of this destruction can be minimized by reducing the frame rate (i.e. increasing the duration between each frame). However, a frame rate of approximately 1 s−1 or faster is required in order to accurately fit post-destruction replenishment curves during quantitative imaging of the limb or heart.1,12
The current study evaluated a real-time imaging strategy that relied on imaging an acoustically-resilient microbubble agent, Sonazoid, with acoustic powers that are higher than conventionally used for real-time imaging (termed intermediate). Imaging at these intermediate powers is ordinarily considered futile due to the fragility of most clinically-used ultrasound contrast agents. Previous studies have revealed that Sonazoid has a higher bulk modulus than most other widely-used microbubbles, and is therefore more durable in a strong ultrasound field.9,20 Accordingly, PCD has shown that Sonazoid compared to Definity requires higher acoustic pressures to produce non-linear signals, but also is more resistant to inertial cavitation (destruction) as the pressure is increased.9 This characteristic is likely attributable to differences not only in the density of the gas cores (C4F10 for Sonazoid; C3F8 for Optison and Definity), but probably more importantly differences in shell composition which is a major factor that determines microbubble viscoelastic properties. We have not definitively identified the lipid characteristic (e.g. hydrocarbon length or hydrogenation status) that explains Sonazoid’s resiliency. However, high-performance liquid chromatography indicates that Sonazoid has the longest fatty acid hydrocarbon length of any agent with 70% being stearic acid (C18:0) or longer.21
Because of the compositional differences in the contrast agents, in this study gradual reduction of the PI during calf CEU imaging revealed that Sonazoid is more resistant than other agents to acoustic destruction. This observation was further supported by in vivo PCD which showed little inertial cavitation with increasing the MI. These data serve to explain why real-time myocardial perfusion imaging with Sonazoid can be achieved with much higher mechanical index imaging than used conventionally.22
Various derivations of the Rayleigh-Plesset equation have been used to mathematically explain the acoustic behavior of bubbles. These models indicate that viscous damping, shell elasticity, shell friction, surface geometry and surface tension affect not only bubble stability but also the extent of stable cavitation.7,23,24 In our study, despite being more resilient to destruction, Sonazoid provided stronger signal at MIs even when destruction was rendered irrelevant by imaging at PI=10. This finding was not in any way due to dose differences. These data can be interpreted to indicate resiliency yet flexibility of this agent, perhaps from strain-softening during oscillation which is a likely explanation for the threshold phenomenon for generating a meaningful signal for this agent.25 Accordingly, when performing image acquisition once per cardiac cycle at end-diastole (which is the minimum acquisition frequency needed to generate accurate post-destruction replenishment curves with CEU and also avoids destruction from unnecessary and unused frames), the highest signal by far was from Sonazoid at intermediate MIs. When performing real-time CEU perfusion imaging at rest or during exercise stress, signal intensity for Optison and Definity did not increase with sequential increase of MI due to the offsetting effects of microbubble destruction which results in characteristic reduction in plateau intensity and a paradoxical increase in β.14
The ability to detect high signal-to-noise with intermediate MI imaging and Sonazoid has several important clinical implications aside from simply providing more robust and reproducible imaging data. Experience using CEU or magnetic resonance perfusion imaging for PAD imaging have noted tremendous variability in flow reserve measurements among relatively homogenous control or disease groups.1,26 This finding is explained by the fact that when the denominator (resting flow) is extremely small compared to hyperemic flow, flow reserve calculations can be dominated by extremely small absolute differences in resting flow. Hence, the degree of hyperemic flow has been a more reliable indicator of the presence of disease. In this study, the ability to obtain robust perfusion data at rest led to improved variability in flow reserve measurement compared to historic data, and implies that resting flow abnormalities in critical limb ischemia could potentially be detected.
There are several limitations of this study that should be highlighted. We did not test a variety of different contrast-specific imaging algorithms, ultrasound systems, or frequencies. However, experiments in pre-clinical animal models have demonstrated that intermediate-power CEU with Sonazoid produced more robust limb perfusion imaging data than Definity using several different imaging frequencies, ultrasound systems, and contrast-specific detection schemes.9 We cannot say that the higher intensity for Sonazoid at PI=10 and intermediate MIs is only from more efficient non-linear oscillation. Phased-array beams produce overlap in scan-line paths during the formation of a single image frame so that a higher intensity within a large region-of-interest may still reflect less microbubble destruction. We did not study Lumason/Sonovue, a lipid-shelled SF6 microbubble commercial contrast agent since, based on our experience, the combination of higher infusion rates needed for limb perfusion imaging (about 50% higher than for myocardial imaging) and extensive amount of data collection would have required multiple vials for this agent. We also did not present data on correlations between Definity and Sonazoid for measuring hyperemic flow or flow reserve due to the low number of comparisons available and the fact that studying only normal controls does not provide a wide spread of values. Because stress CEU was performed on the same day as the rest protocols, our selection of MIs to be used during stress were per protocol (selected “a priori”) rather than optimized for each patient. Finally, it is worth noting that the CEU imaging approach will require validation against a gold standard for quantifying muscle perfusion, such as positron emission tomography imaging at rest and during stress.
In summary, our data indicate that real-time limb CEU imaging with Sonazoid and intermediate MIs is an ideal strategy for assessing limb perfusion in humans at rest and during modest exercise stress. The simplicity and rapid image acquisition for this approach makes it feasible to assess bilateral limb perfusion in a matter of minutes as a widely-available approach for assessing patients with suspected PAD or for assessing the therapeutic impact of established or new treatments.
Supplementary Material
Acknowledgments
Dr. Lindner is supported by grants R01-HL078610, R01-HL111969, and R01-HL120046 from the NIH; grant 14NSBRI1-0025 from the National Space Biomedical Research Institute (NASA). Dr. Moccetti is supported by a grant from the Swiss National Science Foundation. The study was funded in part by a grant from GE Healthcare, Amersham, United Kingdom.
ABBREVIATIONS
- CEU
contrast-enhanced ultrasound
- MI
mechanical index
- PAD
peripheral artery disease
- PI
pulsing interval
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lindner JR, Womack L, Barrett EJ, Weltman J, Price W, Harthun NL, et al. Limb stress-rest perfusion imaging with contrast ultrasound for the assessment of peripheral arterial disease severity. JACC Cardiovasc Imaging. 2008;1:343–50. doi: 10.1016/j.jcmg.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duerschmied D, Olson L, Olschewski M, Rossknecht A, Freund G, Bode C, et al. Contrast ultrasound perfusion imaging of lower extremities in peripheral arterial disease: A novel diagnostic method. Eur Heart J. 2006;27:310–5. doi: 10.1093/eurheartj/ehi636. [DOI] [PubMed] [Google Scholar]
- 3.Duerschmied D, Zhou Q, Rink E, Harder D, Freund G, Olschewski M, et al. Simplified contrast ultrasound accurately reveals muscle perfusion deficits and reflects collateralization in pad. Atherosclerosis. 2009;202:505–12. doi: 10.1016/j.atherosclerosis.2008.05.046. [DOI] [PubMed] [Google Scholar]
- 4.Bonadonna RC, Saccomani MP, Del Prato S, Bonora E, DeFronzo RA, Cobelli C. Role of tissue-specific blood flow and tissue recruitment in insulin-mediated glucose uptake of human skeletal muscle. Circulation. 1998;98:234–41. doi: 10.1161/01.cir.98.3.234. [DOI] [PubMed] [Google Scholar]
- 5.Heinonen I, Kemppainen J, Kaskinoro K, Peltonen JE, Borra R, Lindroos MM, et al. Comparison of exogenous adenosine and voluntary exercise on human skeletal muscle perfusion and perfusion heterogeneity. J Appl Physiol (1985) 2010;108:378–86. doi: 10.1152/japplphysiol.00745.2009. [DOI] [PubMed] [Google Scholar]
- 6.Chin CT, Burns PN. Predicting the acoustic response of a microbubble population for contrast imaging in medical ultrasound. Ultrasound Med Biol. 2000;26:1293–300. doi: 10.1016/s0301-5629(00)00299-4. [DOI] [PubMed] [Google Scholar]
- 7.Sarkar K, Shi WT, Chatterjee D, Forsberg F. Characterization of ultrasound contrast microbubbles using in vitro experiments and viscous and viscoelastic interface models for encapsulation. The Journal of the Acoustical Society of America. 2005;118:539–50. doi: 10.1121/1.1923367. [DOI] [PubMed] [Google Scholar]
- 8.Borden MA, Kruse DE, Caskey CF, Zhao S, Dayton PA, Ferrara KW. Influence of lipid shell physicochemical properties on ultrasound-induced microbubble destruction. IEEE Trans Ultrason Ferroelectr Freq Control. 2005;52:1992–2002. doi: 10.1109/tuffc.2005.1561668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seol SH, Davidson BP, Belcik JT, Mott BH, Goodman RM, Ammi A, et al. Real-time contrast ultrasound muscle perfusion imaging with intermediate-power imaging coupled with acoustically durable microbubbles. J Am Soc Echocardiogr. 2015;28:718–26. e2. doi: 10.1016/j.echo.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davidson BP, Belcik JT, Mott BH, Landry G, Lindner JR. Quantification of residual limb skeletal muscle perfusion with contrast-enhanced ultrasound during application of a focal junctional tourniquet. J Vasc Surg. 2016;63:148–53. doi: 10.1016/j.jvs.2014.06.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dawson D, Vincent MA, Barrett EJ, Kaul S, Clark A, Leong-Poi H, et al. Vascular recruitment in skeletal muscle during exercise and hyperinsulinemia assessed by contrast ultrasound. Am J Physiol Endocrinol Metab. 2002;282:E714–20. doi: 10.1152/ajpendo.00373.2001. [DOI] [PubMed] [Google Scholar]
- 12.Wei K, Jayaweera AR, Firoozan S, Linka A, Skyba DM, Kaul S. Quantification of myocardial blood flow with ultrasound-induced destruction of microbubbles administered as a constant venous infusion. Circulation. 1998;97:473–83. doi: 10.1161/01.cir.97.5.473. [DOI] [PubMed] [Google Scholar]
- 13.Belcik JT, Mott BH, Xie A, Zhao Y, Kim S, Lindner NJ, et al. Augmentation of limb perfusion and reversal of tissue ischemia produced by ultrasound-mediated microbubble cavitation. Circ Cardiovasc Imaging. 2015;8:e002979. doi: 10.1161/CIRCIMAGING.114.002979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leong-Poi H, Swales J, Jayaweera AR, Bin JP, Kaul S, Lindner JR. Effect of microbubble exposure to ultrasound on quantitation of myocardial perfusion. Echocardiography. 2005;22:503–9. doi: 10.1111/j.1540-8175.2005.40001.x. [DOI] [PubMed] [Google Scholar]
- 15.Goh V, Halligan S, Hugill JA, Bartram CI. Quantitative assessment of tissue perfusion using mdct: Comparison of colorectal cancer and skeletal muscle measurement reproducibility. AJR Am J Roentgenol. 2006;187:164–9. doi: 10.2214/AJR.05.0050. [DOI] [PubMed] [Google Scholar]
- 16.Ryu JC, Davidson BP, Xie A, Qi Y, Zha D, Belcik JT, et al. Molecular imaging of the paracrine proangiogenic effects of progenitor cell therapy in limb ischemia. Circulation. 2013;127:710–9. doi: 10.1161/CIRCULATIONAHA.112.116103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bragadeesh T, Sari I, Pascotto M, Micari A, Kaul S, Lindner JR. Detection of peripheral vascular stenosis by assessing skeletal muscle flow reserve. J Am Coll Cardiol. 2005;45:780–5. doi: 10.1016/j.jacc.2004.11.045. [DOI] [PubMed] [Google Scholar]
- 18.Womack L, Peters D, Barrett EJ, Kaul S, Price W, Lindner JR. Abnormal skeletal muscle capillary recruitment during exercise in patients with type 2 diabetes mellitus and microvascular complications. J Am Coll Cardiol. 2009;53:2175–83. doi: 10.1016/j.jacc.2009.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chomas JE, Dayton P, Allen J, Morgan K, Ferrara KW. Mechanisms of contrast agent destruction. IEEE Trans Ultrason Ferroelectr Freq Control. 2001;48:232–48. doi: 10.1109/58.896136. [DOI] [PubMed] [Google Scholar]
- 20.Moran CM, Anderson T, Pye SD, Sboros V, McDicken WN. Quantification of microbubble destruction of three fluorocarbon-filled ultrasonic contrast agents. Ultrasound Med Biol. 2000;26:629–39. doi: 10.1016/s0301-5629(00)00148-4. [DOI] [PubMed] [Google Scholar]
- 21.Hvattum E, Uran S, Sandbaek AG, Karlsson AA, Skotland T. Quantification of phosphatidylserine, phosphatidic acid and free fatty acids in an ultrasound contrast agent by normal-phase high-performance liquid chromatography with evaporative light scattering detection. Journal of pharmaceutical and biomedical analysis. 2006;42:506–12. doi: 10.1016/j.jpba.2006.04.027. [DOI] [PubMed] [Google Scholar]
- 22.Jeetley P, Hickman M, Kamp O, Lang RM, Thomas JD, Vannan MA, et al. Myocardial contrast echocardiography for the detection of coronary artery stenosis: A prospective multicenter study in comparison with single-photon emission computed tomography. J Am Coll Cardiol. 2006;47:141–5. doi: 10.1016/j.jacc.2005.08.054. [DOI] [PubMed] [Google Scholar]
- 23.de Jong N, Hoff L, Skotland T, Bom N. Absorption and scatter of encapsulated gas filled microspheres: Theoretical considerations and some measurements. Ultrasonics. 1992;30:95–103. doi: 10.1016/0041-624x(92)90041-j. [DOI] [PubMed] [Google Scholar]
- 24.Marmottant P, van der Meer S, Emmer M, Versluis M, de jong N, Hilgenfeldt S, et al. A model for large amplitude oscillations of coated bubbles accounting for buckling and rupture. The Journal of the Acoustical Society of America. 2005;118:3499–505. [Google Scholar]
- 25.Emmer M, van Wamel A, Goertz DE, de Jong N. The onset of microbubble vibration. Ultrasound Med Biol. 2007;33:941–9. doi: 10.1016/j.ultrasmedbio.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Jiji RS, Pollak AW, Epstein FH, Antkowiak PF, Meyer CH, Weltman AL, et al. Reproducibility of rest and exercise stress contrast-enhanced calf perfusion magnetic resonance imaging in peripheral arterial disease. Journal of cardiovascular magnetic resonance: official journal of the Society for Cardiovascular Magnetic Resonance. 2013;15:14. doi: 10.1186/1532-429X-15-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.