Abstract
The organization of metabolic multienzyme complexes has been hypothesized to benefit metabolic processes and provide a coordinated way for the cell to regulate metabolism. Historically, their existence has been supported by various in vitro techniques. However, it is only recently that the existence of metabolic complexes inside living cells has come to light to corroborate this long-standing hypothesis. Indeed, subcellular compartmentalization of metabolic enzymes appears to be widespread and highly regulated. On the other hand, it is still challenging to demonstrate the functional significance of these enzyme complexes in the context of the cellular milieu. In this review, we discuss the current understanding of metabolic enzyme complexes by primarily focusing on central carbon metabolism and closely associated metabolic pathways in a variety of organisms, as well as their regulation and functional contributions to cells.
Graphical abstract
Metabolism is a highly orchestrated process, which provides energy and building blocks to the cell. Typically thought of as a complicated map of hundreds of interconnected chemical reactions, metabolism is key to cellular function, growth, and proliferation. Metabolic pathways are mostly thought to be orchestrated by spatially “well-mixed” enzymes.1–4 However, this perception has been challenged for many years.2 Finally, we have begun to understand how metabolic enzymes interact and coordinate with each other in space and time to perform their designed metabolic functions in cells.
Our understanding of metabolic compartmentalization inside cells has enhanced as advanced cell-based techniques are developed. Systems biology along with omics strategies has found that metabolic enzymes often interact with each other in various compartments of the cell and even demonstrate cell-to-cell variability in protein complex composition.5–8 In parallel, fluorescence microscopic techniques in association with biochemical and cellular assays have provided compelling evidence of the complexation of metabolic enzymes in cells.5,9,10 Although subcellular complexation of many enzymes in various metabolic pathways remains to be investigated, it has become clear that metabolism may benefit from the spatial organization of metabolic enzymes in given subcellular locations. Therefore, advancements in cell-based techniques have contributed to our understanding of subcellular localizations of metabolic enzymes and their compartmentalization in cells.
Importantly, the localization of metabolic enzymes into multienzyme complexes can be understood from metabolic and regulatory standpoints. Several metabolic pathways produce and consume chemically unstable or toxic metabolites, so that the proximity of metabolic enzymes to one another would be vital for efficient production of their metabolic products. In addition, spatial and/or temporal concentrations of enzymes and their metabolites are anticipated to generate gradients inside cells.1,11,12 The association of metabolic enzymes into complexes has been hypothesized to facilitate substrate channeling or influence metabolic flux.9,13–15 Thus, the localization of metabolic enzymes to or near each other is thought to play a critical role in regulation of metabolic pathways in cells.
In this review, we summarize the current knowledge of metabolic enzyme complexes primarily implicated in the central pathways of carbon metabolism in a variety of organisms (Figure 1, Table 1). Briefly, we discuss the current understanding of the spatial compartmentalization of metabolic enzymes in glycolysis, pyruvate dehydrogenase complex, mitochondrial oxidative phosphorylation, the tricarboxylic acid (TCA) cycle, fatty acid synthesis, and nucleotide biosynthesis. We also describe prospective multienzyme complexes in polyketide biosynthesis and amino acid biosynthesis. However, we do not include extensive knowledge of macromolecular complexes involved in photosynthesis, nucleic acid metabolism, and polypeptide biosynthesis because these complexes have been extensively reviewed.16–19 Collectively, this review highlights the current status of our understanding of spatial organizations of metabolic enzyme complexes in cells.
Figure 1.
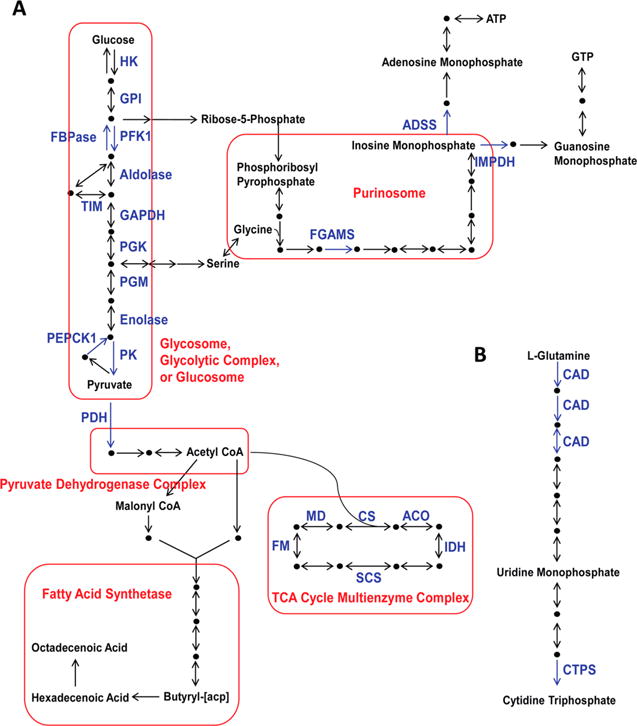
An overview of central carbon metabolism. Enzymes extensively discussed in this review are named in blue, while discussed multienzyme complexes are boxed in red. Metabolites are either shown by their names or black dots. (A) Glucose is consumed through the central pathway of glycolysis, or generated by gluconeogenesis, which shuttles into energy metabolism and anabolic biosynthetic pathways. The product of glycolysis, pyruvate, is shuttled to the pyruvate dehydrogenase complex. Then, the produced acetyl-CoA is directed to either fatty acid synthetase, or the TCA cycle protein complex. Meanwhile, ribose-5-phosphate and serine are produced from glycolytic intermediates and shunted into de novo purine biosynthesis, which is promoted by the purinosome. (B) L-Glutamine is converted to cytidine triphosphate through de novo pyrimidine biosynthesis. Used acronyms: hexokinase (HK); glucose-6-phosphate isomerase (GPI); phosphofructokinase 1 (PFK1); fructose-1,6-bisphosphatase (FBPase); triose phosphate isomerase (TIM); glyceraldehyde-3-phosphate dehydrogenase (GAPDH); phosphoglycerate kinase (PGK); phosphoglycerate mutase (PGM); phosphoenolpyruvate carboxykinase (PEPCK); pyruvate kinase (PK); pyruvate dehydrogenase (PDH); formylglycinamidine ribonucleotide synthase (FGAMS); inosine monophosphate dehydrogenase (IMPDH); adenylosuccinate synthetase (ADSS); citrate synthase (CS); acetyl-CoA oxidase (ACO); isocitrate dehydrogenase (DH); succinyl-CoA synthetase (SCS); fumarase (FM); malate dehydrogenase (MD); carbamoyl-phosphate synthetase, aspartate transcarbamylase, and dihydroorotase (CAD); cytidine triphosphate synthase (CTPS).
Table 1.
Evidence for Multienzyme Metabolic Complexes
| metabolic pathway | in vitro evidence | fixed cell evidence (†colocalization) | live cell evidence († colocalization) | organism |
|---|---|---|---|---|
| glucose metabolism | mass spectrometry26 | immunofluorescence microscopy,25 transmission electron microscopy24,25 | fluorescence microscopy25 | Trypanosomatida |
| co-immunoprecipitation7,38,39 mass spectrometry7,38,39 |
plants | |||
| co-immunoprecipitation34 | fluorescence microscopy33 | yeast | ||
| Immunofluorescence microscopy43,44 | fly | |||
| enzyme inhibitor-binding assays47 | immunofluorescence microscopy46,48† | mammal (erythrocyte) | ||
| immunofluorescence microscopy52,53 | fluorescence microscopy53†, fluorescence recovery after photobleaching,53 intracellular fluorescence resonance energy transfer53† | human (cancer cells) | ||
| pyruvate metabolism | cryoelectron microscopy55 | mammal | ||
| surface plasmon resonance56 | immunofluorescence microscopy62,63 | human (cancer cells) | ||
| TCA cycle | bacterial two-hybrid analysis,67 strep-protein interaction experiment,67,73 in vitro reconstitution68 | bacteria | ||
| cross-linking and mass spectrometry,70 diffusion analysis71 | fluorescence recovery after photobleaching69 | mammal | ||
| mitochondrial oxidative phosphorylation | native PAGE gel75 | yeast | ||
| proteomic mapping81 | fly | |||
| cryoelectron microscopy76 | mammal | |||
| proteomic mapping6,80 | human (embryonic kidney) | |||
| purine biosynthesis | Tango assay91 | fluorescence microscopy88,94†, fluorescence recovery after photobleaching92 | human (cancer cells) | |
| pyrimidine biosynthesis | cryoelectron microscopy113 | immunofluorescence microscopy113 | bacteria | |
| immunofluorescence microscopy114 | fluorescence microscopy116 | yeast | ||
| immunofluorescence microscopy112 | fly | |||
| immunofluorescence microscopy124 | mammal | |||
| immunofluorescence microscopy102,114 | human (cancer cells) | |||
| fatty acid synthesis | X-ray crystallography128 | bacteria | ||
| X-ray crystallography,129 cryoelectron microscopy130 | immunofluorescence microscopy132 | fluorescence microscopy132 | yeast | |
| single particle cryoelectron microscopy131 | mammal | |||
| bioluminescence resonance energy transfer133† | human (cancer cells) | |||
| natural product biosynthesis | mass spectrometry144 | fluorescence microscopy144† | bacteria | |
| co-immunoprecipitiation140 | immunofluorescence microscopy142 | fluorescence microscopy139,140† | fungus | |
| amino acid metabolism | X-ray crystallography153,155 | bacteria | ||
| mass spectrometry156,157 | mammal |
GLUCOSE METABOLISM
Glucose metabolism is the central metabolic pathway, which consists of glycolysis and gluconeogenesis. The conversion of glucose to pyruvate in glycolysis is catalyzed in 10 steps, by 10 enzymes, 3 of which are specific to glycolysis. Conversely, gluconeogenesis produces glucose in 11 steps employing four gluconeogenesis-specific enzymes along with seven enzymes from glycolysis (Figure 1A). The compartmentalization of glycolytic and gluconeogenic enzymes in a variety of species has been investigated over many years (Table 2), suggesting that these enzymes would interact and form a multienzyme complex.20 Here, we review some of the well-characterized protein–protein interactions and their complexes for glucose metabolism in various organisms.
Table 2.
Compartmentalization of Glycolytic Enzymes into Complexes
| organism | enzymes identified to colocalize or associate in cell | subcellular localization |
|---|---|---|
| protists | hexokinase, PGI, PFK, aldolase, TIM, GAPDH, PGK | peroxisome |
| plant | hexokinase, aldolase, enolase | mitochondria |
| yeast | enolase, glucokinase, PGI, PFK, aldolase, TIM, GAPDH, PGM, PK | cytoplasm |
| Human (erythrocyte) | aldolase, GAPDH, PFK, PK | inner cell membrane |
| human (cancer cells) | PFK, FBPase, PKM2, PEPCK1 | cytoplasm |
Glycosome in Protists
The compartmentalization of glycolysis into so-called “glycosomes” has been known for several decades.21,22 Glycosomes are membrane-bound peroxisomes containing enzymes associated with the first six or seven steps of glycolysis in trypanosmatids.22,23 To compensate the missing enzymatic activities for glycolysis, trypanosmatids hijack the rest of the glycolytic enzymes from their host organisms to complete glycolysis. The number of glycosomes per cell varies from about 18 to 65, depending on the species.24,25 Proteomic work has further revealed that, aside from glycolytic enzymes, glycosomes contain various metabolic enzymes in pyruvate metabolism, the TCA cycle, the pentose phosphate pathway, nucleotide metabolism, amino acid metabolism, and steroid metabolism.26 This suggests that the compartmentalization of glycolysis into glycosomes is a way to coordinate multiple metabolic pathways in protists. Collectively, glycosomes in trypanosmatids are membrane-bound peroxisomes which compartmentalize glycolysis and other pathways to coordinate metabolism.
In addition, glycosomes are essential for trypanosmatids. Abolishment of peroxin proteins, which are required for glycosome formation, resulted in depletion of glycosomes, cytoplasmic localization of the glycolytic enzymes, and cell death, indicating that glycosomes are essential for survival.27 Without glycosomes, trypanosmatids die in the presence of glucose; thus glycosomes appear to be vital in regulating glucose utilization.28 Trypanosmatids also regulate the protein composition of the glycosome based on glucose levels,29 although the glycosome does not appear to govern metabolic flux in trypanosmatids.30 Collectively, the glycosome appears to be a mechanism of providing metabolic flexibility to trypanosmatids for their survival.22,30,31
Glycolytic Complexes in Higher Organisms
Glycolytic enzymes in yeast have been found to be associated with the mitochondria as well as cytoskeletal structures. Specifically, all enzymatic activities of glycolysis have been found in isolated yeast mitochondria.32 Under hypoxic conditions, enolase in yeast cells was demonstrated to form punctate structures with glucokinase, glucose-6-phosphate isomerase, phosphofructokinase, aldolase, triose phosphate isomerase, glyceraldehyde-3-phosphate dehydrogenase, phosphoglycerate mutase, and pyruvate kinase.33 In addition, F-actin was found to provide docking sites for the organization of hexokinase, glucose-6-phosphate isomerase, triose phosphate isomerase, glyceralde-hyde-3-phosphate dehydrogenase, phosphoglycerate mutase, and aldolase in yeast.34 Particularly, their association with F-actin appears to increase individual enzyme activities while protecting against the inhibitory effects of trehalose in yeast cells.34 Isotope labeling experiments revealed that the compartmentalization of glycolytic enzymes was associated with increased glucose flux in yeast.33 Alternatively, in budding yeast cells, phosphofructokinase was recently reported to form cytoplasmic filaments, although the functional relevance of the filament structure remains to be elucidated.35,36 Collectively, yeast glycolytic enzymes appear to form a variety of subcellular structures, which are responsible for regulating glycolytic flux in cells.
Several proteomic studies with plant cells have also identified that glycolytic enzymes interact with each other as well as with mitochondria.37–40 In isolated mitochondria from Arabidopsis, all 10 glycolytic enzymes were detected by in vitro enzymatic assays, thus indicating that glycolytic enzymes are concurrent with mitochondria.39,41 The association of glycolytic enzymes to Arabidopsis mitochondria suggests the possibility of substrate channeling of metabolic intermediates between cytoplasmic glycolysis and mitochondria.42 Association or dissociation of various glycolytic enzymes from the mitochondria, including glucose-6-phosphate isomerase, phosphofructokinase, aldolase, triose phosphate isomerase, phosphoglycerate kinase, phosphoglycerate mutase, and pyruvate kinase, was associated with increased or decreased respiration in Arabidopsis cells, respectively.42 Similarly, the association of phosphofructokinase, glyceraldehyde-3-phosphate dehydrogenase, triose phosphate isomerase, phosphoglycerate kinase, phosphoglycerate mutase, and pyruvate kinase was also promoted when respiration increased in potato tubers.42 Importantly, glycolytic enzymes associated with mitochondria in Arabidopsis appear to be enzymatically active, and their complexation seems to mediate substrate channeling of glycolytic intermediates.42 Taken together, plant cells promote association of glycolytic enzymes into the multienzyme complex on mitochondria in a cellular respiration-dependent manner, which allows metabolic intermediates to channel through glycolysis into mitochondria.
Glycolytic enzymes are also compartmentalized in Drosophila flight muscle cells and Caenorhabditis elegans neurons due to their high energy demand. In Drosophila flight muscle, glyceraldehyde-3-phosphate dehydrogenase, aldolase, triose phosphate isomerase, phosphoglycerate kinase, and phosphoglycerate mutase have been individually shown to be localized to the sarcomere of myofibrils.43 However, colocalization or spatial organization of these glycolytic enzymes has not been demonstrated yet in Drosophila flight muscle cells. Interestingly, when the first enzyme in the pentose phosphate pathway, glycerol-3-phosphate dehydrogenase, was knocked out, these glycolytic enzymes did not localize to the sarcomere in Drosophila.44 In addition, in C. elegans, phosphofructokinase 1.1, glyceraldehyde-3-phosphate dehydrogenase, and aldolase colocalized in clusters at presynaptic sites in neurons under hypoxia or neuronal stimulation.45 The localization of phosphofructokinase 1.1 into clusters at presynaptic sites was then hypothesized to be due to energy demands for ATP, and the localization, not necessarily enzymatic activities, of glycolytic enzymes to the presynaptic sites seems to be essential for synaptic function.45 Taken all together, it appears that glycolytic enzyme complexes exist to meet cellular energy needs in high-energy demanding cells, such as Drosophila flight muscle cells and C. elegans neurons.
The formation of a glycolytic complex has also been investigated extensively in mammalian erythrocytes. Aldolase, glyceraldehyde-3-phosphate dehydrogenase, phosphofructokinase, and pyruvate kinase have been found colocalized to the inner surface of the cell membrane of human erythrocytes, in an association with the membrane-bound band 3 protein.46,47 Mouse glycolytic enzymes were found also colocalized to the membrane of mouse erythrocytes with band 3, despite lacking the conserved sequences between mouse and human band 3 proteins.48 Furthermore, the glycolytic enzyme complex in human erythrocytes was shown to interact with β-spectrin, ankyrin, actin, and protein 4.2, indicating that glycolytic enzymes are associated with other nonmetabolic proteins in erythrocytes.49 The association and dissociation of glycolytic enzymes into complexes in mammalian erythrocytes also depended on the oxygenation state of red blood cells as well as the phosphorylation status of band 3.48,50,51 The formation of a glycolytic complex on the erythrocyte membrane is now hypothesized to compartmentalize ATP production in red blood cells.49 Collectively, work thus far in mammalian red blood cells demonstrates the presence of a multienzyme glycolytic complex, but its functional and/or structural significance remains to be further elucidated.
Aside from glycolytic assemblies in erythrocytes, there is developing evidence for glycolytic enzyme complexes in other human cell types. In HeLa cells, subcellular compartmentalization of glyceraldehyde-3-phosphate dehydrogenase appears to be regulated by small ubiquitin related modifier-1 under hypoxic conditions.52 More recently, in various cancer cells, we have demonstrated the formation of a multienzyme metabolic complex in the cytoplasm, termed the “glucosome,” which contains not only glycolytic enzymes but also gluconeogenic enzymes: phosphofructokinase 1, pyruvate kinase, fructose-1,6-bisphosphatase, and phosphoenolpyruvate carboxykinase.53 Interestingly, the functional contributions of glucosome clusters to cellular metabolism appear to be differentiated in a cluster-size dependent fashion. Although in-depth studies may be required, the compartmentalization of glycolytic and gluconeogenic enzymes is hypothesized as a mechanism of glucose flux regulation in human cells.
Collectively, it is important to note here that the described data support the existence of multienzyme metabolic complexes for glycolysis in nature. However, most of the described studies have relied on chemically fixed cells and/or in vitro enzymatic assays from chromatographically fractioned pools of cell lysates or semipurified organelle fractions. Also, the protein components of the identified complexes are mostly from glycolysis, thus excluding gluconeogenic enzymes for the pathway. Evidence has indicated that glycolytic complexes may contain more than the pathway enzymes, which may explain their potential regulatory and/or functional actions in cells. It is clear that there are many challenges still ahead to explore new dimensions of glycolytic enzymes and their complexes inside living cells, which will accelerate our endeavors to determine the functional and/or structural significance of such glycolytic complexes in cells.
MITOCHONDRIAL METABOLISM
Pyruvate Dehydrogenase Complex
Under aerobic conditions, the pyruvate dehydrogenase complex (PDC) catalyzes the decarboxylation of pyruvate to produce acetyl-CoA in the mitochondria (Figure 1A). Three essential enzymes, pyruvate dehydrogenase, dihydrolipoamide acetyltransferase and dihydrolipoamide dehydrogenase, associate with each other to form a multienzyme complex 4.5–9 MDa in size.54 Electron microscopic data have revealed that the PDC is composed of a core of eight dihydrolipoamide acetyltransferase trimers arranged in either a cube (in Escherichia coli) or pentagonal dodecahedron (in eukaryotes and some Grampositive bacteria) and is surrounded by 20–30 pyruvate dehydrogenase heterotetramers and 6–12 dihydrolipoamide dehydrogenase homodimers.55,56 Enzymatically, the lipoyl domain on dihydrolipoamide acetyltransferase plays an essential role by tethering metabolic substrates during the catalytic cycle of acetyl-CoA synthesis. Therefore, extensive in vitro studies have given great understanding to the enzymatic mechanisms and structures of the PDC.
In addition, a specific set of pyruvate dehydrogenase kinases and phosphatases has been identified to regulate the enzymatic activity of PDC in response to metabolic demand.54,57,58 The kinase-mediated phosphorylation of the pyruvate dehydrogenase domain deactivates PDC, whereas the phosphatases reciprocally activates PDC by dephosphorylation in the mitochondria. The activities of the kinases and the phosphatases are found to be thermodynamically regulated by the concentrations of PDC’s substrate, products, cofactors, and hormones like insulin.59,60 Using pharmacological inhibitors of the kinases and phospho-specific antibodies for PDC, the phosphorylation-dependent regulation of mitochondrial PDC activity was visualized in fixed mammalian cells under immunofluorescence microscopy.61 Hence, the PDC activity is under the regulation of a specific set of kinases and phosphatases in cells.
Aside from the mitochondrial matrix, PDC has also been identified in other cellular spaces. First, PDC was found in the mitochondrial outer membrane and intermembrane space, rather than the mitochondrial matrix, in some cancer cells.62 Second, PDC also translocates from the mitochondria to the nucleus in human cells, although the mechanism of transport remains elusive.63,64 Interestingly, nuclear-localized PDC seems to catalyze the formation of acetyl-CoA and influences histone acetylation.63 Therefore, PDC appears to respond to the localized need for metabolites within a cell.
Tricarboxylic Acid Cycle
The tricarboxylic acid (TCA) cycle, also referred to as the citric acid cycle or the Krebs cycle, utilizes two carbon atoms from acetyl-CoA (typically derived from carbohydrates, fatty acids, and amino acids) to generate three molecules of NADH, one molecule of FADH2, and two molecules of CO2 (Figure 1A). This process is essential to aerobic respiration because NADH and FADH2 are required for ATP production in mitochondrial oxidative phosphorylation. As the TCA cycle requires the coordination of eight enzymes, and occurs in the highly crowded environment of the mitochondrial matrix, it has long been hypothesized that the enzymes of the TCA cycle form a multienzyme complex.20
To date, extensive evidence has suggested the existence of a TCA cycle multienzyme complex. Initial in vitro cross-linking studies had found citrate synthase interacted with mitochondrial malate dehydrogenase.65 The enhancement of enzymatic activities of the TCA cycle enzymes were also identified in gently disrupted rat liver mitochondria, compared to that observed in completely disrupted mitochondria, suggesting the compartmentalization of TCA cycle enzymes in the mitochondria.66 More recently, intracellular cross-linking techniques and bacterial two-hybrid studies revealed that three enzymes of the TCA cycle in Bacillus subtilis (i.e., citrate synthase, isocitrate dehydrogenase, and malate dehydrogenase) form a core multienzyme complex.67 Additionally, the core complex interacts further with fumarase, aconitase, and succinyl-CoA synthetase through malate dehydrogenase.67 Isocitrate dehydrogenase of the core complex also interacts with a 2-oxoglutarate dehydrogenase complex.67 In Pseudomonas aeruginosa, a TCA cycle multienzyme complex containing citrate synthase, isocitrate dehydrogenase, malate dehydrogenase, fumarase, aconitase, and succinyl thiokinase was purified by size-exclusion chromatography, and individual enzyme composition was confirmed by in vitro enzyme activity assays.68 In mammalian cells, fluorescence recovery after photobleaching (FRAP) experiments using green fluorescent protein-tagged enzymes revealed citrate synthase (51 kDa), isocitrate dehydrogenase (46 kDa), malate dehydrogenase (36 kDa), and succinyl-CoA synthetase (50 kDa) to have similar apparent diffusion coefficients.69 Considering these enzymes have different multimeric states and sizes, these data support the potential formation of a four enzyme complex in live cells.69 Furthermore, tandem mass spectrometric analysis with cross-linked beef heart mitochondria revealed that all enzymes involved in the TCA cycle were indeed found to be associated with each other, with the strongest interaction between malate dehydrogenase and citrate synthase.70 Collectively, all the evidence strongly supports the association of TCA cycle enzymes into a multienzyme complex in mitochondria.
In addition, the protein-protein interactions or complex formation of the TCA cycle enzymes have been hypothesized to be beneficial for regulating flux through metabolic substrate channeling.20 In vitro microfluidic studies investigating the free diffusion of malate dehydrogenase and citrate synthase demonstrated that the apparent diffusion coefficients of malate dehydrogenase and citrate synthase were influenced by substrate availability.71 Importantly, the rate of citrate production did not change when the TCA cycle enzymes were challenged in vitro with other enzymes using the same substrate, indicating limited free diffusion of metabolic intermediates, i.e., substrate channeling.72 The protein-protein interaction between malate dehydrogenase and isocitrate dehydrogenase was also strengthened by the addition of cofactors and substrates of isocitrate dehydrogenase.73 Taken all together, these data support the hypothesis that a multienzyme complex of the TCA cycle enzymes promotes substrate channeling during the TCA cycle.
Mitochondrial Oxidative Phosphorylation
Mitochondria are often considered as the “powerhouse of the cell” referring to the process of oxidative phosphorylation, which occurs within the inner mitochondrial membrane. In oxidative phosphorylation, the production of ATP is coupled to the generation of a proton gradient via the electron transport chain (ETC) organization. The ETC organization is composed of four macromolecular complexes, termed complexes I, II, III, and IV, coupled with coenzyme Q and cytochrome c. The organization of the ETC has been rigorously investigated, as discussed herein.
Investigations into the compartmentalization of the ETC have established the formation of a “supercomplex”. Initially, the four complexes of the ETC and ATP synthase were thought to be randomly distributed throughout the mitochondrial membrane, and oxidative phosphorylation occurred in a random collision model.74 However, in vitro evidence supported the formation of a supercomplex of the ETC, which is composed of complexes I, III, and IV in mammals, or complexes III and IV in Saccharomyces cerevisiae.75 The mammalian supercomplex model was further strengthened by cryoelectron microscopy visualizing its architecture from sheep heart mitochondria.76 The data were also confirmed by crosslinking studies in mice mitochondria.77 Additionally, the association between complex III and complex IV was found to be mediated by supercomplex assembly factor I.78 Noticeably, complex II is excluded from the respiratory supercomplex; however the reason for this has not been investigated yet.79 Therefore, the compartmentalization of the complexes into a supercomplex is conserved across species for oxidative phosphorylation.
Along with genetic and cryoelectron microscopic techniques, proteomic mapping techniques have recently advanced our understanding of the ETC-ATP synthase organization in mitochondria of living cells. In this technique, live human cells were transfected with mitochondria-targeted ascorbate peroxidase and then treated with biotin-conjugated phenol in the presence of hydrogen peroxide, followed by chemical fixation and pull-down of biotin-labeled protein components for tandem mass spectrometry.6 The portions of the ETC facing either the intermembrane space or mitochondrial matrix were successfully mapped to visualize the orientation of the ETC organization in human mitochondria.6,80 This technique has also been used on a larger scale in Drosophila to profile mitochondria-associated proteins, including the components of the ETC.6,80,81 Collectively, spatial and lateral orientations of protein subunits of the ETC organization corroborate our current understanding of the ETC organization in mammalian mitochondria.
While a clear picture of the ETC supercomplex has come into view, the biological purpose of the supercomplex is less understood. Studies conflict as to whether the supercomplex is functionally capable of oxidative phosphorylation.82–84 On the other hand, various alternative functions for the supercomplex have been proposed, such as substrate channeling or limiting the generation of reactive oxygen species.74 Thus, functional characterization of the ETC supercomplex inside cells largely remains elusive.
NUCLEOTIDE BIOSYNTHESIS
Purine Biosynthesis
Purine nucleotides are essential molecules, used in the cell for a variety of purposes such as DNA and RNA metabolism, cell signaling, and cellular energetics. In rapidly growing cells, the biosynthesis of purine nucleotides is fully promoted via a salvage pathway as well as a de novo pathway. The latter 10-step process is catalyzed by six enzymes including three multifunctional enzymes in human cells, converting phosphoribosyl pyrophosphate into inosine monophosphate (Figure 1A). Interestingly, one enzyme, trifunctional glycinamide ribonucleotide transformylase, catalyzes three nonsequential steps of de novo purine biosynthesis, suggesting that the enzymes in de novo purine biosynthesis may interact with each other and thus form a multienzyme complex to control purine flux.85 Indeed, this hypothesis was initially supported by copurification experiments revealing the activities of multiple purine biosynthetic enzymes in the same fraction of tissue extracts.86,87 However, it has been difficult to experimentally demonstrate direct protein–protein interactions or complex formation in vitro among the pathway enzymes. Nevertheless, earlier experimental data have suggested the potential existence of multienzyme complexes for this pathway in cells.
Recently, a metabolic complex of purine biosynthetic enzymes, namely, the “purinosome,” was identified in living human cells under purine deprivation.88 The reversible nature of purinosome assemblies was demonstrated in response to purine levels, indicating their functional contribution to the cells.88 Later, purinosome formation was positively correlated with increased levels of purine metabolites, compared to cells lacking purinosomes, thus corroborating the metabolic activity of purinosomes in live cells.89,90 Biochemical and biophysical studies have also proposed that three enzymes involved in the first half of the pathway (steps 1–5) form a core structure of the purinosome, while the other three enzymes, catalyzing steps 6 through 10, are dynamically associated with the core complex via protein–protein interactions.91,92 Furthermore, knockout of any purine biosynthetic enzyme resulted in either the reduction or abolishment of purinosome association in human cells.93 More recently, the functional activity of mechanistic target of rapamycin (mTOR) was linked to the spatial association of purinosomes with the mitochondria as well as purine biosynthesis.94,95 Collectively, the formation of purinosomes, which indicates the upregulation of de novo purine biosynthesis, has significantly advanced our understanding of the regulatory mechanisms of de novo purine biosynthesis in human cells.96–98
In addition, we have recently identified a sequestrationmediated downregulation mechanism of de novo purine biosynthesis. The basal level activity of de novo purine biosynthesis has been detected in the absence of purinosome assemblies or under conditions in which purinosome formation was not favorable.89,90,99 These data suggest that purinosomenegative cells maintain a certain level of metabolic activity of de novo purine biosynthesis, and questions if de novo purine biosynthesis is downregulated in human cells. Excitingly, we have identified that AMP-dependent protein kinase (AMPK) promotes the spatial sequestration of one of the purinosome core enzymes into its own self-assemblies for downregulation of de novo purine biosynthesis in HeLa cells.100 Therefore, it has become clear that spatial assemblies of purine biosynthetic enzymes can regulate the metabolic activity of purine metabolism in living human cells.
Furthermore, other enzymes in purine metabolism appear to form cytoplasmic structures in cells. Inosine monophosphate dehydrogenase 2 (IMPDH2) and adenylosuccinate synthase were demonstrated to be part of the purinosome clusters in HeLa cells, indicating the participation of other purine enzymes in the purinosome assembly.90 Alternatively, IMPDH2 was shown to form cytoplasmic rod and ring structures in the cytoplasm of various mammalian cells.101–104 Such rod and ring structures of IMPDH2 were promoted in mammalian cells by glutamine, serine, or glycine starvation.105,106 Conversely, however, the cytoplasmic structures of IMPDH were found to dissociate in mouse pancreatic islets, but not in other tissues, under fasting conditions in mice.103 Collectively, the enzymes which catalyze de novo adenine and guanine biosynthesis appear to be associated with the purinosome, while some are capable of forming their own independent cytoplasmic structures.
Pyrimidine Biosynthesis
De novo pyrimidine biosynthesis is a nine-step pathway catalyzing the conversion of L-glutamine to CTP, utilizing four enzymes, two of which are multifunctional (Figure 1B). Unlike de novo purine biosynthesis, the formation of a sequential multienzyme complex catalyzing pyrimidine biosynthesis has not been systematically investigated, likely because the enzymes are not all cytoplasmic.107 However, there is evidence for the oligomerization of single enzymes involved in pyrimidine biosynthesis.
The multifunctional enzyme catalyzing the first three steps of pyrimidine biosynthesis, with carbamoyl phosphate synthetase, aspartate transcarbamylase, and dihydroorotase (CAD) activities, has been found to form clusters within mammalian cells.96,108–110 The formation of CAD clusters is phosphorylation-dependent.108 Specifically, active mTORC1 has been found to promote the clustering of CAD through ribosomal protein S6 kinase beta-1, resulting in the upregulation of pyrimidine biosynthesis.109–111 Therefore, the compartmentalization of CAD, controlled by post translational modifications, appears to control pyrimidine biosynthesis.
There have also been extensive investigations into the spatial assembly of cytidine triphosphate synthase (CTPS), which catalyzes the final step of pyrimidine biosynthesis. Briefly, CTPS has been shown to form filament structures in bacteria, fly, yeast, and human cells.102,112–118 In vitro investigations of the filament structure of E. coli CTPS have found that CTPS is assembled into tetramers stacked upon each other to promote bacteria curvature as cytoskeletal elements.113,119 In addition, the expression of the transcription factor Myc is positively associated with induction of CTPS filaments, while nonreceptor tyrosine kinase Ack appears to control the formation of CTPS filaments to regulate CTPS activity.120,121 Direct ubiquitination of CTPS seems to be negatively associated with the formation of CTPS filaments in Drosophila and human cell lines.122,123 Nevertheless, it appears that the CTPS filaments are composed of metabolically inactive CTPS.119 This notion was further corroborated by evidence that the formation of CTPS filaments was induced by inhibition of CTPS activity in Drosophila and various vertebrate tissues including human cancer cells.102,115,124 Therefore, it will be interesting to investigate how the other mitochondrial enzyme involved in de novo pyrimidine biosynthesis spatially and/or temporally perform sequential reactions with the cytoplasmic structures of CAD or CTPS in the cell.
LIPID METABOLISM
Fatty Acid Synthesis
Fatty acid synthesis is the process in which the cell utilizes carbohydrates to make fatty acids for lipid membranes, protein modifications, hormone synthesis, and energy storage. In fatty acid synthesis, an activated acetate or an acyl chain is extended by two carbons in four steps, and repeated until forming a chain containing 16 or 18 carbon units (Figure 1A). All the steps in fatty acid synthesis are catalyzed by a multifunctional fatty acid synthase (FAS), which can range in size from 540 kDa to 2.6 MDa.125 Interestingly, type I FAS, expressed in mammals and fungi, contains all enzymatic activities of fatty acid synthesis on one or two polypeptide chains. On the other hand, prokaryotes, chloroplasts, and mitochondria express type II FAS, where each step of fatty acid synthesis is performed by an individual enzyme, requiring the coordination of those enzymes. Since this system has been extensively reviewed recently,125–127 we focus on recent investigations into the structure and the cellular compartmentalization of FAS, which have shed light on the process of fatty acid synthesis.
Essential to fatty acid synthesis is the movement of the growing acyl chain from one active site to the next. Structures of FAS from Thermomyces lanuginosus and S. cerevisiae have revealed that FAS contains a “reaction chamber” in which the acyl carrier protein shuttles the substrate from one active site to the other.128–130 Single particle cryoelectron microscopy has further revealed a variety of conformations of rat FAS, which support dynamic conformational changes of FAS during fatty acid synthesis.131 Thus, the macromolecular structure of FAS provides a mechanistic insight of how recurring sequential reactions are orchestrated from one active site to the other between multiple enzymatic activities.
In addition, the subcellular location of FAS has been investigated along with other lipid biosynthetic enzymes in cells. In yeast, fatty acid synthesis is accomplished by two subunits, Fas1 and Fas2, which colocalize into cytoplasmic clusters in the quiescence stage of growth as visualized by fluorescence microscopy.132 The FAS sequestered into clusters retains its activity, indicating the clusters are not aggregates or misfolded proteins.132 Glucose starvation in yeast also independently promoted the clustering of other biosynthetic enzymes utilizing fatty acids, including acetyl-CoA carboxylase in the cytoplasm, phosphatidylinositol synthase in the endoplasmic reticulum, and phosphatidylserine decarboxylase in the mitochondria.132 Furthermore, in human cells, FAS was identified to interact with ATP-citrate lyase and fatty acid transporters on the peroxisome membrane by bioluminescence resonance energy transfer.133 Therefore, these subcellular localization studies indicate the potential dynamics of FAS to localize with various cellular compartments.
NATURAL PRODUCT BIOSYNTHESIS
Polyketide and Non-Ribosomal Peptide Synthesis
Several natural products, which are commonly used as antibacterials, antifungals, or toxins, are biosynthetically synthesized by polyketide synthases or nonribosomal peptide synthetases, utilizing carboxylic acids or amino acids, respectively.134–138 Like fatty acid synthesis, the biosynthesis of such natural products occurs in a linear fashion by one macromolecular complex containing multiple active sites, or through sequential reactions of many smaller enzymes. Given the mechanistic similarity between fatty acid synthesis and natural product biosynthesis, we briefly summarize here the subcellular localization of metabolic enzymes involved in natural product biosynthesis.
In bacteria, natural product metabolism often occurs in membrane-bound vesicles. For example, in Aspergillus fumigatus and Aspergillus nidulans, enzymes involved in the early steps of melanin biosynthesis are localized to endosomes, whereas enzymes involved in the later steps of the synthesis are localized to the cell wall by the palmitoylation of the enzymes.139,140 Furthermore, these enzymes interact with each other in their respective subcellular locations, possibly allowing substrate channeling for melanin biosynthesis. A similar strategy localizing biosynthetic enzymes in vesicles is also used to synthesize the toxin aflatoxin in Aspergillus parasiticus.141,142 The number of the aflatoxin-synthesizing vesicles is increased in response to the promotion of aflatoxin biosynthesis, followed by the transportation of aflatoxin out of the cell by exocytosis.143 Thus, some organisms have a mechanism to organize biosynthetic enzymes into lipid vesicles for biosynthesis and secretion of complex biomolecules.
In contrast to the vesicle-mediated biosynthesis of natural products, several species of bacteria appear to produce natural products in large nonmembrane bound enzyme complexes. For example, Pseudomonas aeruginosa promotes localization of biosynthetic enzymes involved in siderophore biosynthesis to the cell membrane.144,145 The enzyme complex, so-called “siderosome,” is membrane-associated at the bacterial poles at the early exponential phase of growth through weak protein–protein interactions.144 Since siderophores are essential biomolecules for pathologic bacteria, and serve as iron scavengers for cellular function, it appears that cells do not need to export these biomolecules through the vesicle-mediated exocytosis. In addition, the natural product bacillaene in Bacillus subtilis is produced by a large (~2.5 MDa) hybrid non-ribosomal peptide synthetase and polyketide synthase enzyme complex.146,147 The enzyme complex does not appear to be membrane-bound, but fluorescence microscopy and transmission electron microscopy revealed the complex to associate near the cell membrane.146 Taken together, these examples indicate that enzymes in natural product biosynthesis form spatial organizations for efficient regulation of their metabolic products in cells.
AMINO ACID METABOLISM
Amino acids serve as the building blocks of peptides and proteins, as well as metabolic intermediates. The 20 canonical amino acids can be biosynthetically made by bacteria and plants, nine of which are essential amino acids for mammals. While the knowledge of amino acid metabolism has spanned decades, we are just now beginning to understand how amino acid metabolism is compartmentalized in the cell.148–150 Herein, we review evidence for prospective amino acid metabolic multienzyme complexes, which have yet to be clearly defined.
The biosynthesis of aromatic amino acids in plants, prokaryotes, ascomycete fungi, and apicomplexans is accomplished by the shikimate pathway.151 The shikimate pathway is composed of seven reactions catalyzed by seven enzymes, converting phosphoenolpyruvate and erythrose-4-phosphate to chorismate.148,152 Chorismate is a precursor to the aromatic amino acids, including phenylalanine, tryptophan, and tyrosine. This pathway has been extensively studied in Mycobacterium tuberculosis, wherein the direct interaction between 3-deoxy-D-arabino-heptulosonate-7-phosphate (DAHP) synthase and chorismate mutase, which catalyze the first and seventh steps of the pathway, significantly increased their metabolic activities compared to the enzymes alone.153,154 Crystal structures of DAHP synthase interacting with chorismate mutase revealed that the binding of tryptophan and phenylalanine to DAHP synthase downregulates the activities of both enzymes in the complex.155 Although these enzymes do not catalyze sequential steps, their interaction appears to be vital to the production of aromatic amino acids. It seems that the protein-protein interaction may provide the basis for an interaction of all the enzymes in the shikimate pathway and ultimately other enzymes involved in aromatic amino acid biosynthesis.
The catabolism of the essential branched chain amino acids, leucine, isoleucine, and valine, is accomplished by the branched-chain α-keto acid dehydrogenase complex. The protein complex is composed of a core of dihydrolipoyl transacylase subunits, associated with branched-chain α-keto acid decarboxylase/dehydrogenase, dihydrolipoamide dehydrogenase, branched-chain α-keto acid dehydrogenase complex kinase and phosphatase.156 Affinity chromatographic studies investigating other proteins associated with the complex in mammalian systems revealed weak protein-protein interactions between the branched-chain α-keto acid decarboxylase/dehydrogenase component of the complex and mitochondrial B6-dependent branched chain aminotransferase.156 Furthermore, this interaction increases the decarboxylation of branched-chain α-keto acids, possibly through substrate channeling. Meanwhile, glutamate dehydrogenase and pyruvate carboxylase are also found to be the components of the so called “branched-chain amino acid metabolon”.157 The binding of glutamate dehydrogenase to the metabolon resulted in more efficient channeling of products to oxidative pathways, proposing a functional role of the metabolon in branched chain amino acid catabolism.157 Therefore, the compartmentalization of amino acid catabolism has been hypothesized to promote substrate channeling.
CONCLUDING REMARKS
Metabolism is accomplished through the spatial compartmentalization of metabolic enzymes into vesicles, membranes, cellular organelles, or nonmembrane bound cellular granules in the cytoplasm. As it is reviewed in this article, there is extensive evidence for the formation of metabolic complexes in nature. Along with the rich history studying metabolic enzymes and their complexes in vitro, our understanding of the intracellular compartmentalization of metabolism has significantly advanced in recent years due to the advancement of intracellular biochemical and biophysical techniques. Of particular, recent endeavors on de novo purine biosynthesis in living human cells have shed light on the paradigm that “spatial assemblies of sequential metabolic enzymes can regulate metabolic activities of the pathway in living cells”.88,100
However, our understanding of each multienzyme complex and its functional contributions to cell metabolism is mostly at its infancy. The existence of multienzyme complexes has long been thought to facilitate in substrate channeling and thus influence metabolism.20,86,87,158,159 By localizing active sites close to one another, sequential metabolic enzymes may benefit to not only increase metabolic efficiency, but also limit the diffusion of toxic or unstable intermediates.13–15,40,160–162 However, it has been challenging to structurally and kinetically demonstrate such substrate channels among more than three sequential enzymes. Alternatively, cluster-mediated channeling has been recently proposed to explain the metabolic benefit of the spatial assembly of sequential metabolic enzymes in cells.14,163 In this case, rather than physical coordination between active sites, only colocalization of sequential enzymes seems to be enough to promote metabolic efficiency.14,163 On the other hand, it is also important to note here that metabolic enzyme complexes have been formed to play as intracellular depot systems, demonstrating that the formation of metabolic complex is not the direct indication of metabolic flux enhancement.164 Various spatial assemblies are also formed by single enzymes or only a subset of enzymes of given pathways, further indicating potential functional diversities of spatial metabolic assemblies in cells beyond flux enhancement.35,100,119 It is clear that there is still much to be learned about biological significance of the compartmentalization of metabolic enzymes in cells.
Collectively, a combination of in vitro and intracellular investigations of metabolic enzymes and their complexes will accelerate our endeavors to understand the functional and structural significance of metabolic organizations and their regulatory mechanisms in the context of the cellular milieu. Ultimately, in vitro and cellular biochemistry will provide the fundamental principles of how metabolism is orchestrated in space and time at molecular levels. This new level of understanding will divulge the importance of heretofore unrecognized metabolic compartments as novel targets for therapeutic intervention, thereby contributing to public health and welfare.
Acknowledgments
We would like to thank M. Jeon for her assistance in literature survey and Dr. R Karpel for critically reviewing the manuscript.
Funding
We thank UMBC for providing the start-up funds (S.A.). This work was also supported in part by the 2016 AACR-Bayer Innovation and Discovery Grant (#16-80-44-ANSO; S.A.). D.L.S. was supported by NIH/NIGMS T32GM066706.
Footnotes
ORCID
Songon An: 0000-0003-2189-7374
Notes
The authors declare no competing financial interest.
References
- 1.Ellis RJ. Macromolecular crowding: obvious but underappreciated. Trends Biochem Sci. 2001;26:597–604. doi: 10.1016/s0968-0004(01)01938-7. [DOI] [PubMed] [Google Scholar]
- 2.Cohen RD, Pielak GJ. A cell is more than the sum of its (dilute) parts: A brief history of quinary structure. Protein Sci. 2017;26:403–413. doi: 10.1002/pro.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilson MZ, Gitai Z. Beyond the cytoskeleton: mesoscale assemblies and their function in spatial organization. Curr Opin Microbiol. 2013;16:177–183. doi: 10.1016/j.mib.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barry RM, Gitai Z. Self-assembling enzymes and the origins of the cytoskeleton. Curr Opin Microbiol. 2011;14:704–711. doi: 10.1016/j.mib.2011.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Havugimana PC, Hart GT, Nepusz T, Yang H, Turinsky AL, Li Z, Wang PI, Boutz DR, Fong V, Phanse S, Babu M, Craig SA, Hu P, Wan C, Vlasblom J, Dar VUN, Bezginov A, Clark GW, Wu GC, Wodak SJ, Tillier ERM, Paccanaro A, Marcotte EM, Emili A. A census of human soluble protein complexes. Cell. 2012;150:1068–1081. doi: 10.1016/j.cell.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rhee HW, Zou P, Udeshi ND, Martell JD, Mootha VK, Carr SA, Ting AY. Proteomic mapping of mitochondria in living cells via spatially restricted enzymatic tagging. Science. 2013;339:1328–1331. doi: 10.1126/science.1230593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wan C, Borgeson B, Phanse S, Tu F, Drew K, Clark G, Xiong X, Kagan O, Kwan J, Bezginov A, Chessman K, Pal S, Cromar G, Papoulas O, Ni Z, Boutz DR, Stoilova S, Havugimana PC, Guo X, Malty RH, Sarov M, Greenblatt J, Babu M, Derry WB, Tillier ER, Wallingford JB, Parkinson J, Marcotte EM, Emili A. Panorama of ancient metazoan macromolecular complexes. Nature. 2015;525:339–344. doi: 10.1038/nature14877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ori A, Iskar M, Buczak K, Kastritis P, Parca L, Andrés-Pons A, Singer S, Bork P, Beck M. Spatiotemporal variation of mammalian protein complex stoichiometries. Genome Biol. 2016;17:47. doi: 10.1186/s13059-016-0912-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De La Fuente IM, Martínez L, Pérez-Samartín AL, Ormaetxea L, Amezaga C, Vera-López A. Global self-organization of the cellular metabolic structure. PLoS One. 2008;3:e3100. doi: 10.1371/journal.pone.0003100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Connell JD, Zhao A, Ellington AD, Marcotte EM. Dynamic reorganization of metabolic enzymes into intracellular bodies. Annu Rev Cell Dev Biol. 2012;28:89–111. doi: 10.1146/annurev-cellbio-101011-155841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vöpel T, Makhatadze GI. Enzyme activity in the crowded milieu. PLoS One. 2012;7:e39418. doi: 10.1371/journal.pone.0039418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu I, Mori T, Ando T, Harada R, Jung J, Sugita Y, Feig M. Biomolecular interactions modulate macromolecular structure and dynamics in atomistic model of a bacterial cytoplasm. eLife. 2016;5:e19274. doi: 10.7554/eLife.19274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bauler P, Huber G, Leyh T, McCammon JA. Channeling by proximity: The catalytic advantages of active site colocalization using Brownian Dynamics. J Phys Chem Lett. 2010;1:1332–1335. doi: 10.1021/jz1002007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castellana M, Wilson MZ, Xu Y, Joshi P, Cristea IM, Rabinowitz JD, Gitai Z, Wingreen NS. Enzyme clustering accelerates processing of intermediates through metabolic channeling. Nat Biotechnol. 2014;32:1011–1018. doi: 10.1038/nbt.3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amar P, Legent G, Thellier M, Ripoll C, Bernot G, Nystrom T, Saier MH, Norris V. A stochastic automaton shows how enzyme assemblies may contribute to metabolic efficiency. BMC Syst Biol. 2008;2:27–13. doi: 10.1186/1752-0509-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krewald V, Retegan M, Pantazis DA. Principles of natural photosynthesis. In: Davies TG, Hyvönen M, editors. Fragment-Based Drug Discovery and X-Ray Crystallography. Springer; International Publishing, Switzerland: 2015. pp. 23–48. [Google Scholar]
- 17.Shen JR. The structure of Photosystem II and the mechanism of water oxidation in photosynthesis. Annu Rev Plant Biol. 2015;66:23–48. doi: 10.1146/annurev-arplant-050312-120129. [DOI] [PubMed] [Google Scholar]
- 18.Costa A, Hood IV, Berger JM. Mechanisms for initiating cellular DNA replication. Annu Rev Biochem. 2013;82:25–54. doi: 10.1146/annurev-biochem-052610-094414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de la Cruz J, Karbstein K, Woolford JL., Jr Functions of ribosomal proteins in assembly of eukaryotic ribosomes in vivo. Annu Rev Biochem. 2015;84:93–129. doi: 10.1146/annurev-biochem-060614-033917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Srere PA. Complexes of sequential metabolic enzymes. Annu Rev Biochem. 1987;56:89–124. doi: 10.1146/annurev.bi.56.070187.000513. [DOI] [PubMed] [Google Scholar]
- 21.Opperdoes FR, Borst P. Localization of nine glycolytic enzymes in a microbody-like organelle in Trypanosoma brucei: the glycosome. FEBS Lett. 1977;80:360–364. doi: 10.1016/0014-5793(77)80476-6. [DOI] [PubMed] [Google Scholar]
- 22.Haanstra JR, González-Marcano EB, Gualdrón-López M, Michels PAM. Biogenesis, maintenance and dynamics of glycosomes in trypanosomatid parasites. Biochim Biophys Acta, Mol Cell Res. 2016;1863:1038–1048. doi: 10.1016/j.bbamcr.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 23.Parsons M, Furuya T, Pal S, Kessler P. Biogenesis and function of peroxisomes and glycosomes. Mol Biochem Parasitol. 2001;115:19–28. doi: 10.1016/s0166-6851(01)00261-4. [DOI] [PubMed] [Google Scholar]
- 24.Tetley L, Vickerman K. The glycosomes of trypanosomes: number and distribution as revealed by electron spectroscopic imaging and 3-D reconstruction. J Microsc. 1991;162:83–90. doi: 10.1111/j.1365-2818.1991.tb03118.x. [DOI] [PubMed] [Google Scholar]
- 25.Cull B, Prado Godinho JL, Fernandes Rodrigues JC, Frank B, Schurigt U, Williams RA, Coombs GH, Mottram JC. Glycosome turnover in Leishmania major is mediated by autophagy. Autophagy. 2014;10:2143–2157. doi: 10.4161/auto.36438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Colasante C, Ellis M, Ruppert T, Voncken F. Comparative proteomics of glycosomes from bloodstream form and procyclic culture form Trypanosoma brucei brucei. Proteomics. 2006;6:3275–3293. doi: 10.1002/pmic.200500668. [DOI] [PubMed] [Google Scholar]
- 27.Guerra-Giraldez C, Quijada L, Clayton CE. Compartmentation of enzymes in a microbody, the glycosome, is essential in Trypanosoma brucei. J Cell Sci. 2002;115:2651–2658. doi: 10.1242/jcs.115.13.2651. [DOI] [PubMed] [Google Scholar]
- 28.Furuya T, Kessler P, Jardim A, Schnaufer A, Crudder C, Parsons M. Glucose is toxic to glycosome-deficient trypanosomes. Proc Natl Acad Sci U S A. 2002;99:14177–14182. doi: 10.1073/pnas.222454899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bauer S, Morris JC, Morris MT. Environmentally regulated glycosome protein composition in the African trypanosome. Eukaryotic Cell. 2013;12:1072–1079. doi: 10.1128/EC.00086-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bakker BM, Mensonides FI, Teusink B, van Hoek P, Michels PA, Westerhoff HV. Compartmentation protects trypanosomes from the dangerous design of glycolysis. Proc Natl Acad Sci U S A. 2000;97:2087–2092. doi: 10.1073/pnas.030539197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matthews KR. 25 years of African trypanosome research: From description to molecular dissection and new drug discovery. Mol Biochem Parasitol. 2015;200:30–40. doi: 10.1016/j.molbiopara.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brandina I, Graham J, Lemaitre-Guillier C, Entelis N, Krasheninnikov I, Sweetlove L, Tarassov I, Martin RP. Enolase takes part in a macromolecular complex associated to mitochondria in yeast. Biochim Biophys Ada, Bioenerg. 2006;1757:1217–1228. doi: 10.1016/j.bbabio.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 33.Miura N, Shinohara M, Tatsukami Y, Sato Y, Morisaka H, Kuroda K, Ueda M. Spatial reorganization of Saccharomyces cerevisiae enolase to alter carbon metabolism under hypoxia. Eukaryotic Cell. 2013;12:1106–1119. doi: 10.1128/EC.00093-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Araiza-Olivera D, Chiquete-Felix N, Rosas-Lemus M, Sampedro JG, Pena A, Mujica A, Uribe-Carvajal S. A glycolytic metabolon in Saccharomyces cerevisiaeis stabilized by F-actin. FEBS J. 2013;280:3887–3905. doi: 10.1111/febs.12387. [DOI] [PubMed] [Google Scholar]
- 35.Shen QJ, Kassim H, Huang Y, Li H, Zhang J, Li G, Wang PY, Yan J, Ye F, Liu JL. Filamentation of metabolic enzymes in Saccharomyces cerevisiae. J Genet Genomics. 2016;43:393–404. doi: 10.1016/j.jgg.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li H, Huang Y, Wang PY, Ye F, Liu JL. Data on dynamic study of cytoophidia in Saccharomyces cerevisiae. Data Brief. 2016;8:40–44. doi: 10.1016/j.dib.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aryal UK, Xiong Y, McBride Z, Kihara D, Xie J, Hall MC, Szymanski DB. A proteomic strategy for global analysis of plant protein complexes. Plant Cell. 2014;26:3867–3882. doi: 10.1105/tpc.114.127563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arrivault S, Guenther M, Florian A, Encke B, Feil R, Vosloh D, Lunn JE, Sulpice R, Fernie AR, Stitt M, Schulze WX. Dissecting the subcellular compartmentation of proteins and metabolites in Arabidopsis leaves using non-aqueous fractionation. Mol Cell Proteomics. 2014;13:2246–2259. doi: 10.1074/mcp.M114.038190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giege P. Enzymes of glycolysis are functionally associated with the mitochondrion in Arabidopsis cells. Plant Cell. 2003;15:2140–2151. doi: 10.1105/tpc.012500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sweetlove LJ, Fernie AR. The spatial organization of metabolism within the plant cell. Annu Rev Plant Biol. 2013;64:723–746. doi: 10.1146/annurev-arplant-050312-120233. [DOI] [PubMed] [Google Scholar]
- 41.Duncan O, Taylor NL, Carrie C, Eubel H, Kubiszewski-Jakubiak S, Zhang B, Narsai R, Millar AH, Whelan J. Multiple lines of evidence localize signaling, morphology, and lipid biosynthesis machinery to the mitochondrial outer membrane of Arabidopsis. Plant Physiol. 2011;157:1093–1113. doi: 10.1104/pp.111.183160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Graham JWA, Williams TCR, Morgan M, Fernie AR, Ratcliffe RG, Sweetlove LJ. Glycolytic enzymes associate dynamically with mitochondria in response to respiratory demand and support substrate channeling. Plant Cell. 2007;19:3723–3738. doi: 10.1105/tpc.107.053371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sullivan DT. Analysis of glycolytic enzyme co-localization in Drosophila flight muscle. J Exp Biol. 2003;206:2031–2038. doi: 10.1242/jeb.00367. [DOI] [PubMed] [Google Scholar]
- 44.Wojtas K, Slepecky N, von Kalm L, Sullivan D. Flight muscle function in Drosophila requires colocalization of glycolytic enzymes. Mol Biol Cell. 1997;8:1665–1675. doi: 10.1091/mbc.8.9.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jang S, Nelson JC, Bend EG, Rodríguez-Laureano L, Tueros FG, Cartagenova L, Underwood K, Jorgensen EM, Colón-Ramos DA. Glycolytic enzymes localize to synapses under energy stress to support synaptic function. Neuron. 2016;90:278–291. doi: 10.1016/j.neuron.2016.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Campanella ME, Chu H, Low PS. Assembly and regulation of a glycolytic enzyme complex on the human erythrocyte membrane. Proc Natl Acad Sci U S A. 2005;102:2402–2407. doi: 10.1073/pnas.0409741102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chu H, Low PS. Mapping of glycolytic enzymebinding sites on human erythrocyte band 3. Biochem J. 2006;400:143–151. doi: 10.1042/BJ20060792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Campanella ME, Chu H, Wandersee NJ, Peters LL, Mohandas N, Gilligan DM, Low PS. Characterization of glycolytic enzyme interactions with murine erythrocyte membranes in wild-type and membrane protein knockout mice. Blood. 2008;112:3900–3906. doi: 10.1182/blood-2008-03-146159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Puchulu-Campanella E, Chu H, Anstee DJ, Galan JA, Tao WA, Low PS. Identification of the components of a glycolytic enzyme metabolon on the human red blood cell membrane. J Biol Chem. 2013;288:848–858. doi: 10.1074/jbc.M112.428573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chu H, Breite A, Ciraolo P, Franco RS, Low PS. Characterization of the deoxyhemoglobin binding site on human erythrocyte band 3: implications for O2 regulation of erythrocyte properties. Blood. 2008;111:932–938. doi: 10.1182/blood-2007-07-100180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lewis IA, Campanella ME, Markley JL, Low PS. Role of band 3 in regulating metabolic flux of red blood cells. Proc Natl Acad Sci U S A. 2009;106:18515–18520. doi: 10.1073/pnas.0905999106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Agbor TA, Cheong A, Comerford KM, Scholz CC, Bruning U, Clarke A, Cummins EP, Cagney G, Taylor CT. Small ubiquitin-related modifier (SUMO)-1 promotes glycolysis in hypoxia. J Biol Chem. 2011;286:4718–4726. doi: 10.1074/jbc.M110.115931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kohnhorst CL, Kyoung M, Jeon M, Schmitt DL, Kennedy EL, Ramirez J, Bracey SM, Luu BT, Russell SJ, An S. Identification of a multienzyme complex for glucose metabolism in living cells. J Biol Chem. 2017;292:9191–9203. doi: 10.1074/jbc.M117.783050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Patel MS, Nemeria NS, Furey W, Jordan F. The pyruvate dehydrogenase complexes: structure-based function and regulation. J Biol Chem. 2014;289:16615–16623. doi: 10.1074/jbc.R114.563148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou ZH, McCarthy DB, O’Connor CM, Reed LJ, Stoops JK. The remarkable structural and functional organization of the eukaryotic pyruvate dehydrogenase complexes. Proc Natl Acad Sci U S A. 2001;98:14802–14807. doi: 10.1073/pnas.011597698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Patel MS, Korotchkina LG, Sidhu S. Interaction of E1 and E3 components with the core proteins of the human pyruvate dehydrogenase complex. J Mol Catal B: Enzym. 2009;61:2–6. doi: 10.1016/j.molcatb.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saunier E, Benelli C, Bortoli S. The pyruvate dehydrogenase complex in cancer: An old metabolic gatekeeper regulated by new pathways and pharmacological agents. Int J Cancer. 2016;138:809–817. doi: 10.1002/ijc.29564. [DOI] [PubMed] [Google Scholar]
- 58.Roche TE, Hiromasa Y, Turkan A, Gong X, Peng T, Yan X, Kasten SA, Bao H, Dong J. Essential roles of lipoyl domains in the activated function and control of pyruvate dehydrogenase kinases and phosphatase isoform 1. Eur J Biochem. 2003;270:1050–1056. doi: 10.1046/j.1432-1033.2003.03468.x. [DOI] [PubMed] [Google Scholar]
- 59.Patel MS, Korotchkina LG. Regulation of the pyruvate dehydrogenase complex. Biochem Soc Trans. 2006;34:217–222. doi: 10.1042/BST20060217. [DOI] [PubMed] [Google Scholar]
- 60.Jeoung NH. Pyruvate dehydrogenase kinases: therapeutic targets for diabetes and cancers. Diabetes Metab J. 2015;39:188–197. doi: 10.4093/dmj.2015.39.3.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rardin MJ, Wiley SE, Naviaux RK, Murphy AN, Dixon JE. Monitoring phosphorylation of the pyruvate dehydrogenase complex. Anal Biochem. 2009;389:157–164. doi: 10.1016/j.ab.2009.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hitosugi T, Fan J, Chung TW, Lythgoe K, Wang X, Xie J, Ge Q, Gu TL, Polakiewicz RD, Roesel JL, Chen GZ, Boggon TJ, Lonial S, Fu H, Khuri FR, Kang S, Chen J. Tyrosine phosphorylation of mitochondrial pyruvate dehydrogenase kinase 1 is important for cancer metabolism. Mol Cell. 2011;44:864–877. doi: 10.1016/j.molcel.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sutendra G, Kinnaird A, Dromparis P, Paulin R, Stenson TH, Haromy A, Hashimoto K, Zhang N, Flaim E, Michelakis ED. A nuclear pyruvate dehydrogenase complex is important for the generation of acetyl-CoA and histone acetylation. Cell. 2014;158:84–97. doi: 10.1016/j.cell.2014.04.046. [DOI] [PubMed] [Google Scholar]
- 64.Tang BL. Mitochondrial protein in the nucleus. CellBio. 2015;4:23–29. [Google Scholar]
- 65.Halper LA, Srere PA. Interaction between citrate synthase and mitochondrial malate dehydrogenase in the presence of polyethylene glycol. Arch Biochem Biophys. 1977;184:529–534. doi: 10.1016/0003-9861(77)90462-3. [DOI] [PubMed] [Google Scholar]
- 66.Robinson JB, Inman L, Sumegi B, Srere PA. Further characterization of the Krebs tricarboxylic acid cycle metabolon. J Biol Chem. 1987;262:1786–1790. [PubMed] [Google Scholar]
- 67.Meyer FM, Gerwig J, Hammer E, Herzberg C, Commichau FM, Völker U, Stülke J. Physical interactions between tricarboxylic acid cycle enzymes in Bacillus subtilis evidence for a metabolon. Metab Eng. 2011;13:18–27. doi: 10.1016/j.ymben.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 68.Mitchell CG. Identification of a multienzyme complex of the tricarboxylic acid cycle enzymes containing citrate synthase isoenzymes from Pseudomonas aeruginosa. Biochem J. 1996;313:769–774. doi: 10.1042/bj3130769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Haggie PM, Verkman AS. Diffusion of tricarboxylic acid cycle enzymes in the mitochondrial matrix in vivo: evidence for restricted mobility of a multienzyme complex. J Biol Chem. 2002;277:40782–40788. doi: 10.1074/jbc.M207456200. [DOI] [PubMed] [Google Scholar]
- 70.Wu F, Minteer S. Krebs cycle metabolon: structural evidence of substrate channeling revealed by cross-linking and mass spectrometry. Angew Chem, Int Ed. 2015;54:1851–1854. doi: 10.1002/anie.201409336. [DOI] [PubMed] [Google Scholar]
- 71.Wu F, Pelster LN, Minteer SD. Krebs cycle metabolon formation: metabolite concentration gradient enhanced compartmentation of sequential enzymes. Chem Commun. 2015;51:1244–1247. doi: 10.1039/c4cc08702j. [DOI] [PubMed] [Google Scholar]
- 72.Bulutoglu B, Garcia KE, Wu F, Minteer SD, Banta S. Direct evidence for metabolon formation and substrate channeling in recombinant TCA cycle enzymes. ACS Chem Biol. 2016;11:2847–2853. doi: 10.1021/acschembio.6b00523. [DOI] [PubMed] [Google Scholar]
- 73.Bartholomae M, Meyer FM, Commichau FM, Burkovski A, Hillen W, Seidel G. Complex formation between malate dehydrogenase and isocitrate dehydrogenase from Bacillus subtilisis regulated by tricarboxylic acid cycle metabolites. FEBS J. 2014;281:1132–1143. doi: 10.1111/febs.12679. [DOI] [PubMed] [Google Scholar]
- 74.Enriquez JA, Lenaz G. Coenzyme q and the respiratory chain: coenzyme q pool and mitochondrial super-complexes. Mol Syndromol. 2014;5:119–140. doi: 10.1159/000363364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schägger H, Pfeiffer K. Supercomplexes in the respiratory chains of yeast and mammalian mitochondria. EMBO J. 2000;19:1777–1783. doi: 10.1093/emboj/19.8.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Letts JA, Degliesposti G, Fiedorczuk K, Skehel M, Sazanov LA. Purification of ovine respiratory complex I results in a highly active and stable preparation. J Biol Chem. 2016;291:24657–24675. doi: 10.1074/jbc.M116.735142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schweppe DK, Chavez JD, Lee CF, Caudal A, Kruse SE, Stuppard R, Marcinek DJ, Shadel GS, Tian R, Bruce JE. Mitochondrial protein interactome elucidated by chemical cross-linking mass spectrometry. Proc Natl Acad Sci U S A. 2017;114:1732–1737. doi: 10.1073/pnas.1617220114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cogliati S, Calvo E, Loureiro M, Guaras AM, Nieto-Arellano R, Garcia-Poyatos C, Ezkurdia I, Mercader N, Vazquez J, Enriquez JA. Mechanism of super-assembly of respiratory complexes III and IV. Nature. 2016;539:579–582. doi: 10.1038/nature20157. [DOI] [PubMed] [Google Scholar]
- 79.Winge DR. Sealing the mitochondrial respirasome. Mol Cell Biol. 2012;32:2647–2652. doi: 10.1128/MCB.00573-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hung V, Zou P, Rhee HW, Udeshi ND, Cracan V, Svinkina T, Carr SA, Mootha VK, Ting AY. Proteomic mapping of the human mitochondrial intermembrane space in live cells via ratiometric APEX tagging. Mol Cell. 2014;55:332–341. doi: 10.1016/j.molcel.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen CL, Hu Y, Udeshi ND, Lau TY, Wirtz-Peitz F, He L, Ting AY, Carr SA, Perrimon N. Proteomic mapping in live Drosophila tissues using an engineered ascorbate peroxidase. Proc Natl Acad Sci U S A. 2015;112:12093–12098. doi: 10.1073/pnas.1515623112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Trouillard M, Meunier B, Rappaport F. Questioning the functional relevance of mitochondrial supercomplexes by time-resolved analysis of the respiratory chain. Proc Natl Acad Sci U S A. 2011;108:e1027–e1034. doi: 10.1073/pnas.1109510108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lapuente-Brun E, Moreno-Loshuertos R, Acín-Pérez R, Latorre-Pellicer A, Colás C, Balsa E, Perales-Clemente E, Quirós PM, Calvo E, Rodríguez-Hernández MA, Navas P, Cruz R, Carracedo Á, López-Otín C, Pérez-Martos A, Fernández-Silva P, Fernández-Vizarra E, Enríquez JA. Supercomplex assembly determines electron flux in the mitochondrial electron transport chain. Science. 2013;340:1567–1570. doi: 10.1126/science.1230381. [DOI] [PubMed] [Google Scholar]
- 84.Blaza JN, Serreli R, Jones AJY, Mohammed K, Hirst J. Kinetic evidence against partitioning of the ubiquinone pool and the catalytic relevance of respiratory-chain supercomplexes. Proc Natl Acad Sci U S A. 2014;111:15735–15740. doi: 10.1073/pnas.1413855111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cnu SY, Henderson JF. Inhibition of the phosphoribosyl-formylglycineamidine synthetase of Ehrlich ascites tumor cells by glutamine analogues. Biochem Pharmacol. 1972;21:401–406. doi: 10.1016/0006-2952(72)90351-6. [DOI] [PubMed] [Google Scholar]
- 86.Rowe PB, McCairns E, Madsen G, Sauer D, Elliott H. De novo purine synthesis in avian liver. Co-purification of the enzymes and properties of the pathway. J Biol Chem. 1978;253:7711–7721. [PubMed] [Google Scholar]
- 87.Caperelli CA, Benkovic PA, Chettur G, Benkovic SJ. Purification of a complex catalyzing folate cofactor synthesis and transformylation in de novo purine biosynthesis. J Biol Chem. 1980;255:1885–1890. [PubMed] [Google Scholar]
- 88.An S, Kumar R, Sheets ED, Benkovic SJ. Reversible compartmentalization of de novo purine biosynthetic complexes in living cells. Science. 2008;320:103–106. doi: 10.1126/science.1152241. [DOI] [PubMed] [Google Scholar]
- 89.An S, Deng Y, Tomsho JW, Kyoung M, Benkovic SJ. Microtubule-assisted mechanism for functional metabolic macromolecular complex formation. Proc Natl Acad Sci U S A. 2010;107:12872–12876. doi: 10.1073/pnas.1008451107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhao H, Chiaro CR, Zhang L, Smith PB, Chan CY, Pedley AM, Pugh RJ, French JB, Patterson AD, Benkovic SJ. Quantitative analysis of purine nucleotides indicates that purinosomes increase de novo purine biosynthesis. J Biol Chem. 2015;290:6705–6713. doi: 10.1074/jbc.M114.628701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Deng Y, Gam J, French JB, Zhao H, An S, Benkovic SJ. Mapping protein-protein proximity in the purinosome. J Biol Chem. 2012;287:36201–36207. doi: 10.1074/jbc.M112.407056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kyoung M, Russell SJ, Kohnhorst CL, Esemoto NN, An S. Dynamic architecture of the purinosome involved in human de novo purine biosynthesis. Biochemistry. 2015;54:870–880. doi: 10.1021/bi501480d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Baresova V, Krijt M, Skopova V, Souckova O, Kmoch S, Zikanova M. CRISPR-Cas9 induced mutations along de novo purine synthesis in HeLa cells result in accumulation of individual enzyme substrates and affect purinosome formation. Mol Genet Metab. 2016;119:270–277. doi: 10.1016/j.ymgme.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 94.French JB, Jones SA, Deng H, Pedley AM, Kim D, Chan CY, Hu H, Pugh R, Zhao H, Zhang Y, Huang TJ, Fang Y, Zhuang X, Benkovic SJ. Spatial colocalization and functional link of purinosomes with mitochondria. Science. 2016;351:733737. doi: 10.1126/science.aac6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ben-Sahra I, Hoxhaj G, Ricoult SJH, Asara JM, Manning BD. mTORC1 induces purine synthesis through control of the mitochondrial tetrahydrofolate cycle. Science. 2016;351:728733. doi: 10.1126/science.aad0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chitrakar I, Kim-Holzapfel DM, Zhou W, French JB. Higher order structures in purine and pyrimidine metabolism. J Struct Biol. 2017;197:354–364. doi: 10.1016/j.jsb.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 97.Zhao H, French JB, Fang Y, Benkovic SJ. The purinosome, a multi-protein complex involved in the de novo biosynthesis of purines in humans. Chem Commun. 2013;49:4444–4449. doi: 10.1039/c3cc41437j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pedley AM, Benkovic SJ. A new view into the regulation of purine metabolism: The purinosome. Trends Biochem Sci. 2016;197:354–364. doi: 10.1016/j.tibs.2016.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yamaoka T, Yano M, Kondo M, Sasaki H, Hino S, Katashima R, Moritani M, Itakura M. Feedback inhibition of amidophosphoribosyltransferase regulates the rate of cell growth via purine nucleotide, DNA, and protein syntheses. J Biol Chem. 2001;276:21285–21291. doi: 10.1074/jbc.M011103200. [DOI] [PubMed] [Google Scholar]
- 100.Schmitt DL, Cheng YJ, Park J, An S. Sequestration-mediated downregulation of de novo purine biosynthesis by AMPK. ACS Chem Biol. 2016;11:1917–1924. doi: 10.1021/acschembio.6b00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gunter JH, Thomas EC, Lengefeld N, Kruger SJ, Worton L, Gardiner EM, Jones A, Barnett NL, Whitehead JP. Characterisation of inosine monophosphate dehydrogenase expression during retinal development: differences between variants and isoforms. Int J Biochem Cell Biol. 2008;40:1716–1728. doi: 10.1016/j.biocel.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 102.Carcamo WC, Satoh M, Kasahara H, Terada N, Hamazaki T, Chan JYF, Yao B, Tamayo S, Covini G, Mühlen, von Mühlen CA, Chan EKL. Induction of cytoplasmic rods and rings structures by inhibition of the CTP and GTP synthetic pathway in mammalian cells. PLoS One. 2011;6:e29690. doi: 10.1371/journal.pone.0029690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chang CC, Lin WC, Pai LM, Lee HS, Wu SC, Ding ST, Liu JL, Sung LY. Cytoophidium assembly reflects upregulation of IMPDH activity. J Cell Sci. 2015;128:3550–3555. doi: 10.1242/jcs.175265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Keppeke GD, Calise SJ, Chan EKL, Andrade LEC. Assembly of IMPDH2-based, CTPS-based, and mixed rod/ring structures is dependent on cell type and conditions of induction. J Genet Genomics. 2015;42:287–299. doi: 10.1016/j.jgg.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 105.Calise SJ, Carcamo WC, Krueger C, Yin JD, Purich DL, Chan EKL. Glutamine deprivation initiates reversible assembly of mammalian rods and rings. Cell Mol Life Sci. 2014;71:2963–2973. doi: 10.1007/s00018-014-1567-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Calise SJ, Purich DL, Nguyen T, Saleem DA, Krueger C, Yin JD, Chan EKL. Rod and ring” formation from IMP dehydrogenase is regulated through the one-carbon metabolic pathway. J Cell Sci. 2016;129:3042–3052. doi: 10.1242/jcs.183400. [DOI] [PubMed] [Google Scholar]
- 107.Evans DR, Guy HI. Mammalian pyrimidine biosynthesis: fresh insights into an ancient pathway. J Biol Chem. 2004;279:33035–33038. doi: 10.1074/jbc.R400007200. [DOI] [PubMed] [Google Scholar]
- 108.Sigoillot FD, Kotsis DH, Serre V, Sigoillot SM, Evans DR, Guy HI. Nuclear localization and mitogen-activated protein kinase phosphorylation of the multifunctional protein CAD. J Biol Chem. 2005;280:25611–25620. doi: 10.1074/jbc.M504581200. [DOI] [PubMed] [Google Scholar]
- 109.Robitaille AM, Christen S, Shimobayashi M, Cornu M, Fava LL, Moes S, Prescianotto-Baschong C, Sauer U, Jenoe P, Hall MN. Quantitative phosphoproteomics reveal mTORC1 activates de novo pyrimidine synthesis. Science. 2013;339:1320–1323. doi: 10.1126/science.1228771. [DOI] [PubMed] [Google Scholar]
- 110.Ben-Sahra I, Howell JJ, Asara JM, Manning BD. Stimulation of de novo pyrimidine synthesis by growth signaling through mTOR and S6K1. Science. 2013;339:1323–1328. doi: 10.1126/science.1228792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Huang M, Graves LM. De novo synthesis of pyrimidine nucleotides; emerging interfaces with signal transduction pathways. Cell Mol Life Sci. 2003;60:321–336. doi: 10.1007/s000180300027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liu JL. Intracellular compartmentation of CTP synthase in Drosophila. J Genet Genomics. 2010;37:281–296. doi: 10.1016/S1673-8527(09)60046-1. [DOI] [PubMed] [Google Scholar]
- 113.Ingerson-Mahar M, Briegel A, Werner JN, Jensen GJ, Gitai Z. The metabolic enzyme CTP synthase forms cytoskeletal filaments. Nat Cell Biol. 2010;12:739–746. doi: 10.1038/ncb2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Noree C, Sato BK, Broyer RM, Wilhelm JE. Identification of novel filament-forming proteins in Saccharomyces cerevisiae and Drosophila melanogaster. J Cell Biol. 2010;190:541–551. doi: 10.1083/jcb.201003001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chen K, Zhang J, Tastan ÖY, Deussen ZA, Siswick MYY, Liu JL. Glutamine analogs promote cytoophidium assembly in human and Drosophila cells. J Genet Genomics. 2011;38:391–402. doi: 10.1016/j.jgg.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 116.Zhang J, Hulme L, Liu JL. Asymmetric inheritance of cytoophidia in Schizosaccharomyces pombe. Biol Open. 2014;3:1092–1097. doi: 10.1242/bio.20149613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liu JL. The Cytoophidium and its kind: filamentation and compartmentation of metabolic enzymes. Annu Rev Cell Dev Biol. 2016;32:349–372. doi: 10.1146/annurev-cellbio-111315-124907. [DOI] [PubMed] [Google Scholar]
- 118.Aughey GN, Liu JL. Metabolic regulation via enzyme filamentation. Crit Rev Biochem Mol Biol. 2016;51:282–293. doi: 10.3109/10409238.2016.1172555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Barry RM, Bitbol AF, Lorestani A, Charles EJ, Habrian CH, Hansen JM, Li HJ, Baldwin EP, Wingreen NS, Kollman JM, Gitai Z. Large-scale filament formation inhibits the activity of CTP synthetase. eLife. 2014;3:e03638. doi: 10.7554/eLife.03638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Strochlic TI, Stavrides KP, Thomas SV, Nicolas E, O’Reilly AM, Peterson JR. Ack kinase regulates CTP synthase filaments during Drosophila oogenesis. EMBO Rep. 2014;15:1184–1191. doi: 10.15252/embr.201438688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Aughey GN, Grice SJ, Liu JL. The interplay between Myc and CTP Synthase in Drosophila. PLoS Genet. 2016;12:e1005867. doi: 10.1371/journal.pgen.1005867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang PY, Lin WC, Tsai YC, Cheng ML, Lin YH. Regulation of CTP synthase filament formation during DNA endoreplication in Drosophila. Genetics. 2015;201:1511–1523. doi: 10.1534/genetics.115.180737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pai LM, Wang PY, Lin WC, Chakraborty A, Yeh CT, Lin YH. Ubiquitination and filamentous structure of cytidine triphosphate synthase. Fly. 2016;10:108–114. doi: 10.1080/19336934.2016.1182268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gou KM, Chang CC, Shen QJ, Sung LY, Liu JL. CTP synthase forms cytoophidia in the cytoplasm and nucleus. Exp Cell Res. 2014;323:242–253. doi: 10.1016/j.yexcr.2014.01.029. [DOI] [PubMed] [Google Scholar]
- 125.Finzel K, Lee DJ, Burkart MD. Using modern tools to probe the structure-function relationship of fatty acid synthases. ChemBioChem. 2015;16:528–547. doi: 10.1002/cbic.201402578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ameer F, Scandiuzzi L, Hasnain S, Kalbacher H, Zaidi N. De novo lipogenesis in health and disease. Metab, Clin Exp. 2014;63:895–902. doi: 10.1016/j.metabol.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 127.Beld J, Lee DJ, Burkart MD. Fatty acid biosynthesis revisited: structure elucidation and metabolic engineering. Mol BioSyst. 2015;11:38–59. doi: 10.1039/c4mb00443d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jenni S, Leibundgut M, Boehringer D, Frick C, Mikolasek B, Ban N. Structure of fungal fatty acid synthase and implications for iterative substrate shuttling. Science. 2007;316:254–261. doi: 10.1126/science.1138248. [DOI] [PubMed] [Google Scholar]
- 129.Lomakin IB, Xiong Y, Steitz TA. The crystal structure of yeast fatty acid synthase, a cellular machine with eight active sites working together. Cell. 2007;129:319–332. doi: 10.1016/j.cell.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 130.Gipson P, Mills DJ, Wouts R, Grininger M, Vonck J, Kuhlbrandt W. Direct structural insight into the substrate-shuttling mechanism of yeast fatty acid synthase by electron cryomicroscopy. Proc Natl Acad Sci U S A. 2010;107:9164–9169. doi: 10.1073/pnas.0913547107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Brignole EJ, Smith S, Asturias FJ. Conformational flexibility of metazoan fatty acid synthase enables catalysis. Nat Struct Mol Biol. 2009;16:190–197. doi: 10.1038/nsmb.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Suresh HG, da Silveira Dos Santos AX, Kukulski W, Tyedmers J, Riezman H, Bukau B, Mogk A. Prolonged starvation drives reversible sequestration of lipid biosynthetic enzymes and organelle reorganization in Saccharomyces cerevisiae. Mol Biol Cell. 2015;26:1601–1615. doi: 10.1091/mbc.E14-11-1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hillebrand M, Gersting SW, Lotz-Havla AS, Schafer A, Rosewich H, Valerius O, Muntau AC, Gartner J. Identification of a new fatty acid synthesis-transport machinery at the peroxisomal membrane. J Biol Chem. 2012;287:210–221. doi: 10.1074/jbc.M111.272732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pang B, Wang M, Liu W. Cyclization of polyketides and non-ribosomal peptides on and off their assembly lines. Nat Prod Rep. 2016;33:162–173. doi: 10.1039/c5np00095e. [DOI] [PubMed] [Google Scholar]
- 135.Strieker M, Tanovć A, Marahiel MA. Nonribosomal peptide synthetases: structures and dynamics. Curr Opin Struct Biol. 2010;20:234–240. doi: 10.1016/j.sbi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 136.Challis GL, Naismith JH. Structural aspects of non-ribosomal peptide biosynthesis. Curr Opin Struct Biol. 2004;14:748–756. doi: 10.1016/j.sbi.2004.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bender CL, Rangaswamy V, Loper J. Polyketide production by plant-associated pseudomonads. Annu Rev Phytopathol. 1999;37:175–196. doi: 10.1146/annurev.phyto.37.1.175. [DOI] [PubMed] [Google Scholar]
- 138.Kistler HC. Cellular compartmentalization of secondary metabolism. Front Microbiol. 2015;6:68. doi: 10.3389/fmicb.2015.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Upadhyay S, Xu X, Lowry D, Jackson JC, Roberson RW, Lin X. Subcellular compartmentalization and trafficking of the biosynthetic machinery for fungal melanin. Cell Rep. 2016;14:2511–2518. doi: 10.1016/j.celrep.2016.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Upadhyay S, Xu X, Lin X. Interactions between melanin enzymes and their atypical recruitment to the secretory pathway by palmitoylation. mBio. 2016;7:e01925. doi: 10.1128/mBio.01925-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Roze LV, Chanda A, Linz JE. Compartmentalization and molecular traffic in secondary metabolism: A new understanding of established cellular processes. Fungal Genet Biol. 2011;48:35–48. doi: 10.1016/j.fgb.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chanda A, Roze LV, Kang S, Artymovich KA, Hicks GR, Raikhel NV, Calvo AM, Linz JE. A key role for vesicles in fungal secondary metabolism. Proc Natl Acad Sci U S A. 2009;106:19533–19538. doi: 10.1073/pnas.0907416106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chanda A, Roze LV, Linz JE. A possible role for exocytosis in aflatoxin export in Aspergillus parasiticus. Eukaryotic Cell. 2010;9:1724–1727. doi: 10.1128/EC.00118-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Imperi F, Visca P. Subcellular localization of the pyoverdine biogenesis machinery of Pseudomonas aeruginosa: a membrane-associated “siderosome. FEBS Lett. 2013;587:3387–3391. doi: 10.1016/j.febslet.2013.08.039. [DOI] [PubMed] [Google Scholar]
- 145.Gasser V, Guillon L, Cunrath O, Schalk IJ. Cellular organization of siderophore biosynthesis in Pseudomonas aeruginosa: evidence for siderosomes. J Inorg Biochem. 2015;148:27–34. doi: 10.1016/j.jinorgbio.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 146.Straight PD, Fischbach MA, Walsh CT, Rudner DZ, Kolter R. A singular enzymatic megacomplex from Bacillus subtilis. Proc Natl Acad Sci U S A. 2007;104:305–310. doi: 10.1073/pnas.0609073103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Butcher RA, Schroeder FC, Fischbach MA, Straight PD, Kolter R, Walsh CT, Clardy J. The identification of bacillaene, the product of the PksX megacomplex in Bacillus subtilis. Proc Natl Acad Sci U S A. 2007;104:1506–1509. doi: 10.1073/pnas.0610503104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Maeda H, Dudareva N. The shikimate pathway and aromatic amino acid biosynthesis in plants. Annu Rev Plant Biol. 2012;63:73–105. doi: 10.1146/annurev-arplant-042811-105439. [DOI] [PubMed] [Google Scholar]
- 149.Umbarger HE. Amino acid biosynthesis and its regulation. Annu Rev Biochem. 1978;47:533–606. doi: 10.1146/annurev.bi.47.070178.002533. [DOI] [PubMed] [Google Scholar]
- 150.Miflin BJ, Lea PJ. Amino acid metabolism. Annu Rev Plant Physiol. 1977;28:299–329. [Google Scholar]
- 151.Braus GH. Aromatic amino acid biosynthesis in the yeast Saccharomyces cerevisiae: a model system for the regulation of a eukaryotic biosynthetic pathway. Microbiol Rev. 1991;55:349–370. doi: 10.1128/mr.55.3.349-370.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mir R, Jallu S, Singh TP. The shikimate pathway: review of amino acid sequence, function and threedimensional structures of the enzymes. Crit Rev Microbiol. 2015;41:172–189. doi: 10.3109/1040841X.2013.813901. [DOI] [PubMed] [Google Scholar]
- 153.Sasso S, Ökvist M, Roderer K, Gamper M, Codoni G, Krengel U, Kast P. Structure and function of a complex between chorismate mutase and DAHP synthase: efficiency boost for the junior partner. EMBO J. 2009;28:2128–2142. doi: 10.1038/emboj.2009.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Blackmore NJ, Nazmi AR, Hutton RD, Webby MN, Baker EN, Jameson GB, Parker EJ. Complex formation between two biosynthetic enzymes modifies the allosteric regulatory properties of both. J Biol Chem. 2015;290:18187–18198. doi: 10.1074/jbc.M115.638700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Munack S, Roderer K, Ökvist M, Kamarauskaite J, Sasso S, van Eerde A, Kast P, Krengel U. Remote control by inter-enzyme allostery: a novel paradigm for regulation of the shikimate pathway. J Mol Biol. 2016;428:1237–255. doi: 10.1016/j.jmb.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 156.Islam MM, Wallin R, Wynn RM, Conway M, Fujii H, Mobley JA, Chuang DT, Hutson SM. A novel branched-chain amino acid metabolon. Protein-protein interactions in a supramolecular complex. J Biol Chem. 2007;282:11893–11903. doi: 10.1074/jbc.M700198200. [DOI] [PubMed] [Google Scholar]
- 157.Islam MM, Nautiyal M, Wynn RM, Mobley JA, Chuang DT, Hutson SM. Branched-chain amino acid metabolon: interaction of glutamate dehydrogenase with the mitochondrial branched-chain aminotransferase (BCATm) J Biol Chem. 2010;285:265–276. doi: 10.1074/jbc.M109.048777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Welch GR. Organized Multienzyme Systems: Catalytic Properties. Academic Press; New York: 1985. [Google Scholar]
- 159.Winkel BSJ. Metabolic channeling in plants. Annu Rev Plant Biol. 2004;55:85–107. doi: 10.1146/annurev.arplant.55.031903.141714. [DOI] [PubMed] [Google Scholar]
- 160.Tullman-Ercek D. Metabolism: “channeling” Hans Krebs. Nat Chem Biol. 2015;11:180–181. doi: 10.1038/nchembio.1758. [DOI] [PubMed] [Google Scholar]
- 161.Kurakin A. Scale-free flow of life: on the biology, economics, and physics of the cell. Theor Biol Med Modell. 2009;6:6. doi: 10.1186/1742-4682-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Poshyvailo L, von Lieres E, Kondrat S. Does metabolite channeling accelerate enzyme-catalyzed cascade reactions? PLoS One. 2017;12:e0172673. doi: 10.1371/journal.pone.0172673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lee H, DeLoache WC, Dueber JE. Spatial organization of enzymes for metabolic engineering. Metab Eng. 2012;14:242–251. doi: 10.1016/j.ymben.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 164.Petrovska I, Nüske E, Munder MC, Kulasegaran G, Malinovska L, Kroschwald S, Richter D, Fahmy K, Gibson K, Verbavatz JM, Alberti S. Filament formation by metabolic enzymes is a specific adaptation to an advanced state of cellular starvation. eLife. 2014;3:e02409. doi: 10.7554/eLife.02409. [DOI] [PMC free article] [PubMed] [Google Scholar]


