Abstract
Background and Aims
Since its market release, gabapentin has been presumed to have no abuse potential and subsequently has been prescribed widely off-label, despite increasing reports of gabapentin misuse. This review estimates and describes the prevalence and effects of, motivations behind, and risk factors for gabapentin misuse, abuse, and diversion.
Methods
Databases were searched for peer-reviewed articles demonstrating gabapentin misuse, characterized by taking a larger dosage than prescribed or taking gabapentin without a prescription, and diversion. All types of studies were considered; grey literature was excluded. Thirty-three articles met inclusion criteria, consisting of 23 case studies and 11 epidemiological reports. Published reports came from the USA, the UK, Germany, Finland, India, South Africa, and France, and two analyzed websites not specific to a particular country.
Results
Prevalence of gabapentin misuse in the general population was reported to be 1%, 40–65% among individuals with prescriptions, and between 15–22% within populations of people who abuse opioids. An array of subjective experiences reminiscent of opioids, benzodiazepines, and psychedelics were reported over a range of doses, including those within clinical recommendations. Gabapentin was primarily misused for recreational purposes, self-medication, or intentional self-harm and was misused alone or in combination with other substances, especially opioids, benzodiazepines, and/or alcohol. Individuals with histories of drug abuse were most often involved in its misuse.
Conclusions
Epidemiological and case report evidence suggests that the antiepileptic and analgesic medication gabapentin is being misused internationally at a rate of about 1%, with substance abuse populations at special risk for misuse/abuse.
Keywords: gabapentin, prescription drug misuse, systematic review, diversion, substance abuse
Introduction
Gabapentin is an analog of GABA (1); however, it does not bind to GABAA or GABAB receptors (or benzodiazepine, opioid or cannabinoid receptors), but it can increase GABA and can decrease glutamate concentrations (2, 3). Its mechanisms of antiepileptic and analgesic actions are unknown, although some have speculated, in the case of the latter, that gabapentin may reduce the release of pain-related peptides and may decrease opioid-induced hyperalgesia (4). However, a unique gabapentin binding protein has been identified (5, 6) as a subunit of the voltage-dependent calcium channel complex (7), suggesting a less specific mechanism of action through modulation of neurosignaling.
Gabapentin was approved in 1993 by the US Food and Drug Administration (FDA) initially only for treatment of epilepsy as an adjunct to anticonvulsant therapy, but in 2004 was also approved as an analgesic for post-herpetic neuralgia (8). The European Medicines Agency approved gabapentin in 2006 for epilepsy and certain types of neuropathic pain (9) and the UK National Institute for Clinical Excellence (NICE) recommends gabapentin as a first-line treatment for all neuropathic pain (10). Because its mechanism of action is unclear and it is assumed to have no abuse potential, gabapentin is widely used off-label to treat an array of disorders, including insomnia, various neuropathic pain conditions, drug and alcohol addiction, anxiety, bipolar disorder, borderline personality disorder, menopausal conditions, vertigo, pruritic disorders, and migraines. In fact, estimates of the off-label usage of gabapentin are reported to range from 83–95% of all gabapentin use (11, 12), which is estimated to account for over 90% of its sales (8). Due to illegal marketing (promoting off-label uses) of gabapentin, Pfizer was fined $420 million after it was acquired from Warner-Lambert (13).
Gabapentin is safely tolerated over a very broad range of doses from approximately 800–1800 mg/day (although package inserts suggest that patients may be treated with doses as high as 3600 mg/day). In clinical practice, dosing is typically titrated starting from lower doses (i.e., <400 mg/day) and moving rapidly upward. The European Medicines Agency (14) and the Physician Prescribing Information generally recommends dosing up to 1800 mg in adults. While substantially higher doses have been tested in clinical trials, no additional clinical benefit has been observed (15). However, other studies have examined gabapentin as acute doses in the higher dose range, and it was well tolerated. At least one imaging study has reported that gabapentin (1200 and 2400 mg) significantly (and rapidly) increased measurable concentrations of brain gamma-aminobutryric acid (GABA), one of its presumed mechanisms of action (3). Hart and colleagues (2004) examined gabapentin (600 and 1200 mg) for its potential to reduce the reinforcing effects of cocaine in the human laboratory (16). Their data reveal reductions in ratings of anxiety with both gabapentin doses (in the absence of cocaine) compared to placebo. Lile (2013) examined 600 and 1200 mg yielding significant differences from placebo on numerous outcomes, including liking, take again and good effects (17). Bisaga and Evans (2006) examined gabapentin in combination with alcohol at acute doses of 1000 and 2000 mg (18). In this dose range, gabapentin produced some direct effect on psychomotor function but was still safely tolerated in combination with alcohol.
Despite initial views that gabapentin had no abuse potential (19–23), there have been numerous published case reports of gabapentin abuse by substance abusers in the community and penal system (24–36). The purpose of this review is to describe the international scope of gabapentin abuse (i.e., prevalence, risk factors, motivations behind misuse, how it is misused, illicit value, effects experienced) and to identify implications for practice and future research.
Methods
Definitions
The definitions presented here were used to guide article selection and are used throughout the present article to facilitate discussion. Gabapentin refers to the capsules, tablets, and oral solutions of which gabapentin (1-(aminomethyl)cyclohexaneacetic acid) is the active ingredient. This definition includes the prodrug of gabapentin, gabapentin enacarbil. When discussing case reports, the dose and formulation of gabapentin will be specified, when that information is available. Misuse is defined as the use of a drug in a manner or for a purpose other than indicated, including, but not limited to, taking another person’s medication, unprescribed or non-recommended route of administration, or a higher dosage than prescribed (37); thus, missing prescribed doses or dose reduction is not included. Abuse consists of persistent use of a drug despite negative consequences (37). Dependence refers to the physical and psychological elements associated with abuse, which include compulsion, withdrawal, and tolerance (37). Diversion is defined as the unauthorized selling or sharing of prescription medications, which can be either intentional (e.g., selling personal medication to someone without a prescription for that particular drug) or unintentional (e.g., theft). Diversion can occur at any point along the drug manufacturing and delivery process, however, at the core of this definition is unlawful movement of licit and regulated pharmaceuticals to the illicit marketplace (38, 39).
Search strategy and article selection
This review sought to identify peer-reviewed, published manuscripts describing cases of gabapentin misuse and/or abuse in accordance with PRISMA guidelines. The databases PubMed, Web of Science (all databases), CINAHL, PsycINFO, and Cochrane were searched utilizing terms and strategies specific to each database (Appendix 1) developed in collaboration with a qualified librarian and peer-reviewed by two additional medical librarians. All searches were conducted between May and August 2015. Only those articles written in English that described occurrences of gabapentin misuse/abuse among human populations were included. Studies describing only gabapentin toxicity, withdrawal, or dependence without misuse/abuse were excluded, as were articles describing only pregabalin misuse/abuse. Grey literature, as defined by the Institute of Medicine (40), was excluded; a well-constructed preliminary examination in Google Scholar provided over 21,000 results, exclusion of which highlighted a vast body of evidence of gabapentin misuse. Snowball sampling (i.e., reviewing references of included papers) was then used to identify any additional articles that may have been excluded after applying index-based filters.
Data extraction was performed by the first author; all of the selected articles were reviewed by the second and third authors to assess whether they met inclusion criteria. Any disagreements regarding inclusion were discussed among all authors until agreement was reached.
Results
The initial search yielded 1,128 unique citations, of which 1,067 were excluded based on title or abstract (Figure 1). Sixty-one articles were read in their entirety to assess whether they met inclusion criteria. Twenty-eight were excluded because they did not actually describe gabapentin misuse, abuse, or diversion. The remaining 31 articles met all inclusion criteria. Snowball sampling identified 351 unique publications; 346 were excluded based on title or abstract, 2 met criteria and were included in the review. In total, this systematic review analyzed 33 articles. There were 47 case studies of gabapentin misuse/abuse found in 23 published articles from 1993 to 2015 and 11 epidemiological reports published over the same time frame (one article described both types (41)). Notably, one review article was included in this paper not due to the content of the review, but rather a statement in the introduction, which mentioned a personal communication of large-scale gabapentin abuse occurring within a drug using population in Pittsburgh, Pennsylvania (26).
Figure 1.
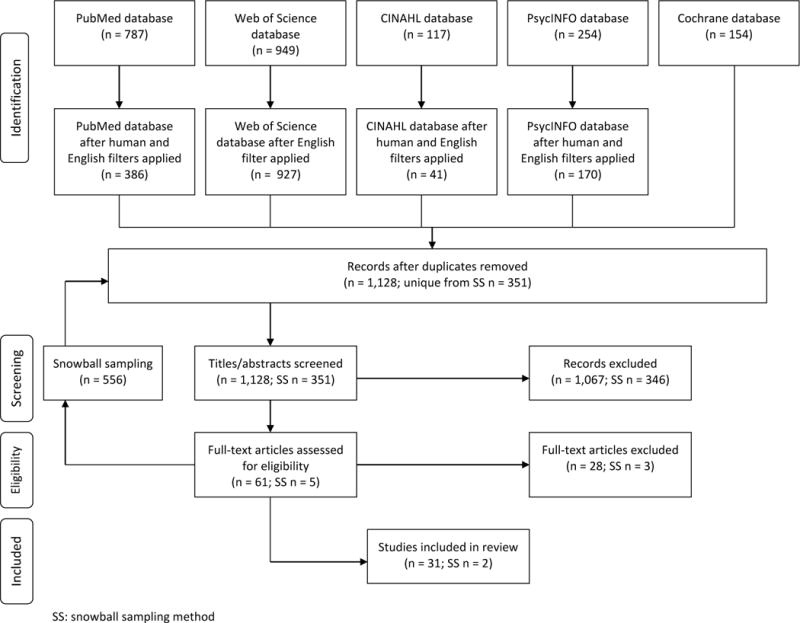
Flow diagram of systematic article selection
The present review attempted to summarize rigorously conducted and well-presented findings on gabapentin misuse/abuse. As such, the quality of case reports could not be evaluated; therefore, this presentation focused on epidemiological and toxicological studies using case studies as secondary data. It would be detrimental to have excluded case reports, as they provide rich context from which the population data may arise. Therefore, unless clearly noted in the manuscript text that the article was a case report, the reader could assume that the study was sample-based.
Study base and data sources
The 11 epidemiological studies (all cross-sectional) selected for this analysis obtained data from unique sources (Table 1); four publications involved substance misuse/abuse populations (42–45), two examined toxicology records (41, 46), one used a population-based sample (47), two involved reports to a poison center (48, 49), and two analyzed websites with qualitative information regarding gabapentin abuse (50, 51).
Table 1.
Summary of gabapentin misuse in reviewed articles
| Study, year, and reference | Country | Type of study | Sample size and characteristics | Prevalence of gabapentin misuse/abuse | Dose | Cost, Source, Diversion | Other substances in simultaneous combination | Motives | Effects experienced | Route of administration |
|---|---|---|---|---|---|---|---|---|---|---|
| Baird 2013 (42) | Scotland | Paper survey | N=129 from six substance misuse clinics | 19% | Not mentioned | Not mentioned | Methadone; possibly benzodiazepines | To become intoxicated, to potentiate the effects of methadone | Feeling ‘high’ or ‘stoned’ | Not mentioned |
| Hakkinen 2014 (46) | Finland | Analysis of toxicological autopsies | N=22,421 medico-legal autopsies with toxicology samples; 8 cases of gabapentin abuse; 75.0% of gabapentin abuse cases were male; median age of gabapentin abuse cases (range): 30 (24–47) | 0.31% involved in postmortem cases; 18% of those were related to drug abuse | For abuse cases, median concentration in postmortem femoral blood: 12 mg/L (range=0.62–45) | Not mentioned | Alcohol (37.5% of gabapentin abuse cases); opioids (87.5% of gabapentin abuse cases) | Not mentioned | Not mentioned | Not mentioned |
| Kapil 2013 (47) | UK | Online survey | N=1500 market research panel members; 49.1% male; 9.1% age 16–20, 40.5% age 21–39, 21.1% age 40–49, 29.3% age 50–59 yo | 1.1% lifetime prevalence | Not mentioned | 57.8% received from family or acquaintances; 47.3% from the Internet; 7.8% abroad | Not mentioned | Not mentioned | Not mentioned | Not mentioned |
| Klein-Schwartz 2003 (49) | USA | Analysis of poison control cases | N=20 gabapentin exposures reported to three poison control centers; 60% female; mean age for asymptomatic cases (± SD): 21.8 ± 29.0; mean age for symptomatic cases (± SD): 23.0 ±13.9 | 20 of 77 gabapentin-involved cases were gabapentin-only | Mean dose (± SD) for asymptomatic cases: 1906 mg ±2238; mean dose for symptomatic cases: 6320 mg ±10926 | 65% involved the patient’s own medication | 52 of 77 cases involved co-ingestants, but did not specify what they were and were excluded from analysis | 55% was intentional suicide attempt; 5 cases of therapeutic error; 4 unintentional (general) cases | Drowsiness (x8), ataxia (x2), tachycardia (x2), dizziness (x3), hypotension (x2), nystagmus (x1), nausea/vomiting (x2), diarrhea (x1), syncope (x1), bradycardia (x1), none (x5) | Not mentioned |
| Peterson 2009* (41) | USA | Analysis of blood samples | N=23,479 driving impairment cases in Washington state from 2003–2007; 50% male; mean age (± SD): 43.0 ±10.9 | 0.6% were positive for gabapentin | Mean concentration (±SD): 8.4 mg/L ±5.4; median: 7.0 | Not mentioned | Only 9 of the gabapentin cases were positive for gabapentin only. Of the remainder, 44% also contained benzodiazepines, 43% opioids, antidepressants 43%, other CNS depressants 36%, antiepileptic drugs 25%, 15% cannabinoids, 11% stimulants, and 6% ethanol. | Not mentioned | Not mentioned | Not mentioned |
| Schifano 2011 (50) | Online review | Qualitative analysis of websites | N=108 websites in English, German, Spanish, Italian, Dutch, Norwegian, Finnish, and Swedish | Not mentioned | Varying doses mentioned in subjective reports ranging from 900 to 4800 mg | Mentioned online pharmacies as a source, but likely not sole source | Baclofen, cannabis, alcohol, SSRIs, LSD, ampthetamine, GHB | Not clear, but likely recreational use | Reminiscent of “amphetamine rush,” “fully sedated opiate buzz,” “disassociation like DXM,” “talkative,” “comparable to cannabis,” “buzz slightly reminiscent of MDMA” | Oral and intramuscular |
| Seale 2014 (51) | Online review | Brief summary of website findings | Drug forums and pharmacist blogs | Not mentioned | Not mentioned | Not mentioned | Buprenorphine/naloxone | To get “high” | Not mentioned | Not mentioned |
| Smith 2012 (43) | Scotland | -Qualitative reports -Prescribing data -Clinical data -Postmortem examinations | -Qualitative reports arose from from clinical experiences and a police report, unreported sample size -Prescribing data: arose from Tayside region in Scotland from 1993–2011, unreported sample size -Clinical data arose from substance misuse services in Tayside, Scotland in 2009, n=251 of those who had used misuse services for 4+ years -Postmortem examinations came from Central, Tayside, and Fife Scotland in 2011, n=1400 | Of 251 individuals in substance misuse clinics, 5.2% receiving prescribed gabapentin; Of 1400 postmortem toxicology examinations, 48 included gabapentin, of which 36 also included methadone and/or morphine | The 5.2% receiving prescription gabapentin have a mean dose of 1343 mg | Can purchase on the street market for approximately 1 GBP per 300 mg; gabapentin is being used as a cutting agent in heroin according to a police report | Nonmedical use of prescription analgesics, morphine, methadone | Not clear, but likely recreational use | Euphoria, improved sociability, a marijuana-like ‘high’, relaxation, sense of calm, ‘zombie-like’ effects | Not mentioned |
| Smith 2015 (44) | USA | Questionnaire | N=503 nonmedical prescription opioid users from 2008 to 2014; 77.8% of gabapentin misuse cases were female | 15% in past 6 months | Not mentioned | Physicians (52%) and drug dealers (36%); costs less than 1 USD per pill | Unclear if simultaneous use, but were more likely than non-gabapentin users to report past 30-day use of: immediate-release oxycodone, buprenorphine, benzodiazepines | Recreational, “to get high” | Not mentioned | Not mentioned |
| Wilens 2015 (45) | USA | Survey | N=162 opioid dependent patients seeking detoxification; 51% male; mean age (±SD): 33 (±10) | 22% received prescription gabapentin; 40% of which reported using more than prescribed; 13% used unprescribed gabapentin; in total, 22% misused gabapentin (either more than prescribed or taking unprescribed) | Not mentioned | Not mentioned | Not mentioned | Not mentioned | Not mentioned | Not mentioned |
| Wills 2014 (48) | USA | Medical chart review | N=347 poison center reports; 69.5% female; median age (IQR): 30 (20–44) | 116 cases of gabapentin overdose | Median dose: 6000 (IQR: 2700–12000) | Not mentioned | Co-ingestion cases were excluded | Not mentioned | 10% neuromuscular symptoms, 2% seizures, 41% CNS symptoms, 6% GI symptoms, 11% cardiac symptoms, 16% blood pressure, 5% metabolic signs | Not mentioned |
| Case Reports | ||||||||||
| Study, year, and reference | Country | Gender and age | History of substance abuse | Dose | Cost | Source/Diversion | Other substances in simultaneous combination | Motives | Effects experienced | Route of administration |
| Barrueto 2002 (52) | USA | 34 yo male | No | 8000 mg/day | Not mentioned | Patient’s own medication | None | Manage pain | Withdrawal | Presumed oral, but not explicitly mentioned |
| Cantrell 2015 (24) | USA | 47 yo female | Yes (D) | Up to 15.6 g once | Not mentioned | Daughter’s medication | None | Not clear | Death | Oral |
| Fernandez 1996 (53) | USA | 32 yo male | Not mentioned | 91 g once | Not mentioned | Unclear if patient’s own medication or not | Valproic acid, alcohol | Suicide | Nystagmus, slurred speech, dizziness, drowsy | Oral |
| Fischer 1994 (25) | USA | 16 yo female | Yes (D) | 48.9 g once | Not mentioned | Father’s medication | None | Self harm | Dizziness, liquid stool, lethargy, dysphoria, emotional lability | Oral |
| Howland 2014 (26) | USA | Not mentioned | Yes (D) | Not mentioned | Not mentioned | Mentioned street market for selling gabapentin | Opiate agents | “get high” | Not mentioned | Not mentioned |
| Jones 2002 (58) | USA | 46 yo female | Not mentioned | 2 additional doses over prescribed once | Not mentioned | Patient’s own medication | None | Not mentioned | Somnolence, hypoxia, tremulous, and hyperreflexic | Presumed oral, but not explicitly mentioned |
| Koschny 2014 (59) | Germany | 21 yo female | Not mentioned | 16 g once | Not mentioned | Not mentioned | Carvedilol, amlodipine, amitriptyline, torsemide, nicotinic acid, ketoprofen | Suicide | Cardiac failure | Oral |
| Kruszewski 2009 (27) | USA | 38 yo male | Yes (A) | 4800+ mg/day | Not mentioned | Patient’s own medication | Not clear – possibly alcohol, buspirone, bupropion | Control moods and anxiety | Delirium, addiction | Presumed oral, but not explicitly mentioned |
| Markowitz 1997 (28) | USA | 41 yo female | Yes (D) | 600–1500 mg/day | Not mentioned | Husband’s medication | None | Substitute for crack cocaine | Reduced crack cocaine cravings, relaxation | Presumed oral, but not explicitly mentioned |
| Middleton 2011 (60) | USA | 62 yo female | No | Up to 45 g once | Not mentioned | Unclear, possibly patient’s own medication | Clonazepam | Suicide | Death | Not explicitly mentioned |
| Peterson** 2009 (41) | USA | 44 yo male | Not mentioned | < 2.0 mg/L | Not mentioned | Implies no medication because states the patient is “self-medicating”, but no indication of source | Quetiapine | Self medication for bipolar disorder | Lack of focus, lethargy | Presumed oral, but not explicitly mentioned. |
| Pittenger 2007 (29) | USA | 1. 33 yo male 2. 63 yo male |
1. Yes (A, D) 2.Yes (A) |
1. 3600 mg/day 2. 4900 mg/day |
Not mentioned for either case | Both patients used their own medication | 1. Not clear – possibly cannabis,
paroxetine, quetiapine 2. Not clear – possibly oxycodone |
1. Control mood and withdrawals 2. Not mentioned |
1. Felt calmer and reduced alcohol cravings,
withdrawal 2. Withdrawal |
Presumed oral for both cases, but not explicitly mentioned for either |
| Rasimas 2006 (54) | USA | 44 yo female | Not mentioned | 7 mg/L once | Not mentioned | Unclear, possibly patient’s own medication | Nefazodone, possibly alcohol | Intentional self-poisoning | Tachycardia, lethargy, depressed mental status | Oral |
| Reccoppa*** 2004 (30) | USA | 29–45 yo males | Yes (D) | 300–400 mg | Not mentioned | Some misused their own medication, others misused others’ prescriptions | Not clear – possibly tricyclic antidepressants, SSRIs, valproic acid, carbamazepine | “get high” | Altered mental state like snorting cocaine | Nasal insufflation |
| Reeves 2014 (32) | USA | 38 yo male | Yes (D) | 2400 mg once | Not mentioned | Not mentioned | Buprenorphine/naloxone | “get high” | Euphoria | Presumed oral, but not explicitly mentioned |
| Reeves 2014 (31) | USA | 1. 42 yo male 2. Female 3. Unknown 4. Unknown 5. Unknown |
1–2. Yes (D) 3–5. Not mentioned |
1. Up to 1500 mg each dose 2. Up to 1200 mg each dose 3–5. 900–1800 mg each dose |
Sold or traded for illicit drugs; specific price not mentioned | Sold or traded, or patients received their own prescription by exaggerating symptoms or false prescriptions | 1. Quetiapine 2. Quetiapine and alcohol 3–5. Quetiapine |
1–2. Substitute/replace
cocaine 3–5. Not mentioned |
1–2. Sedation and
euphoria 3–5. Not mentioned |
Presumed oral for all cases, but not explicitly mentioned |
| Roberge 2002 (33) | USA | 44 yo female | Yes (D) | “handful” once | Not mentioned | Patient’s own medication | Mexilitine, valproic acid, alcohol | “get high” | Slurred speech, somnolence, anisocoria, sluggishly reactive pupils, depressed gag reflex, obtundation | Oral |
| Rohman 2014 (34) | UK | 26 yo male | Yes (D) | 1600 mg once | Not mentioned | Friend | None | Recreational | Dystonia | Oral |
| Satish 2015 (35) | India | 26 yo male | Yes (D) | 400 mg – 2 g/day | Not mentioned | Initially a friend, unclear if patient eventually received own prescription | None | Recreational | Dependency, sense of well-being, increased energy, improved mood and sleep quality, increased attention span | Presumed oral, but not explicitly mentioned |
| Schauer 2013 (57) | USA | 59 yo female | No | 90 g once | Not mentioned | Patient’s own medication | Hydrocodone/acetaminophen | Suicide | Nausea and mild sedation | Oral |
| Spiller 2002 (55) | USA | 61 yo female | Not mentioned | Up to 54 g once | Not mentioned | Patient’s own medication | Quetiapine | Not clear - possibly suicide | Coma, respiratory depression | Oral |
| Stopforth 1997 (56) | South Africa | 17 yo female | Not mentioned | 12 g once | Not mentioned | Not clear, but likely own patient’s medication since she was epileptic | Lamotrigine | Not mentioned | Drowsy with slurred speech | Oral |
| Victorri-Vigneau 2007 (36) | France | 67 yo female | Yes (A) | 7200+ mg/day | Not mentioned | Patient’s own medication | Not clear – possibly naproxen, amitriptyline | Not mentioned | Withdrawal, dependency | Presumed oral, but not explicitly mentioned |
Article is a mixed methods analysis of qualitative and quantitative data. Therefore, this article appears in both the first and second sections of this table.
Article described 4 cases, only one of which may have been gabapentin misuse and is therefore the only incident included in summary.
Article combined information for 5 cases.
A = alcohol abuse; CNS = central nervous system; D = drug abuse; DXM = dextromethorphan; GBP = British pound; GHB = 4-hydroxybutanoic acid; GI = gastrointestinal; IQR = interquartile range; LSD = lysergic acid diethylamide; MDMA = 3,4-methylenedioxyamphetamine; SD = standard deviation; SSRIs = selective serotonin reuptake inhibitors; USD = United States dollar.
Over half of the case report articles (n=14) arose from patients presenting to a hospital or general clinic with overdose or withdrawal-like symptoms (24, 25, 29, 33, 34, 36, 52–59); two came from substance abuse clinics (26, 31), three from psychiatric facilities (27, 28, 35), two from the penal system (30, 32), one from postmortem toxicology findings (60), and one from poison center reports (49).
Demographic and geographical distribution
Five epidemiology/toxicology papers provided demographic characteristics of their sample. Two toxicology studies using poison center data indicated slightly higher representation of females (60–65%) (48, 49), while another study among opioid dependent patients found no significant difference in representation by gender (51% male, p=0.58)(45). One article noted that females were significantly more likely to misuse gabapentin than males in a cohort of opioid users (percent difference=17.3%, 95% confidence interval=10.4–24.6%) (44). A toxicology paper by Peterson (2009) observed no difference in gender in the likelihood of being a positive gabapentin driving impairment case (50% male)(41). Among case studies, males had slightly higher representation than females (15 males vs. 13 females), although gender was incompletely specified in two reports (31, 49). The mean age of samples ranged between 21 and 43 in studies in which it was reported (41, 45, 46, 48, 49). The calculated mean age of case reports was 41.
Published reports came from the United States (67%, n=22), the United Kingdom (12%, n=4), Germany (3%, n=1), Finland (3%, n=1), India (3%, n=1), South Africa (3%, n=1), France (3%, n=1), and two analyzed websites not specific to a particular country (6%). While all of the articles in this review described gabapentin misuse/abuse, 12 (36%) were documented reports of overdose involving gabapentin (24, 25, 33, 48, 49, 53–57, 59, 60).
Misuse and abuse of gabapentin
Prevalence
Only one article gave an estimate of lifetime prevalence of gabapentin abuse in the general population; Kapil and colleagues (2013) surveyed a UK population-based sample of 1500 and found that 1.1% reported ever misusing gabapentin (47).
Over half of the studies described gabapentin misuse that occurred among samples with a history of or current substance misuse/abuse/dependence (n=6), the majority of which discussed opioid misuse, specifically (n=5). Smith (2012) and Baird (2013) gave reports of gabapentin misuse within Scottish populations that attended substance misuse clinics, which likely included individuals who abuse alcohol and/or drugs (42, 43). Recent cross-sectional studies of opioid abuse samples in the US and UK estimated gabapentin misuse to be between 15–22% (42, 44, 45) and gabapentin abuse with a prescription ranged from 40–65% (44, 45, 47, 49). There was little evidence of gabapentin abuse among those with a positive history of alcohol abuse or dependence. In fact, Wilens and colleagues (2015) conducted a survey among opioid dependent individuals seeking substance detoxification in the US and found no gabapentin abuse among those undergoing alcohol detoxification (45). Conversely, for opioid dependent patients, 40% reported using more gabapentin than prescribed and 13% reported using unprescribed gabapentin (45).
In Scotland in 2010, approximately 1% of all drug-related deaths were directly attributed to gabapentin (42). Further, two articles assessed toxicological results in primarily substance misusing populations; the first examined 23,479 impaired driving cases in the US and found gabapentin was involved in 0.6% of them (41), while a Finnish study reviewed 13,766 medico-legal postmortem investigations and identified gabapentin in 0.3% of the cases (46).
Doses, Cost, and Diversion
Studies indicate gabapentin is misused/abused over a wide range of doses, from within therapeutic range (900–3600 mg/day) to supratherapeutic doses. All but two articles discussed the dosage involved in gabapentin misuse (42, 47). Evidence from the US suggested that gabapentin misuse among individuals with prescriptions for gabapentin involved a higher amount than prescribed (45, 46, 61). For example, as previously mentioned, a US study found that 22% of a sample of 162 opioid-dependent patients had a prescription for gabapentin, of which 40% indicated they used more than prescribed (45). Potential explanations for this trend are tolerance and addiction as described in two clinical case discussions from France and the US, respectively (27, 36). Interestingly, according to American and European case reports, those who used gabapentin, but did not have a prescription for it, often took doses that fell within clinical guidelines, regardless of motivations behind use, though the doses were not spread out over the course of a day and it was unclear how often an individual dosed per day (31, 34).
Over half of the articles (n=7) mentioned or referred to diversion of gabapentin. Studies in the UK and US identified health services/physicians as one of the major sources of misused gabapentin, with rates ranging from 52–63% (the 63% also may include baclofen and pregabalin) (44, 47). Other sources included family or acquaintances, Internet, bought abroad (47), and drug dealers (44).
Case reports support these findings from epidemiological studies. Reports from India, the UK and US also identify family members or acquaintances as gabapentin sources. Behaviors that are markers of abuse liability, such as doctor shopping, exaggeration of symptoms, and fabrication of prescriptions, were reported in case studies from France and the US (31, 36). Due to widespread gabapentin abuse in a US correctional facility, Reccoppa and colleagues (2004) inventoried dispensed medications and found only 19 of 96 prescriptions in the possession of the inmate receiving the prescription (30).
There is a street market demand for gabapentin. An American case study stated that, “{gabapentin} tablets were sometimes sold or traded for illicit drugs” (31). In Scotland, the Drug and Crime Enforcement Agency identified the growing use of gabapentin as a cutting agent in heroin (43). In the UK and US, epidemiological studies reported the illicit market value for gabapentin ranged from <1–7 USD per pill depending on strength (42–44).
Combination with other substances
Three toxicology studies elucidated the most commonly found substances with gabapentin. The first, by Häkkinen and colleagues (2014), examined Finnish postmortem toxicological samples positive for gabapentin from 2010–2011 and found that all cases classified as gabapentin abuse also involved the use of alcohol and/or opioids (most commonly buprenorphine and tramadol) (46). Peterson (2009) conducted a study in the US, also utilizing toxicological data, which examined the presence of gabapentin in driving impairment cases. Only 7% of gabapentin-positive blood samples detected solely gabapentin; the remainder were polysubstance cases, with benzodiazepines (44%), opioids (43%), antidepressants (43%), other CNS depressants (e.g., trazodone, zolpidem; 36%), antiepileptics (25%), cannabinoids (15%), stimulants (11%), and ethanol (6%) (41). Smith and colleagues (2012) stated that postmortem toxicology reports in Scotland revealed 75% of those identifying gabapentin also included morphine and/or methadone, which the authors said may be indicative of recent opioid dependence (43). The toxicology studies, while helpful for providing a picture of what classes of medicines were commonly found in combination with gabapentin, did not address unprescribed mixing of licit or illicit drugs.
Alternatively, several epidemiological studies did identify simultaneous combination of gabapentin with other substances for the explicit purpose of misusing them. One article discussed the misuse of gabapentin in combination with buprenorphine for the purpose of “getting high” (44). Similarly, Baird and colleagues (2014) stated that 38% of a substance misuse sample in Scotland took gabapentin (and/or pregabalin) in combination with prescribed methadone to potentiate the effects of methadone (42).
Studies in US and UK substance abuse populations, by Smith (2015) and Smith (2012) respectively, identified a greater likelihood for those misusing gabapentin to also be misusing prescription opioids (43, 44). Smith (2015) also found that individuals who reported using gabapentin to get “high” were also more likely to be misusing benzodiazepines (44), which supports the finding by Peterson (2009; discussed earlier) that benzodiazepines were the most commonly detected class of drugs in combination with gabapentin (41).
Use of gabapentin and ethanol were commonly reported together; in addition to the two toxicology studies discussed earlier (41, 46), another mentioned the misuse of gabapentin in combination with alcohol (50). An international review of recreational gabapentin misuse anecdotes described other substances that have been reported in conjunction with misused gabapentin including cannabis, SSRIs, LSD, amphetamine, and GHB (gamma-Hydroxybutyric acid) (50).
Case studies have corroborated the epidemiological findings and have also identified buprenorphine/naloxone and quetiapine as combinations of abuse with gabapentin (31, 32, 51).
Motives
A variety of motivations behind gabapentin misuse were identified, many that related to substance abuse behaviors in general, which included: recreational use (42–44, 50), control mood and/or anxiety (41), potentiate the effects of drug abuse treatment (42), and intentional self harm (49). Case reports substantiated those intentions (25, 27–35, 51, 53, 57, 59, 60), and also identified the following: pain (52), reduce cravings for/manage withdrawal from other drugs (28, 29, 35), substitute for other drugs (28, 31, 32), and addicted to gabapentin (27, 36).
Effects Experienced
Only three epidemiological studies mentioned the effects sought by misusing gabapentin (42, 43, 50); these findings were not presented as inference from a sample, rather examples accumulated from individual reporting. Six case reports also described feelings achieved from gabapentin misuse/abuse (28–32, 35). Therefore, the two types of articles were combined in this section to provide a comprehensive catalog of individual effects experienced and consequently should be interpreted with caution.
Several case studies mentioned experiencing euphoria after gabapentin misuse that was reminiscent of, but not as strong as, opioids (31, 32, 35). This feeling was achieved in combination with other drugs (e.g., buprenorphine/naloxone, methadone, baclofen, quetiapine, alcohol) (31, 32, 42, 50) as well as by using gabapentin alone (35, 43), in dosages ranging from 1500–12000 mg, though only three articles give actual amounts misused (31, 32, 35). One case study described individuals snorting gabapentin powder from capsules and experiencing a high similar to that felt after snorting cocaine (30). Another commonly reported sensation from gabapentin misuse was sedation/relaxation/calmness, which was described in six studies (28, 29, 31, 32, 43, 50). As with euphoria achieved from gabapentin misuse, sedation/relaxation/calmness was experienced in combination with other substances (e.g., quetiapine, alcohol, cannabis, buprenorphine/naloxone) (29, 31, 32) or by taking gabapentin alone (28, 50), and over a range of dosages (e.g., 600–4800 mg). Other effects experienced included: improved sociability (43, 50), marijuana-like “high” (43, 50), cocaine-like “high” (30), “amphetamine rush” (50), disassociation (50), MDMA-like “high” (50), increased energy and focus (35), improved quality of sleep (35), and becoming more talkative (50).
Discussion
Gabapentin has been presumed to have no abuse potential historically (19–23), however, this review reports evidence to the contrary. Of the 11 population-based studies and 23 case reports included here, nearly one-third report gabapentin misuse/abuse for recreational purposes and epidemiological studies from the US and UK estimate abuse rates between 40–65% just among individuals with a gabapentin prescription. Studies from the UK indicate that gabapentin has developed a prominent place as a drug of abuse; in Scottish prisons, gabapentin is among the top-requested prescription drugs of abuse (42). However, the rise in popularity of recreationally used gabapentin is occurring in the US, as well. Smith and colleagues (2015) describe a near 3000% increase in the use of gabapentin to get “high” from 2008 to 2014 among a cohort of 503 prescription drug users in the Central Appalachian region of the US (44).
Motivations for misused gabapentin can be classified largely into three basic categories: recreational (e.g., get high or substitute for more expensive drugs), self-harm, and self-medication (e.g., for pain or withdrawal symptoms from other substances). The majority of case reports involved individuals who had prescriptions for gabapentin, but took higher dosages than they were prescribed. Descriptive reports on gabapentin reveal an array of subjective experiences evocative of opioids (e.g., euphoria, talkativeness, increased energy, sedation), benzodiazepines (e.g., sedation), and psychedelics (e.g., dissociation). These effects do not appear to be specific to a particular dose and may occur well within the therapeutic range. No pattern was observed in terms of dose taken or interactions between dose and motive or dose and effects achieved, which may be partially explained by the unpredictable pharmacokinetics and non-linear bioavailability of gabapentin (62). To date, no carefully controlled human laboratory studies have been published that sought to examine and characterize the abuse potential profile of gabapentin in comparison to other prototypic drugs of abuse. Overall, further empirical research is clearly needed to better evaluate and characterize gabapentin psychopharmacology and the risks associated with gabapentin use, especially among those using it recreationally.
It is difficult to ascertain risk factors for gabapentin misuse/abuse except history of or current drug abuse, particularly opioids, is likely one from reports available to date. While no studies to date have formally assessed a history of or current substance abuse (especially drug abuse) as a risk factor for gabapentin misuse, it was the most common characteristic detected here. This is particularly important because it indicates that the increasing trend in gabapentin abuse, notably among populations with opioid misuse, has the potential to affect an estimated 0.6–0.8% of the world’s population aged 15–64 that has used opioids in the past year (63). It is important to note, however, that this review may overrepresent individuals who have abused substances, illustrating the importance of examining gabapentin misuse in the general population. Further, grey literature was excluded, which may have provided more information from which to infer risk factors for misuse, along with other characteristics of gabapentin misuse/abuse. Still, the present review emphasizes the paucity of peer-reviewed research on this important emerging topic, and provides key starting points for subsequent examination.
Gabapentin is relatively inexpensive and, in fact, many individuals can acquire it for free or a drastically reduced price under subsidy plans (64–66). Further, due to its widespread off-label prescribing worldwide (8, 11, 12), it is relatively easy to receive gabapentin by prescription, as illustrated by physicians and the health care system being the primary source of misused gabapentin in the US and UK. These factors have enabled the market to be flooded with gabapentin and it has been referred to among the drug using population as “a cheap man’s high” (personal communication). It is important that prescribers recognize the current diversion of gabapentin and dispense judiciously.
Gabapentin requires a prescription, but generally has no additional controls (66–69); however, pregabalin, its close structural relative, which was approved after gabapentin, was placed into Schedule V (abuse potential) in the US (70) and included in the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA)-Europol annual report on new psychoactive substances of abuse (71). It was found that pregabalin had euphoric and sedative properties similar to other frequently abused substances; moreover, as it is known that tolerance and physical dependence (with withdrawal symptoms upon discontinuation) may occur in response to repeated dosing, these factors may contribute to the escalation or continued misuse of gabapentin in those abusing the drug for its psychoactive effects (72). Our review, and other non-abuse reports falling outside the scope of this study (73–79), identified that gabapentin, too, produces these effects (i.e., tolerance, physical dependence, and withdrawal) thereby warranting reevaluation of its abuse potential. However, it is important to consider in reexamination that gabapentin may be an appropriate treatment for many individuals (e.g., those in alcohol withdrawal, chronic pain, epilepsy) that may face impediments to receiving their medication upon increased control. Therefore, a risk-benefit analysis is necessary prior to any abuse potential labeling.
From published reports presented here, gabapentin is most often misused in combination with other substances, especially opioids, benzodiazepines, and alcohol, although details in this area are sparse and necessitate systematic data collection and analysis. Concomitant use is particularly important because gabapentin is often co-prescribed with opioids, and pain patients often receive prescriptions for benzodiazepines due to anxiety and/or difficulty sleeping. Moreover, its uncontrolled status leads doctors to believe that it lacks abuse potential; thus, they may feel confident in their prescribing of gabapentin to patients with substance use histories. NHS England released advice for gabapentin prescribers that strongly recommends using it as approved, offering alternative interventions for conditions outside the licensing indications (69). Finally, benzodiazepines have been used to treat delirium resulting from gabapentin withdrawal (29) and gabapentin has been used to treat withdrawal from both benzodiazepines (80) and alcohol (19, 21). These findings suggest that these three agents may share a common neuropharmacological pathway for abuse and dependence; however, further research is necessary to explore this hypothesis.
In summary, findings from the present review suggest that gabapentin is misused/abused internationally for recreation, self-medication, or self-harm, with an array of subjective experiences. Substance abuse populations, especially individuals with a history of or current opioid misuse, appear to be at particular risk for misuse/abuse. Further studies to identify risk factors for gabapentin misuse and to characterize gabapentin’s abuse liability are recommended.
Supplementary Material
Acknowledgments
The authors would like to thank Robert Shapiro for his assistance in developing the search strategy for this review.
Funding:
This work is supported by a grant awarded to JRH by the National Institute on Drug Abuse (R01DA033862). JRH has received consulting fees from Pinney Associates and unrestricted research grant funding from Purdue Pharma. SLW has received honoraria and travel reimbursement for developing and delivering educational talks through an arms-length unrestricted educational grant from Reckitt Benckiser Pharmaceuticals to PCM Scientific, UK; SLW has also received honoraria from the same grant for organizing and serving as a conference chairperson. SLW has received past salary support from a research grant from Braeburn Pharmaceuticals. SLW has received consulting fees for advising pharmaceutical companies on product development and study design, including Braeburn, Camurus, Pfizer, Novartis, Sun Pharma, Astra Zeneca and World Meds, Inc.; none of this involves gabapentinoid compounds.
Footnotes
Competing interests:
RVS has no competing interests to declare.
References
- 1.Maneuf YP, Gonzalez MI, Sutton KS, Chung FZ, Pinnock RD, Lee K. Cellular and molecular action of the putative GABA-mimetic, gabapentin. Cellular and molecular life sciences: CMLS. 2003;60:742–750. doi: 10.1007/s00018-003-2108-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dougherty JA, Rhoney DH. Gabapentin: a unique anti-epileptic agent. Neurological research. 2001;23:821–829. doi: 10.1179/016164101101199414. [DOI] [PubMed] [Google Scholar]
- 3.Petroff OA, Hyder F, Rothman DL, Mattson RH. Effects of gabapentin on brain GABA, homocarnosine, and pyrrolidinone in epilepsy patients. Epilepsia. 2000;41:675–680. doi: 10.1111/j.1528-1157.2000.tb00227.x. [DOI] [PubMed] [Google Scholar]
- 4.Compton P, Kehoe P, Sinha K, Torrington MA, Ling W. Gabapentin improves cold-pressor pain responses in methadone-maintained patients. Drug and alcohol dependence. 2010;109:213–219. doi: 10.1016/j.drugalcdep.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suman-Chauhan N, Webdale L, Hill DR, Woodruff GN. Characterisation of [3H]gabapentin binding to a novel site in rat brain: homogenate binding studies. European journal of pharmacology. 1993;244:293–301. doi: 10.1016/0922-4106(93)90155-3. [DOI] [PubMed] [Google Scholar]
- 6.Thurlow RJ, Brown JP, Gee NS, Hill DR, Woodruff GN. [3H]gabapentin may label a system-L-like neutral amino acid carrier in brain. European journal of pharmacology. 1993;247:341–345. doi: 10.1016/0922-4106(93)90204-m. [DOI] [PubMed] [Google Scholar]
- 7.Gee NS, Brown JP, Dissanayake VU, Offord J, Thurlow R, Woodruff GN. The novel anticonvulsant drug, gabapentin (Neurontin), binds to the alpha2delta subunit of a calcium channel. The Journal of biological chemistry. 1996;271:5768–5776. doi: 10.1074/jbc.271.10.5768. [DOI] [PubMed] [Google Scholar]
- 8.Mack A. Examination of the evidence for off-label use of gabapentin. Journal of managed care pharmacy: JMCP. 2003;9:559–568. doi: 10.18553/jmcp.2003.9.6.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.European Medicines Agency. Summary information on referral opinion pursuant to Article 30 of Council Directive 2001/83/EC for Neurontin and associated names (see Annex I) International Non-Proprietary Name (INN) Gabapentin: Background information. 2006 [Google Scholar]
- 10.National Institute for Health and Clinical Excellence. Neuropathic pain in adults: pharmacological management in non-specialist settings. 2013 [PubMed] [Google Scholar]
- 11.Hamer AM, Haxby DG, McFarland BH, Ketchum K. Gabapentin use in a managed medicaid population. Journal of managed care pharmacy: JMCP. 2002;8:266–271. doi: 10.18553/jmcp.2002.8.4.266. [DOI] [PubMed] [Google Scholar]
- 12.Radley DC, Finkelstein SN, Stafford RS. Off-label prescribing among office-based physicians. Archives of internal medicine. 2006;166:1021–1026. doi: 10.1001/archinte.166.9.1021. [DOI] [PubMed] [Google Scholar]
- 13.Newman M. Bitter pills for drug companies. BMJ (Clinical research ed) 2010;341:c5095. doi: 10.1136/bmj.c5095. [DOI] [PubMed] [Google Scholar]
- 14.European Medicines Agency. Annex III: Summary of Product Characteristics, Labelling, and Package Leaflet [Google Scholar]
- 15.Pfizer. Neurontin US Physician Prescribing Information. 2014 [Google Scholar]
- 16.Hart CL, Ward AS, Collins ED, Haney M, Foltin RW. Gabapentin maintenance decreases smoked cocaine-related subjective effects, but not self-administration by humans. Drug and alcohol dependence. 2004;73:279–287. doi: 10.1016/j.drugalcdep.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 17.Lile JA, Kelly T, Hays L. Separate and combined effects of gabapentin and Δ 9-THC doses in cannabis users discriminating Δ 9-THC. College on Problems of Drug Dependence; San Diego, California: 2013. [Google Scholar]
- 18.Bisaga A, Evans SM. The acute effects of gabapentin in combination with alcohol in heavy drinkers. Drug and alcohol dependence. 2006;83:25–32. doi: 10.1016/j.drugalcdep.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 19.Bonnet U, Banger M, Leweke FM, Maschke M, Kowalski T, Gastpar M. Treatment of alcohol withdrawal syndrome with gabapentin. Pharmacopsychiatry. 1999;32:107–109. doi: 10.1055/s-2007-979203. [DOI] [PubMed] [Google Scholar]
- 20.Lavigne JE, Heckler C, Mathews JL, Palesh O, Kirshner JJ, Lord R, et al. A randomized, controlled, double-blinded clinical trial of gabapentin 300 versus 900 mg versus placebo for anxiety symptoms in breast cancer survivors. Breast cancer research and treatment. 2012;136:479–486. doi: 10.1007/s10549-012-2251-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Myrick H, Malcolm R, Brady KT. Gabapentin treatment of alcohol withdrawal. The American journal of psychiatry. 1998;155:1632. [PubMed] [Google Scholar]
- 22.Nunes EV. Gabapentin: a new addition to the armamentarium for alcohol dependence? JAMA internal medicine. 2014;174:78–79. doi: 10.1001/jamainternmed.2013.11973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Voris J, Smith NL, Rao SM, Thorne DL, Flowers QJ. Gabapentin for the treatment of ethanol withdrawal. Substance abuse. 2003;24:129–132. doi: 10.1080/08897070309511541. [DOI] [PubMed] [Google Scholar]
- 24.Cantrell FL, Mena O, Gary RD, McIntyre IM. An acute gabapentin fatality: a case report with postmortem concentrations. International journal of legal medicine. 2015;129:771–775. doi: 10.1007/s00414-015-1193-3. [DOI] [PubMed] [Google Scholar]
- 25.Fischer JH, Barr AN, Rogers SL, Fischer PA, Trudeau VL. Lack of serious toxicity following gabapentin overdose. Neurology. 1994;44:982–983. doi: 10.1212/wnl.44.5.982. [DOI] [PubMed] [Google Scholar]
- 26.Howland RH. Gabapentin: can it be misused? Journal of psychosocial nursing and mental health services. 2014;52:12–15. doi: 10.3928/02793695-20131213-01. [DOI] [PubMed] [Google Scholar]
- 27.Kruszewski SP, Paczynski RP, Kahn DA. Gabapentin-induced delirium and dependence. Journal of psychiatric practice. 2009;15:314–319. doi: 10.1097/01.pra.0000358318.73684.df. [DOI] [PubMed] [Google Scholar]
- 28.Markowitz JS, Finkenbine R, Myrick H, King L, Carson WH. Gabapentin abuse in a cocaine user: implications for treatment? Journal of clinical psychopharmacology. 1997;17:423–424. doi: 10.1097/00004714-199710000-00012. [DOI] [PubMed] [Google Scholar]
- 29.Pittenger C, Desan PH. Gabapentin abuse, and delirium tremens upon gabapentin withdrawal. The Journal of clinical psychiatry. 2007;68:483–484. doi: 10.4088/jcp.v68n0320a. [DOI] [PubMed] [Google Scholar]
- 30.Reccoppa L, Malcolm R, Ware M. Gabapentin abuse in inmates with prior history of cocaine dependence. The American journal on addictions/American Academy of Psychiatrists in Alcoholism and Addictions. 2004;13:321–323. doi: 10.1080/10550490490460300. [DOI] [PubMed] [Google Scholar]
- 31.Reeves RR, Burke RS. Abuse of combinations of gabapentin and quetiapine. The primary care companion for CNS disorders. 2014:16. doi: 10.4088/PCC.14l01660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reeves RR, Ladner ME. Potentiation of the effect of buprenorphine/naloxone with gabapentin or quetiapine. The American journal of psychiatry. 2014;171:691. doi: 10.1176/appi.ajp.2014.13111526. [DOI] [PubMed] [Google Scholar]
- 33.Roberge RJ, Francis EH., 3rd Use of naloxone in valproic acid overdose: case report and review. The Journal of emergency medicine. 2002;22:67–70. doi: 10.1016/s0736-4679(01)00438-3. [DOI] [PubMed] [Google Scholar]
- 34.Rohman L, Hebron A. Acute dystonic reaction caused by gabapentin. The Journal of emergency medicine. 2014;46:e89. doi: 10.1016/j.jemermed.2013.08.024. [DOI] [PubMed] [Google Scholar]
- 35.Satish R, Kandasamy A, Jayarajan D, Benegal V. Gabapentin dependence in a patient with opioid dependence syndrome. The Journal of neuropsychiatry and clinical neurosciences. 2015;27:e64. doi: 10.1176/appi.neuropsych.13110339. [DOI] [PubMed] [Google Scholar]
- 36.Victorri-Vigneau C, Guerlais M, Jolliet P. Abuse, dependency and withdrawal with gabapentin: a first case report. Pharmacopsychiatry. 2007;40:43–44. doi: 10.1055/s-2006-958522. [DOI] [PubMed] [Google Scholar]
- 37.World Health Organization. Lexicon of alcohol and drug terms published by the World Health Organization. 2015 [Google Scholar]
- 38.Inciardi JA, Surratt HL, Kurtz SP, Burke JJ. The diversion of prescription drugs by health care workers in Cincinnati, Ohio. Substance use & misuse. 2006;41:255–264. doi: 10.1080/10826080500391829. [DOI] [PubMed] [Google Scholar]
- 39.Larance B, Degenhardt L, Lintzeris N, Winstock A, Mattick R. Definitions related to the use of pharmaceutical opioids: extramedical use, diversion, non-adherence and aberrant medication-related behaviours. Drug and alcohol review. 2011;30:236–245. doi: 10.1111/j.1465-3362.2010.00283.x. [DOI] [PubMed] [Google Scholar]
- 40.Eden J, Levit L, Berg A, Morton S, editors. Institute of Medicine Committee on Standards for Systematic Reviews of Comparative Effectiveness R. Finding What Works in Health Care: Standards for Systematic Reviews. Washington (DC): National Academies Press (US) Copyright 2011 by the National Academy of Sciences All rights reserved; 2011. [PubMed] [Google Scholar]
- 41.Peterson BL. Prevalence of gabapentin in impaired driving cases in Washington State in 2003–2007. Journal of analytical toxicology. 2009;33:545–549. doi: 10.1093/jat/33.8.545. [DOI] [PubMed] [Google Scholar]
- 42.Baird CR, Fox P, Colvin LA. Gabapentinoid abuse in order to potentiate the effect of methadone: a survey among substance misusers. European addiction research. 2014;20:115–118. doi: 10.1159/000355268. [DOI] [PubMed] [Google Scholar]
- 43.Smith BH, Higgins C, Baldacchino A, Kidd B, Bannister J. Substance misuse of gabapentin. The British journal of general practice: the journal of the Royal College of General Practitioners. 2012;62:406–407. doi: 10.3399/bjgp12X653516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith RV, Lofwall MR, Havens JR. Abuse and diversion of gabapentin among nonmedical prescription opioid users in Appalachian Kentucky. The American journal of psychiatry. 2015;172:487–488. doi: 10.1176/appi.ajp.2014.14101272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilens T, Zulauf C, Ryland D, Carrellas N, Catalina-Wellington I. Prescription medication misuse among opioid dependent patients seeking inpatient detoxification. The American journal on addictions/American Academy of Psychiatrists in Alcoholism and Addictions. 2015;24:173–177. doi: 10.1111/ajad.12159. [DOI] [PubMed] [Google Scholar]
- 46.Hakkinen M, Vuori E, Kalso E, Gergov M, Ojanpera I. Profiles of pregabalin and gabapentin abuse by postmortem toxicology. Forensic science international. 2014;241:1–6. doi: 10.1016/j.forsciint.2014.04.028. [DOI] [PubMed] [Google Scholar]
- 47.Kapil V, Green JL, Le Lait MC, Wood DM, Dargan PI. Misuse of the gamma-aminobutyric acid analogues baclofen, gabapentin and pregabalin in the UK. British journal of clinical pharmacology. 2014;78:190–191. doi: 10.1111/bcp.12277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wills B, Reynolds P, Chu E, Murphy C, Cumpston K, Stromberg P, et al. Clinical outcomes in newer anticonvulsant overdose: a poison center observational study. Journal of medical toxicology: official journal of the American College of Medical Toxicology. 2014;10:254–260. doi: 10.1007/s13181-014-0384-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Klein-Schwartz W, Shepherd JG, Gorman S, Dahl B. Characterization of gabapentin overdose using a poison center case series. Journal of toxicology Clinical toxicology. 2003;41:11–15. doi: 10.1081/clt-120018265. [DOI] [PubMed] [Google Scholar]
- 50.Schifano F, D’Offizi S, Piccione M, Corazza O, Deluca P, Davey Z, et al. Is there a recreational misuse potential for pregabalin? Analysis of anecdotal online reports in comparison with related gabapentin and clonazepam data. Psychotherapy and psychosomatics. 2011;80:118–122. doi: 10.1159/000321079. [DOI] [PubMed] [Google Scholar]
- 51.Seale JP, Dittmer T, Sigman EJ, Clemons H, Johnson JA. Combined abuse of clonidine and amitriptyline in a patient on buprenorphine maintenance treatment. Journal of addiction medicine. 2014;8:476–478. doi: 10.1097/ADM.0000000000000081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barrueto F, Jr, Green J, Howland MA, Hoffman RS, Nelson LS. Gabapentin withdrawal presenting as status epilepticus. Journal of toxicology Clinical toxicology. 2002;40:925–928. doi: 10.1081/clt-120016965. [DOI] [PubMed] [Google Scholar]
- 53.Fernandez MC, Walter FG, Petersen LR, Walkotte SM. Gabapentin, valproic acid, and ethanol intoxication: elevated blood levels with mild clinical effects. Journal of toxicology Clinical toxicology. 1996;34:437–439. doi: 10.3109/15563659609013815. [DOI] [PubMed] [Google Scholar]
- 54.Rasimas JJ, Burkhart KK. Cardiac conduction disturbances after an overdose of nefazodone and gabapentin. The American journal of emergency medicine. 2006;24:886–888. doi: 10.1016/j.ajem.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 55.Spiller HA, Dunaway MD, Cutino L. Massive gabapentin and presumptive quetiapine overdose. Veterinary and human toxicology. 2002;44:243–244. [PubMed] [Google Scholar]
- 56.Stopforth J. Overdose with gabapentin and lamotrigine. South African medical journal = Suid-Afrikaanse tydskrif vir geneeskunde. 1997;87:1388. [PubMed] [Google Scholar]
- 57.Schauer SG, Varney SM. Gabapentin overdose in a military beneficiary. Military medicine. 2013;178:e133–135. doi: 10.7205/MILMED-D-12-00301. [DOI] [PubMed] [Google Scholar]
- 58.Jones H, Aguila E, Farber HW. Gabapentin toxicity requiring intubation in a patient receiving long-term hemodialysis. Annals of internal medicine. 2002;137:74. doi: 10.7326/0003-4819-137-1-200207020-00029. [DOI] [PubMed] [Google Scholar]
- 59.Koschny R, Lutz M, Seckinger J, Schwenger V, Stremmel W, Eisenbach C. Extracorporeal life support and plasmapheresis in a case of severe polyintoxication. Journal of Emergency Medicine. 2014;47:527–531. doi: 10.1016/j.jemermed.2014.04.044. [DOI] [PubMed] [Google Scholar]
- 60.Middleton O. Suicide by gabapentin overdose. Journal of forensic sciences. 2011;56:1373–1375. doi: 10.1111/j.1556-4029.2011.01798.x. [DOI] [PubMed] [Google Scholar]
- 61.Smith RV, Lofwall MR, Havens JR. Gabapentin focus groups among illicit drug users. Unpublished. [Google Scholar]
- 62.Bockbrader HN, Wesche D, Miller R, Chapel S, Janiczek N, Burger P. A comparison of the pharmacokinetics and pharmacodynamics of pregabalin and gabapentin. Clinical pharmacokinetics. 2010;49:661–669. doi: 10.2165/11536200-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 63.United Nations Office on Drugs and Crime. World Drug Report. 2012 [Google Scholar]
- 64.The Henry J. Kaiser Family Foundation. Medicaid Benefits: Prescription Drugs [Google Scholar]
- 65.Ministry of Health Singapore. Drug Subsidies. 2015 [Google Scholar]
- 66.Australian Government Department of Health The Pharmacy Benefits Scheme. Gabapentin [Google Scholar]
- 67.Smith N, Quansah K, Chelak K, Fitzsimmons H. Narcotics, Benzodiazepines, Stimulants, and Gabapentin: Policies, Initiatives, and Practices Across Canada. Ottawa: Canadian Agency for Drugs and Technologies in Health; 2014. 2014. [Google Scholar]
- 68.Drug Enforcement Administration Department of Justice. Controlled Substances. 2015 [Google Scholar]
- 69.Public Health England and NHS England. Advice for prescribers on the risk of the misuse of pregabalin and gabapentin. 2014 [Google Scholar]
- 70.Drug Enforcement Administration Department of Justice. Schedules of controlled substances: placement of pregabalin into schedule V. Final rule, Federal register. 2005;70:43633–43635. [PubMed] [Google Scholar]
- 71.EMCDDA-Europol. Annual Report on the implementation of Council Decision 2005/387/JHA; Annex 2 - New psychoactive substances reported to the EMCDDA and Europol for the first time in 209 underf the term of Council. 2009 [Google Scholar]
- 72.Papazisis G, Tzachanis D. Pregabalin’s abuse potential: a mini review focusing on the pharmacological profile. International journal of clinical pharmacology and therapeutics. 2014;52:709–716. doi: 10.5414/CP202118. [DOI] [PubMed] [Google Scholar]
- 73.Cora-Locatelli G, Greenberg BD, Martin JD, Murphy DL. Rebound psychiatric and physical symptoms after gabapentin discontinuation. The Journal of clinical psychiatry. 1998;59:131. doi: 10.4088/jcp.v59n0308a. [DOI] [PubMed] [Google Scholar]
- 74.Finch CK, Eason J, Usery JB. Gabapentin withdrawal syndrome in a post-liver transplant patient. Journal of pain & palliative care pharmacotherapy. 2010;24:236–238. doi: 10.3109/15360288.2010.493927. [DOI] [PubMed] [Google Scholar]
- 75.Hellwig TR, Hammerquist R, Termaat J. Withdrawal symptoms after gabapentin discontinuation. American journal of health-system pharmacy: AJHP: official journal of the American Society of Health-System Pharmacists. 2010;67:910–912. doi: 10.2146/ajhp090313. [DOI] [PubMed] [Google Scholar]
- 76.Norton JW. Gabapentin withdrawal syndrome. Clinical neuropharmacology. 2001;24:245–246. doi: 10.1097/00002826-200107000-00011. [DOI] [PubMed] [Google Scholar]
- 77.Rosebush PI, MacQueen GM, Mazurek MF. Catatonia following gabapentin withdrawal. Journal of clinical psychopharmacology. 1999;19:188–189. doi: 10.1097/00004714-199904000-00019. [DOI] [PubMed] [Google Scholar]
- 78.Tran KT, Hranicky D, Lark T, Jacob N. Gabapentin withdrawal syndrome in the presence of a taper. Bipolar disorders. 2005;7:302–304. doi: 10.1111/j.1399-5618.2005.00200.x. [DOI] [PubMed] [Google Scholar]
- 79.Mah L, Hart M. Gabapentin withdrawal: case report in an older adult and review of the literature. Journal of the American Geriatrics Society. 2013;61:1635–1637. doi: 10.1111/jgs.12427. [DOI] [PubMed] [Google Scholar]
- 80.Crockford D, White WD, Campbell B. Gabapentin use in benzodiazepine dependence and detoxification. Canadian journal of psychiatry Revue canadienne de psychiatrie. 2001;46:287. doi: 10.1177/070674370104600315. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


