Abstract
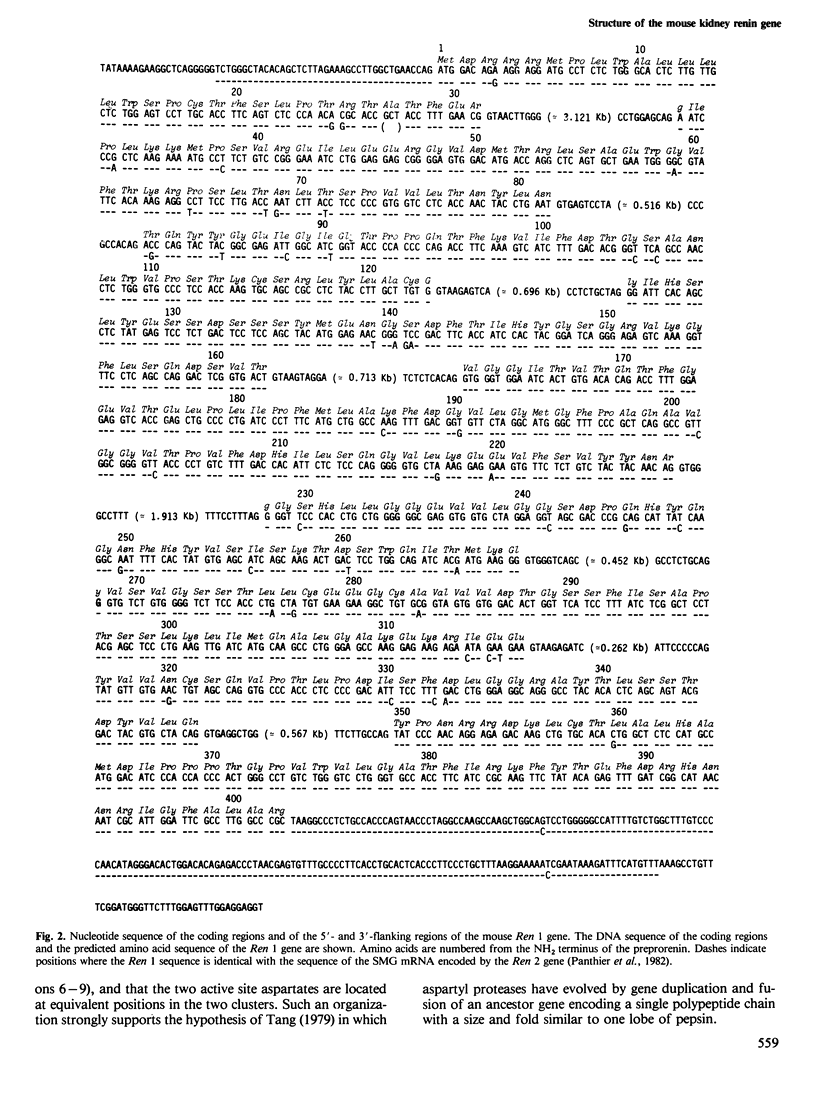
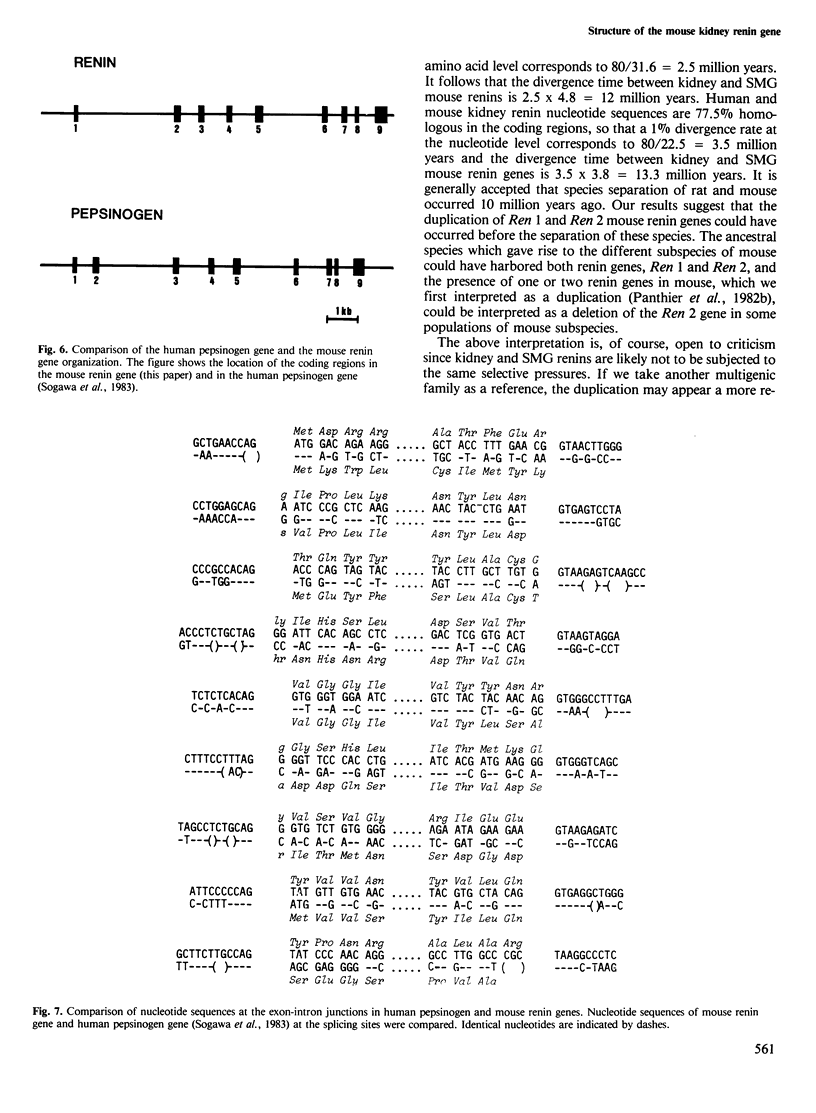
Overlapping recombinant clones that appear to encompass the entire renin gene, named Ren 1, have been isolated from a library of BALB/c mouse genomic DNA fragments. Based on restriction endonuclease mapping and DNA sequence analysis, Ren 1 spans 9.6 kb and contains nine exons interrupted by eight intervening sequences of highly variable size. The first exon, encoding the signal peptide of preprorenin, is separated from the eight following exons by a 3-kb intron. These eight exons are organized into two clusters of four separated by a 2-kb intron. DNA stretches encoding the aspartyl residues, which are part of the active site of renin, are located at homologous positions in both clusters. Our results show that aspartyl protease genes have arisen by duplication and fusion of an ancestral gene containing five exons. The estimated date of the duplication event of the mouse renin genes Ren 1 and Ren 2 is discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Blundell T., Sibanda B. L., Pearl L. Three-dimensional structure, specificity and catalytic mechanism of renin. Nature. 1983 Jul 21;304(5923):273–275. doi: 10.1038/304273a0. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Benoist C., O'Hare K., Gannon F., Chambon P. Ovalbumin gene: evidence for a leader sequence in mRNA and DNA sequences at the exon-intron boundaries. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4853–4857. doi: 10.1073/pnas.75.10.4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu I. N., Delbaere L. T., James M. N., Hofmann T. Penicillopepsin from Penicillium janthinellum crystal structure at 2.8 A and sequence homology with porcine pepsin. Nature. 1977 Mar 10;266(5598):140–145. doi: 10.1038/266140a0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Misono K. S., Chang J. J., Inagami T. Amino acid sequence of mouse submaxillary gland renin. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4858–4862. doi: 10.1073/pnas.79.16.4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins J. J., Burt D. W., Windass J. D., McTurk P., George H., Brammar W. J. Molecular cloning of two distinct renin genes from the DBA/2 mouse. EMBO J. 1982;1(11):1461–1466. doi: 10.1002/j.1460-2075.1982.tb01338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollo R., Auffray C., Sikorav J. L., Rougeon F. Mouse heavy chain variable regions: nucleotide sequence of a germ-line VH gene segment. Nucleic Acids Res. 1981 Aug 25;9(16):4099–4109. doi: 10.1093/nar/9.16.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollo R., Rougeon F. Gene conversion and polymorphism: generation of mouse immunoglobulin gamma 2a chain alleles by differential gene conversion by gamma 2b chain gene. Cell. 1983 Feb;32(2):515–523. doi: 10.1016/0092-8674(83)90471-3. [DOI] [PubMed] [Google Scholar]
- Panthier J. J., Foote S., Chambraud B., Strosberg A. D., Corvol P., Rougeon F. Complete amino acid sequence and maturation of the mouse submaxillary gland renin precursor. Nature. 1982 Jul 1;298(5869):90–92. doi: 10.1038/298090a0. [DOI] [PubMed] [Google Scholar]
- Panthier J. J., Holm I., Rougeon F. The mouse Rn locus: S allele of the renin regulator gene results from a single structural gene duplication. EMBO J. 1982;1(11):1417–1421. doi: 10.1002/j.1460-2075.1982.tb01332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panthier J. J., Rougeon F. Kidney and submaxillary gland renins are encoded by two non-allelic genes in Swiss mice. EMBO J. 1983;2(5):675–678. doi: 10.1002/j.1460-2075.1983.tb01483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccini N., Knopf J. L., Gross K. W. A DNA polymorphism, consistent with gene duplication, correlates with high renin levels in the mouse submaxillary gland. Cell. 1982 Aug;30(1):205–213. doi: 10.1016/0092-8674(82)90026-5. [DOI] [PubMed] [Google Scholar]
- Sepulveda P., Marciniszyn J., Jr, Liu D., Tang J. Primary structure of porcine pepsin. III. Amino acid sequence of a cyanogen bromide fragment, CB2A, and the complete structure of porcine pepsin. J Biol Chem. 1975 Jul 10;250(13):5082–5088. [PubMed] [Google Scholar]
- Sheppard H. W., Gutman G. A. Allelic forms of rat kappa chain genes: evidence for strong selection at the level of nucleotide sequence. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7064–7068. doi: 10.1073/pnas.78.11.7064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogawa K., Fujii-Kuriyama Y., Mizukami Y., Ichihara Y., Takahashi K. Primary structure of human pepsinogen gene. J Biol Chem. 1983 Apr 25;258(8):5306–5311. [PubMed] [Google Scholar]
- Soubrier F., Panthier J. J., Corvol P., Rougeon F. Molecular cloning and nucleotide sequence of a human renin cDNA fragment. Nucleic Acids Res. 1983 Oct 25;11(20):7181–7190. doi: 10.1093/nar/11.20.7181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J. Evolution in the structure and function of carboxyl proteases. Mol Cell Biochem. 1979 Jul 31;26(2):93–109. doi: 10.1007/BF00232887. [DOI] [PubMed] [Google Scholar]
- Tang J., James M. N., Hsu I. N., Jenkins J. A., Blundell T. L. Structural evidence for gene duplication in the evolution of the acid proteases. Nature. 1978 Feb 16;271(5646):618–621. doi: 10.1038/271618a0. [DOI] [PubMed] [Google Scholar]
- Wilson A. C., Carlson S. S., White T. J. Biochemical evolution. Annu Rev Biochem. 1977;46:573–639. doi: 10.1146/annurev.bi.46.070177.003041. [DOI] [PubMed] [Google Scholar]
- Wilson C. M., Erdös E. G., Dunn J. F., Wilson J. D. Genetic control of renin activity in the submaxillary gland of the mouse. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1185–1189. doi: 10.1073/pnas.74.3.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C. M., Taylor B. A. Genetic regulation of thermostability of mouse submaxillary gland renin. J Biol Chem. 1982 Jan 10;257(1):217–223. [PubMed] [Google Scholar]


