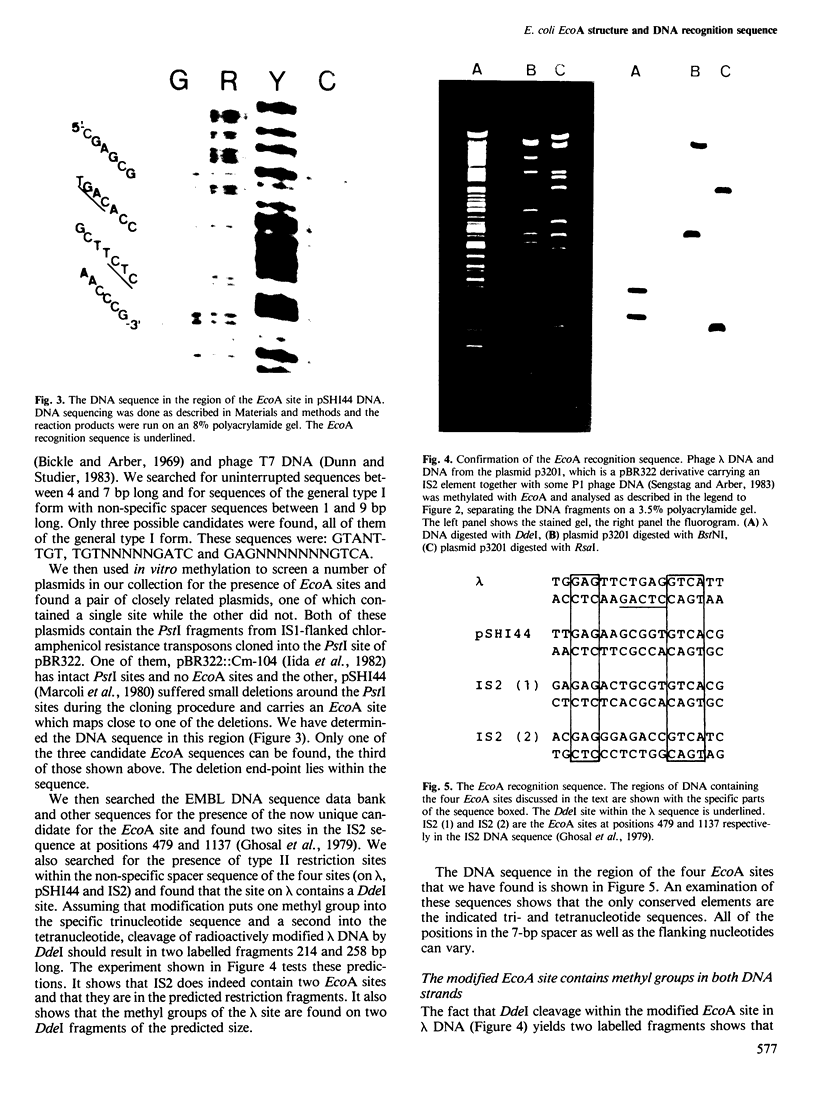
Abstract
The EcoA restriction enzyme from Escherichia coli 15T- has been isolated. It proves to be an unusual enzyme, clearly related functionally to the classical type I restriction enzymes. The basic enzyme is a two subunit modification methylase. Another protein species can be purified which by itself has no enzymatic activities but which converts the modification methylase to an ATP and S-adenosylmethionine-dependent restriction endonuclease. The DNA recognition sequence of EcoA has an overall structure that is very similar to previously determined type I sequences. It is: 5'-GAGNNNNNNNGTCA-3' 3'-CTCNNNNNNNCAGT-5' where N can be any nucleotide. Modification methylates the adenosyl residue in the specific trinucleotide and the adenosyl residue in the lower strand of the specific tetranucleotide.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arber W., Rifat A., Wauters-Willems D., Kühnlein U. Host specificity of DNA produced by Escherichia coli. XVI. Phage lambda DNA carries a single site of affinity for A-specific restriction and modification. Mol Gen Genet. 1972;115(3):195–207. doi: 10.1007/BF00268883. [DOI] [PubMed] [Google Scholar]
- Arber W., Wauters-Willems D. Host specificity of DNA produced by Escherichia coli. XII. The two restriction and modification systems of strain 15T-. Mol Gen Genet. 1970;108(3):203–217. doi: 10.1007/BF00283350. [DOI] [PubMed] [Google Scholar]
- Bickle T., Arber W. Host-controlled restriction and modification of filamentous 1- and F-specific bacteriophages. Virology. 1969 Nov;39(3):605–607. doi: 10.1016/0042-6822(69)90112-3. [DOI] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Bullas L. R., Colson C. DNA restriction and modification systems in Salmonella. III. SP, a Salmonella potsdam system allelic to the SB system in Salmonella typhimurium. Mol Gen Genet. 1975 Aug 27;139(3):177–188. [PubMed] [Google Scholar]
- Bullas L. R., Colson C., Van Pel A. DNA restriction and modification systems in Salmonella. SQ, a new system derived by recombination between the SB system of Salmonella typhimurium and the SP system of Salmonella potsdam. J Gen Microbiol. 1976 Jul;95(1):166–172. doi: 10.1099/00221287-95-1-166. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. Complete nucleotide sequence of bacteriophage T7 DNA and the locations of T7 genetic elements. J Mol Biol. 1983 Jun 5;166(4):477–535. doi: 10.1016/s0022-2836(83)80282-4. [DOI] [PubMed] [Google Scholar]
- Eskin B., Linn S. The deoxyribonucleic acid modification and restriction enzymes of Escherichia coli B. II. Purification, subunit structure, and catalytic properties of the restriction endonuclease. J Biol Chem. 1972 Oct 10;247(19):6183–6191. [PubMed] [Google Scholar]
- Ghosal D., Sommer H., Saedler H. Nucleotide sequence of the transposable DNA-element IS2. Nucleic Acids Res. 1979 Mar;6(3):1111–1122. doi: 10.1093/nar/6.3.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover S. W., Colson C. Genetics of host-controlled restriction and modification in Escherichia coli. Genet Res. 1969 Apr;13(2):227–240. doi: 10.1017/s0016672300002901. [DOI] [PubMed] [Google Scholar]
- Glover S. W. Functional analysis of host-specificity mutants in Escherichia coli. Genet Res. 1970 Apr;15(2):237–250. doi: 10.1017/s0016672300001567. [DOI] [PubMed] [Google Scholar]
- Gough J. A., Murray N. E. Sequence diversity among related genes for recognition of specific targets in DNA molecules. J Mol Biol. 1983 May 5;166(1):1–19. doi: 10.1016/s0022-2836(83)80047-3. [DOI] [PubMed] [Google Scholar]
- Hadi S. M., Bickle T. A., Yuan R. The role of S-adenosylmethionine in the cleavage of deoxyribonucleic acid by the restriction endonuclease from Escherichia coli K. J Biol Chem. 1975 Jun 10;250(11):4159–4164. [PubMed] [Google Scholar]
- Hadi S. M., Bächi B., Shepherd J. C., Yuan R., Ineichen K., Bickle T. A. DNA recognition and cleavage by the EcoP15 restriction endonuclease. J Mol Biol. 1979 Nov 5;134(3):655–666. doi: 10.1016/0022-2836(79)90372-3. [DOI] [PubMed] [Google Scholar]
- Hubacek J., Glover S. W. Complementation analysis of temperature-sensitive host specificity mutations in Escherichia coli. J Mol Biol. 1970 May 28;50(1):111–127. doi: 10.1016/0022-2836(70)90108-7. [DOI] [PubMed] [Google Scholar]
- Kan N. C., Lautenberger J. A., Edgell M. H., Hutchison C. A., 3rd The nucleotide sequence recognized by the Escherichia coli K12 restriction and modification enzymes. J Mol Biol. 1979 May 15;130(2):191–209. doi: 10.1016/0022-2836(79)90426-1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lark C., Arber W. Host specificity of DNA produced by Escherichia coli. 13. Breakdown of cellular DNA upon growth in ethionine of strains with r plus-15, r plus-P1 or r plus-N3 restriction phenotypes. J Mol Biol. 1970 Sep 14;52(2):337–348. doi: 10.1016/0022-2836(70)90034-3. [DOI] [PubMed] [Google Scholar]
- Lautenberger J. A., Edgell M. H., Hutchison C. A., 3rd, Godson G. N. The DNA sequence on bacteriophage G4 recognized by the Escherichia coli B restriction enzyme. J Mol Biol. 1979 Jul 15;131(4):871–875. doi: 10.1016/0022-2836(79)90206-7. [DOI] [PubMed] [Google Scholar]
- Lautenberger J. A., Kan N. C., Lackey D., Linn S., Edgell M. H., Hutchison C. A., 3rd Recognition site of Escherichia coli B restriction enzyme on phi XsB1 and simian virus 40 DNAs: an interrupted sequence. Proc Natl Acad Sci U S A. 1978 May;75(5):2271–2275. doi: 10.1073/pnas.75.5.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautenberger J. A., Linn S. The deoxyribonucleic acid modification and restriction enzymes of Escherichia coli B. I. Purification, subunit structure, and catalytic properties of the modification methylase. J Biol Chem. 1972 Oct 10;247(19):6176–6182. [PubMed] [Google Scholar]
- Lida S., Marcoli R., Bickle T. A. Phenotypic reversion of an IS1-mediated deletion mutation: a combined role for point mutations and deletions in transposon evolution. EMBO J. 1982;1(6):755–759. doi: 10.1002/j.1460-2075.1982.tb01242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcoli R., Iida S., Bickle T. A. The DNA sequence of an IS/-flanked transposon coding for resistance to chloramphenicol and fusidic acid. FEBS Lett. 1980 Jan 28;110(1):11–14. doi: 10.1016/0014-5793(80)80011-1. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Meselson M., Yuan R., Heywood J. Restriction and modification of DNA. Annu Rev Biochem. 1972;41:447–466. doi: 10.1146/annurev.bi.41.070172.002311. [DOI] [PubMed] [Google Scholar]
- Murray N. E., Gough J. A., Suri B., Bickle T. A. Structural homologies among type I restriction-modification systems. EMBO J. 1982;1(5):535–539. doi: 10.1002/j.1460-2075.1982.tb01205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piekarowicz A., Bickle T. A., Shepherd J. C., Ineichen K. The DNA sequence recognised by the HinfIII restriction endonuclease. J Mol Biol. 1981 Feb 15;146(1):167–172. doi: 10.1016/0022-2836(81)90372-7. [DOI] [PubMed] [Google Scholar]
- Ravetch J. V., Horiuchi K., Zinder N. D. Nucleotide sequence of the recognition site for the restriction-modification enzyme of Escherichia coli B. Proc Natl Acad Sci U S A. 1978 May;75(5):2266–2270. doi: 10.1073/pnas.75.5.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers S. G., Weiss B. Exonuclease III of Escherichia coli K-12, an AP endonuclease. Methods Enzymol. 1980;65(1):201–211. doi: 10.1016/s0076-6879(80)65028-9. [DOI] [PubMed] [Google Scholar]
- Sain B., Murray N. E. The hsd (host specificity) genes of E. coli K 12. Mol Gen Genet. 1980;180(1):35–46. doi: 10.1007/BF00267350. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Hong G. F., Hill D. F., Petersen G. B. Nucleotide sequence of bacteriophage lambda DNA. J Mol Biol. 1982 Dec 25;162(4):729–773. doi: 10.1016/0022-2836(82)90546-0. [DOI] [PubMed] [Google Scholar]
- Sengstag C., Arber W. IS2 insertion is a major cause of spontaneous mutagenesis of the bacteriophage P1: non-random distribution of target sites. EMBO J. 1983;2(1):67–71. doi: 10.1002/j.1460-2075.1983.tb01382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer R., Schaller H. Nucleotide sequence of the recognition site of the B-specific restriction modification system in E. coli. Mol Gen Genet. 1979 Jan 11;168(3):331–335. doi: 10.1007/BF00271504. [DOI] [PubMed] [Google Scholar]
- Yuan R. Structure and mechanism of multifunctional restriction endonucleases. Annu Rev Biochem. 1981;50:285–319. doi: 10.1146/annurev.bi.50.070181.001441. [DOI] [PubMed] [Google Scholar]