Abstract
Antineoplastic targeted therapies, such as EGFR inhibitors, tyrosine kinase inhibitors and BRAF inhibitors, frequently lead to systemic and cutaneous side effects, significantly affecting patient’s quality of life. Patients with new targeted therapies have an increased risk of developing skin reactions. The new molecular target therapies developed in the last decades can induce severe skin reactions, which may require dose reduction or discontinuation of treatment and consequently, a decrease in patient’s quality of life.
The present paper describes toxic cutaneous reactions associated with the most frequently used molecular therapies (epidermal growth factor receptor inhibitors, tyrosine kinase inhibitors, BRAF-inhibitors), frequency of occurrence and methods of diagnosis and treatment, in order to offer a clinically efficient management for maintaining a good quality of life, with compliance to treatment and good therapeutic efficacy.
Knowledge of cutaneous adverse reactions in new therapies is mandatory in order to have a proper management of oncologic patients. Recognizing target therapy toxicities by both oncologists and dermatologists, understanding therapeutic mechanisms and choosing optimum treatments for oncologic patients are critical. A correct evaluation of skin toxicity can allow for an adequate decision regarding treatment dose or discontinuation, impacting therapy response and patient survival.
Keywords:targeted therapies, epidermal growth factor receptor inhibitors, tyrosine kinase inhibitors, BRAF inhibitors, toxic cutaneous reactions, patient quality of life
INTRODUCTION
Antineoplastic therapies have numerous side effects, both systemic and cutaneous. Patients treated with either classical chemotherapeutic agents or with new targeted therapies have an increased risk of developing skin reactions.
Classical chemotherapy induces well known skin reactions. These include infusion reactions, diffuse or localized cutaneous pigmentary changes, radiation dermatitis, hand-foot syndrome, nail changes (changes in pigmentation, onycholysis, paronychia), mucosal changes, stomatitis, alopecia, photosensitivity, cutaneous erythematosus lupus, drug rashes, exfoliative dermatitis, erythema multforme. Some agents may also cause severe skin reactions: Stevens Johnson syndrome, toxic epidermal necrolysis, ulcers, Raynaud’s syndrome, reactive dermatomyositis paraneoplastic skin syndromes – pemfigus like, porphyria, reactivation of varicella zoster virus.
The new molecular target therapies developed in the last decades (epidermal growth factor receptor inhibitors, tyrosine kinase inhibitors, monoclonal antibodies, BRAF-inhibitors) can induce severe skin reactions, which may require dose reduction or discontinuation of treatment and consequently, a decrease in the patient’s quality of life (1-3).
The correct diagnosis of an adverse reaction secondary to antineoplastic agents requires a differential diagnosis with other drug reactions, specific dermatological entities, cutaneous metastases and paraneoplastic syndromes. A multi di sciplinary approach, with oncologists and dermatologists, aims to improve quality of life and treatment adherence.
The present paper describes toxic cutaneous reactions associated with the most often used mole cular therapies, frequency of occurrence and methods of diagnosis and treatment, in order to offer a clinically efficient management for maintaining a good quality of life, with compliance to treatment and good therapeutic efficacy.
Epidermal growth factor receptor inhibitors (anti EGFR)
Molecular therapies targeting epidermal growth factor receptors (EGFR) have proved their efficacy in treating multiple types of cancer (lung PRIDE syndrome comprises the most frequent reactions associated with anti-EGFR reactions (papules, pustules, paronychia, hair growth disorders, pruritus, skin and mucosal xerosis).Unfortunately, some patients can develop severe acneiform reactions that may lead to dose reduction or treatment discontinuation.Some studies show a positive correlation between acneiform rash severity and treatment response.„Correlation Between the Severity of Cetuximab- Induced Skin Rash and Clinical Outcome for Head and Neck Cancer Patients: The RTOG Experience”, a study with 602 enrolled patients, demonstrated a higher survival rate for patients with grade 2-4 cutaneous reactions after treatment with Cetuximab, probably due to the decrease of distant metastases (7).Two meta-analyses, published in 2012 and 2013, have demonstrated that skin toxicity is a predictive and independent survival factor, and patients who developed moderate to severe cutaneous reactions had a higher treatment response rate.Further studies are needed to demonstrate if dermatotoxicities can represent a reliable criteria for treatment response monitoring regarding anti-EGFR therapies (8, 9).Other cutaneous and mucosal reactions due to anti-EGFR therapy include oral and nasal aphthosis, ulcerations, mucositis, stomatitis and photosensitivity. Rarely, exfoliative dermatitis, Stevens-Johnson syndrome, toxic epidermal necro lysis, ocular complications (dry eye syndrome, corneal erosions), vasculitis, purpura, and anaphylactoid reactions may develop (1).
Bacterial infection, most often with Staphylococcus aureus, may determine a sudden worsening of a cutaneous rash and an alteration of the clinical status (4, 10).
Skin rashes secondary to anti-EGFR therapy could have a negative impact on disease-related quality of life and may influence the efficacy and duration of treatment. Available therapies for such reactions include empirical topical and systemic antibiotic therapies. Testing for specific bacterial colonization is not mandatory, unless patients exhibit worsened symptoms or reactions that become refractory to treatment. The management of cutaneous drug reactions implies preventive and specific dermatologic therapies, customized for each patient, according to lesion type, location and severity. The collaboration between oncologists and dermatologists is advisable for most patients. Preventive measures include adequate hydration of dry areas (non-alcoholic emollients, urea creams), decrease in sun exposure, avoiding prolonged skin contact with water, irritants and solvents. Topical agents may be used to reduce the reaction severity. Mild reactions may receive clindamycin and hydrocortisone based creams or ointments. A study published by Yamazaki et al. offers objective evidence to support the use of topical agents in cutaneous reactions after anti- EGFR TKI therapies, early use permitting further anti-EGFR treatment (7).
Oral antibiotics can be prophylactically use (such as minocycline 100 mg/day, tetracycline 500 mg/day), leading to a decrease in pruritus and erythema intensity, consequently reducing skin irritation. Moderate reactions may be treated with hydrocortisone, clindamycin, erythromycin, pimecrolimus, along with oral antibiotics therapies, including cyclins (doxycycline 50-100 mg/day). Tetracycline is widely used in cutaneous reactions because of its anti-inflammatory properties, inhibiting lymphocyte proliferation, neutrophil migration and interleukin-6 synthesis, as well for its antibacterial properties.
Using these methods, 80% of anti-EGFR therapy-related reactions can be easily managed. Most often, modifying treatment dose will not be necessary. If cutaneous toxic effects are not reduced in 2-4 weeks, despite correct topical and oral treatment, then treatment dosage may be reduced or anti-EGFR therapy may be ceased. In severe reactions (grade 2 or higher), if a rash infection is suspected or if skin reactions are refractory to topical treatment, systemic corticoid therapy, such as Prednisone (12-25 mg/day, one week, with dose tapering), should be initiated. Recommended systemic antibiotherapy is tetracycline, doxycycline and minocycline. Doxycycline doses may range from 50-100 mg/day, lower doses having mainly an anti-inflammatory effect, in order to reduce the incidence of antibiotherapy resistance and digestive adverse effects. Higher doses of doxycycline (100 mg twice daily) are reserved for severe reactions. Studies showed that doxycycline did not reduce adverse reactions related to anti-EGFR therapies; however, they significantly reduced their severity. If the rash is worse despite treatment or persistent, a bacterial superinfection should to be evaluated. Retinoids represent another therapeutic option, because of their anti-inflammatory effects; however, patients may face a worsening of mucosal and cutaneous dryness due to anti-EGFR therapies. Higher retinoid doses are associated with desquamation, paronychia and photosensitivity. Minimal therapeutic doses must be prescribed to avoid these side effects. New therapies that are currently under investigation include the vitamin K3 analogue, menadione (1, 4-6, 10-12). Xerosis, desquamations and skin fissures require ammonium lactate 12%, salicylic acid 6% and 20% urea based emollients. Pruritus may be alleviated using topical cortico steroids, menthol, oral antihistamines, and in severe cases gabapentin and pregabalin can be used. Studies show that concomitant antineoplastic therapies (radiotherapy and chemotherapy and Cetuximab) do not produce a higher risk for mucositis, acneiform reactions or post-radiothe rapy dermatitis impacting quality of life (13-15).
Small molecule tyrosine kinase inhibitors
Sorafenib and Sunitinib are tyrosine kinase inhibitors that stop angiogenesis and tumor proliferation by blocking the vascular endothelial growth factor receptor (VEGFR), platelet derived growth factor receptor (PDGFR) and cytokinic receptor (KIT). Sunitinib is approved for renal cancers, gastrointestinal stromal tumors, pancreatic neuroendocrine tumors, whereas Sorafenib, which inhibits RAF kinase, is used in renal, hepatic and thyroid cancer. Patients may present adverse effects such as arterial hypertension, diarrhea and cutaneous reactions by inhibiting PDGFR and VEGFR, and direct vascular injury, determining direct extravasation in skin and mucosae, both molecules displaying the same types of toxicities (1, 16, 17).
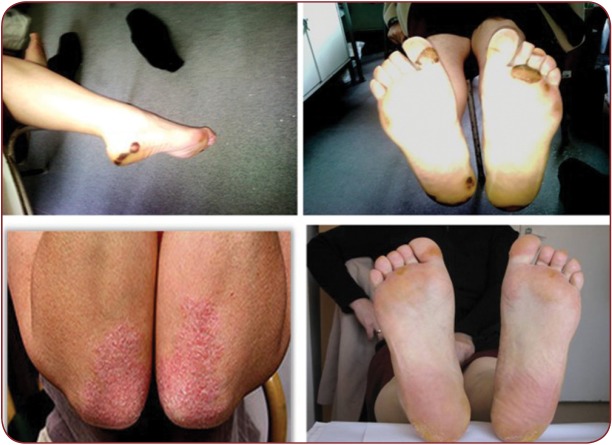
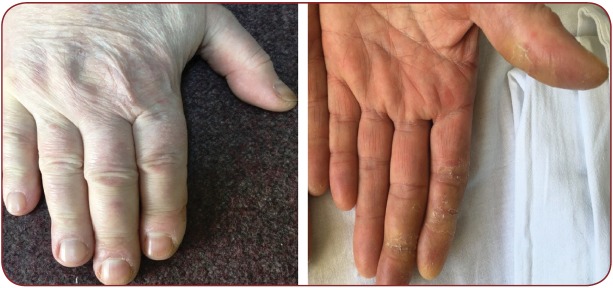
Hand-foot syndrome (Figure 3) is the most frequently encountered cutaneous adverse reaction in patients undergoing treatment with tyrosine kinase inhibitors. Reaction severity is dose-dependent. It presents as painful vesicles that subsequently transform in hyperkeratosis on areas exposed to friction or traumas (heals, soles, toes, interphalangeal joints, elbows, knees). Lesions usually appear after 2-4 weeks of treatment (Figure 4). Associated symptoms (tingling, paresthe sias, burning sensation and pain) significantly affect patient’s quality of life, leading to either treatment cessation or dose reduction. This may negatively impact primary disease management. Hand-foot syndrome pathogenesis is unknown (1, 16, 17).
Other adverse reactions include stomatitis (with an early onset), alopecia, hair fragility, hyperpigmentation (Sorafenib) or depigmentation (Sunitinib), which is reversible one month after treatment cessation, proliferative lesions (squamous cell carcinomas, keratoachantomas and inflamed actinic keratosis), facial and scalp erythema (similar to seborrheic dermatitis), facial edema and yellow skin pigmentation (for Sunitinib), which are reversible. Cutaneous hemorrhages, periungal erythema, erythema multiforme, dysesthesia and Stevens-Johnson syndrome are less frequent (1, 16, 17).
Preventive measures against hand-foot syndrome include limitation of hot water usage for hands and feet, cooling with cold water or a cold damp towel, avoiding heat generators, saunas, sun exposure, use of thick socks or gloves, avoiding contact with chemical substances (e.g., laundry detergent, domestic cleansing products) and limiting any physical strain that may lead to pressure or friction on palms and soles.
Topical agents are used for grade 1-2 reactions, whereas topical associated with oral therapies are used for grade 3 reactions. Hyperkeratotic areas can be treated with daily applications of 10-20% urea based creams or 0.1% tazarotene creams, while 0.05% clobetasole propionate is used on erythematous areas. Urea is a keratolytic agent, increasing skin moisture by softening the dry or rough layers of the skin. Tazarotene is a topical retinoid that reduces cutaneous proliferation and inflammation. Topical agents must be used at most twice daily. Excessive use may lead to irritation. Other options include salicylic acid and ammonium lactate. Topical corticotherapy or anesthetics (lidocaine creams or patches) can be used on painful vesicles or areas on palms and soles. Non-steroidal anti-inflammatory drugs (ibuprofen, naproxen and celecoxib), pregabalin or codeine are oral analgesics used in severe cases. Such cases warrant a reduction in Sunitinib/ Sorafenib dose or even temporary treatment discontinuation. Studies show that patients exhibiting adverse reactions to Sunitinib and Sorafenib have a higher treatment efficacy. Such correlations may lead to identifying patient groups that benefit more from treatment with small molecule tyrosine kinase inhibitors (1, 16-20).
BRAF inhibitors
Approximately 40-60% of melanoma patients have a BRAF mutation, that leads to the activation of a signaling cascade in the MAP kinase pathway (mitogen-activated protein kinases) involved in cell proliferation, differentiation, survival, stress response and apoptosis (1, 21).
Vemurafenib is a strong BRAF mutation inhibitor. Dabrafenib is a reversible, ATP-competitive, selective BRAF inhibitor. Both are approved for the treatment of V600 BRAF positive metastatic melanoma. Both Vemurafenib and Dabrafenib are generally well tolerated. Adverse reactions are due to a paradoxical activation of MAPK pathway (22-25).
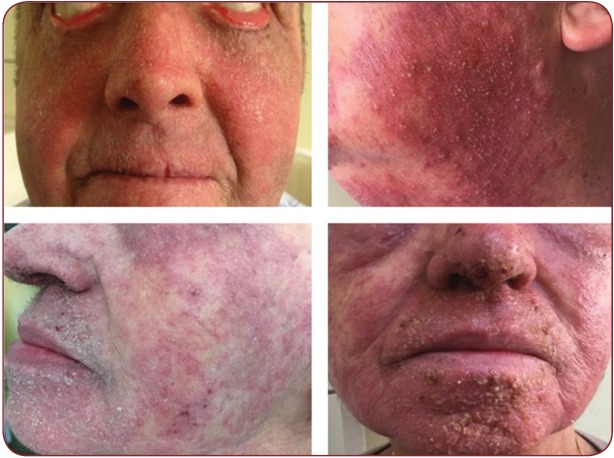
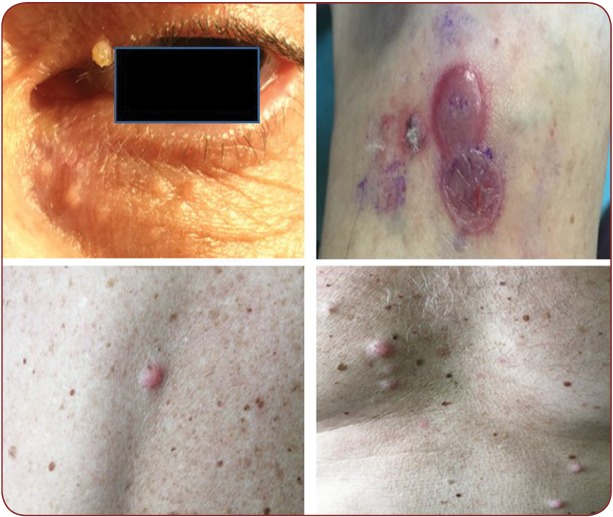
Cutaneous reactions due to BRAF inhibitors are papular eruptions, photosensitivity, xerosis, pruritus, paronychia, alopecia and hair changes, hyperkeratotic lesions. Vemurafenib and Dabrafenib patients can develop warts, seborrheic keratoses, hypertrophic actinic keratoses, eczemas, hand-foot syndrome, papillomas, keratoachantomas and squamous cell carcinomas, most of which are related to Vemurafenib. Dabrafenib is an appropriate therapeutic option in patients that do not tolerate Vemurafenib (Figure 5). Trametinib is a MAPK inhibitor approved for inoperable melanomas with V600E BRAF or V600K mutations. Cutaneous adverse reactions are less frequent and comprise acneiform eruptions, pruritus and xerosis. Squamous cell carcinoma secondary to Trametinib administration is uncommon comparing to BRAF inhibitors. Dabrafenib plus Trametinib and Vemurafenib plus Cobimetinib associations have similar efficacy, but with a lower incidence of skin toxicities or either malignant or hyperproliferative disorders. This is due to the paradoxical activation of the MAPK pathway. Addition of a MEK inhibitor leads to the inhibition of RAS signaling in MAPK pathway, which prevents cellular proliferation. Fever is the most important adverse reaction of the Dabrafenib plus Trametinib combination and may lead to dose reduction or treatment discontinuation (1, 21-25). An interdisciplinary collaboration between oncologists and dermatologists is necessary. Knowledge of cutaneous adverse reactions in new therapies is mandatory in order to have a proper management of oncologic patients. As such, patients receiving BRAF inhibitors must be examined on a regular basis by a dermatologist since an early diagnosis and adequate treatment can improve patient quality of life and overall survival rates (1, 21, 22).
FIGURE 1.
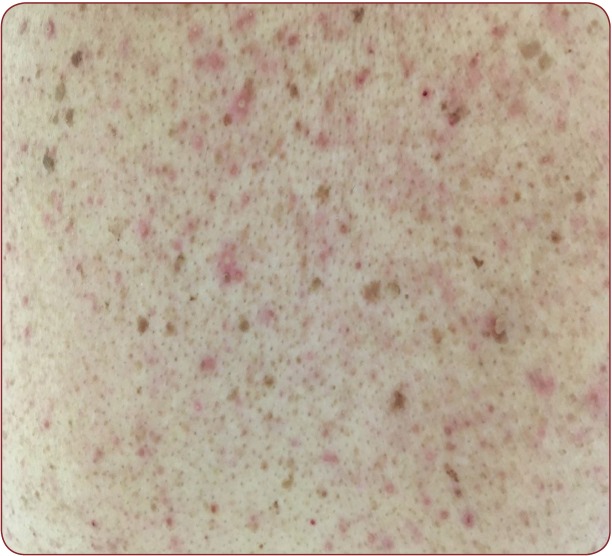
FIGURE 1. Skin rash consisting of erythematous papules and pustules after anti EGFR therapy (Cetuximab)
FIGURE 2.
FIGURE 2. Skin rash consisting of erythematous violaceous papules and pustules, with fi ne scales, yellow crusts and telangiectasias after anti EGFR therapy (Cetuximab)
FIGURE 3.
FIGURE 3. Up: vesicles and tension bullae on a well defi ned erythematous background on soles (HFS); lower left: well defi ned erythemato-squamous plaques on elbows; lower right: well defi ned erythematous plaques on soles
FIGURE 4.
FIGURE 4. Erythema on the palmar and dorsal aspects of hands, including the fi ngers and nail folds, with desquamation, hyperkeratosis and fi ssures
FIGURE 5.
FIGURE 5. Upper left: periocular papillomas; upper right: erythema multiforme-like lesions, soft bullae with serous fl uid content, surrounded by and erythemato-edematous ring, on a red-violaceous background (targetoid lesions); lower left and right: small, slightly erythematous papules and nodules, (metastases versus dermatofi bromas)
CONCLUSION
Antineoplastic targeted therapies such as EGFR inhibitors, tyrosine kinase inhibitors and BRAF inhibitors frequently lead to dermatologic adverse reactions, which significantly affect patient’s quality of life. This may determine unwanted dose modifications. A correct evaluation of skin toxicity can allow for an adequate decision regarding treatment dose or discontinuation, impacting therapy response and patient survival. Today, dose modifications are made using the adverse reactions criteria according to the National Cancer Institute’s CTCEA.
Although useful and widely accepted, this system is yet to be validated. Therefore, patient evaluations can be subjective. Other possible methods to describe cutaneous toxicities are: DERETT journal (DErmatologic REaction Targeted Therapy–Patient Symptom Experience Diary), where patients take notes of adverse reactions, using the FACT-EGFRI-18 scale for anti-EGFR therapies, HFS-14 (The Hand-Foot Syndrome 14) for hand-foot syndrome. These instruments used in order to measure the impact of dermatological adverse events on quality of life, together with medical criteria, can deliver important data in order for adequate decisions to be made for optimal antineoplastic therapy dosing and maintaining a good quality of life. Recognizing target therapy toxicities by both oncologists and dermatologists, understanding therapeutic mecha nisms and choosing optimum treatments for oncologic patients is critical for both oncologists and dermatologists.
Conflict of interests: none declared.
Financial support: none declared.
Contributor Information
Dana Lucia STANCULEANU, Medical Oncology Department, “Prof. Dr. Al. Trestioreanu” Institute of Oncology, Bucharest, Romania ; Oncology Department, ”Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania.
Daniela ZOB, Medical Oncology Department, “Prof. Dr. Al. Trestioreanu” Institute of Oncology, Bucharest, Romania.
Oana Catalina TOMA, Medical Oncology Department, “Prof. Dr. Al. Trestioreanu” Institute of Oncology, Bucharest, Romania.
Bogdan GEORGESCU, Medical Oncology Department, “Prof. Dr. Al. Trestioreanu” Institute of Oncology, Bucharest, Romania.
Laura PAPAGHEORGHE, Dermatology Department, Coltea Clinical Hospital, Bucharest, Romania.
Raluca Ioana MIHAILA, Medical Oncology Department, “Prof. Dr. Al. Trestioreanu” Institute of Oncology, Bucharest, Romania.
REFERENCES
- Lupu I, Voiculescu N, Bacalbasa N, Cojocaru I, Vrancian V, Giurcaneanu C. - Cutaneous complications of molecular targeted therapy used in oncology. Journal of Medicine and Life. 2016;9:19–25. [PMC free article] [PubMed] [Google Scholar]
- Veronica J Shi, Lauren L Levy, Jennifer N Choi. - Cutaneous manifestations of nontargeted and targeted chemotherapies. Seminars in Oncology. 2016;43:419–425. doi: 10.1053/j.seminoncol.2016.02.018. [DOI] [PubMed] [Google Scholar]
- Macdonald JB, Macdonald B, Goli; LE, LoRusso P, Sekulic A. - Cutaneous adverse eff ects of targeted therapies: Part I: Inhibitors of the cellular membrane. J Am Acad Dermatol. 2015;72:203–208. doi: 10.1016/j.jaad.2014.07.032. [DOI] [PubMed] [Google Scholar]
- Fabbrocini G, Panariello L, Caro G, Cacciapuoti S. - Acneiform Rash Induced by EGFR Inhibitors: Review of the Literature and New Insights. Skin Appendage Disorders. 2015;1:31–37. doi: 10.1159/000371821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrieta O, Carmona A, de Jesus Vega MT, Lopez-Mejia M, Cardona AF. - Skin communicates what we deeply feel: antibiotic prophylactic treatment to reduce epidermal growth factor receptor inhibitors induced rash in lung cancer (the Pan Canadian rash trial). Annals of Translational Medicine. 2016;4:313. doi: 10.21037/atm.2016.08.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y-T, Chen K-L, Chu C-Y. - Treatment strategies of epidermal growth factor receptor inhibitor-induced skin toxicities: pre-emptive or reactive? Annals of Translational Medicine. 2016;4:318. doi: 10.21037/atm.2016.08.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Ad V, Zhang Q, Harari P, Axelrod R, et al. - Correlation Between the Severity of Cetuximab-Induced Skin Rash and Clinical Outcome for Head and Neck Cancer Patients: The RTOG Experience. International Journal of Radiation Oncology Biology Physics. 2016;95:1346–1354. doi: 10.1016/j.ijrobp.2016.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrelli F, Borgonovo K, Barni S. - The predictive role of skin rash with cetuximab and panitumumab in colorectal cancer patients: a systematic review and meta-analysis of published trials. Target Oncol. 2013;8:173–181. doi: 10.1007/s11523-013-0257-x. [DOI] [PubMed] [Google Scholar]
- Petrelli F, Borgonovo K, Cabiddu M, Lonati V, Barni S. - Relationship between skin rash and outcome in non-small-cell lung cancer patients treated with anti-EGFR tyrosine kinase inhibitors: a literature-based meta-analysis of 24 trials. Lung Cancer. 2012;78:8–15. doi: 10.1016/j.lungcan.2012.06.009. [DOI] [PubMed] [Google Scholar]
- Braden RL, Anadkat MJ. . Support Care Cancer. 2016;24:3943. doi: 10.1007/s00520-016-3231-1. [DOI] [PubMed] [Google Scholar]
- Deplanque G, Gervais R, Vergnenegre A, Falchero L, Souquet PJ, Chavaillon JM, et al. - Doxycycline for prevention of erlotinib-induced rash in patients with non-small-cell lung cancer (NSCLC) after failure of fi rst-line chemotherapy: A randomized, open-label trial. Journal of the American Academy of Dermatology. 2016;74:1077–1085. doi: 10.1016/j.jaad.2016.01.019. [DOI] [PubMed] [Google Scholar]
- Petrelli F, Borgonovo K, Barni S. - Preventing or treating anti-EGFR related skin rash with antibiotics? Annals of Translational Medicine. 2016;4:312. doi: 10.21037/atm.2016.07.01. [DOI] [PMC free article] [PubMed] [Google Scholar]