Abstract
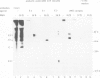
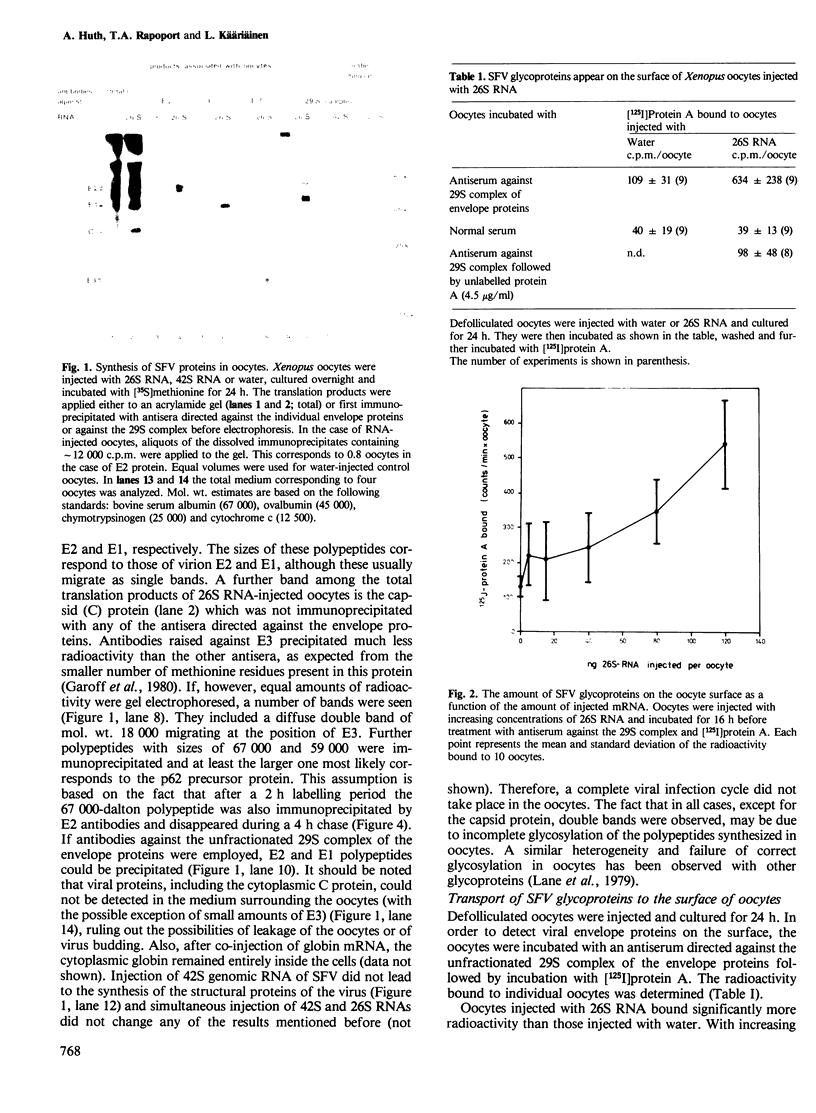
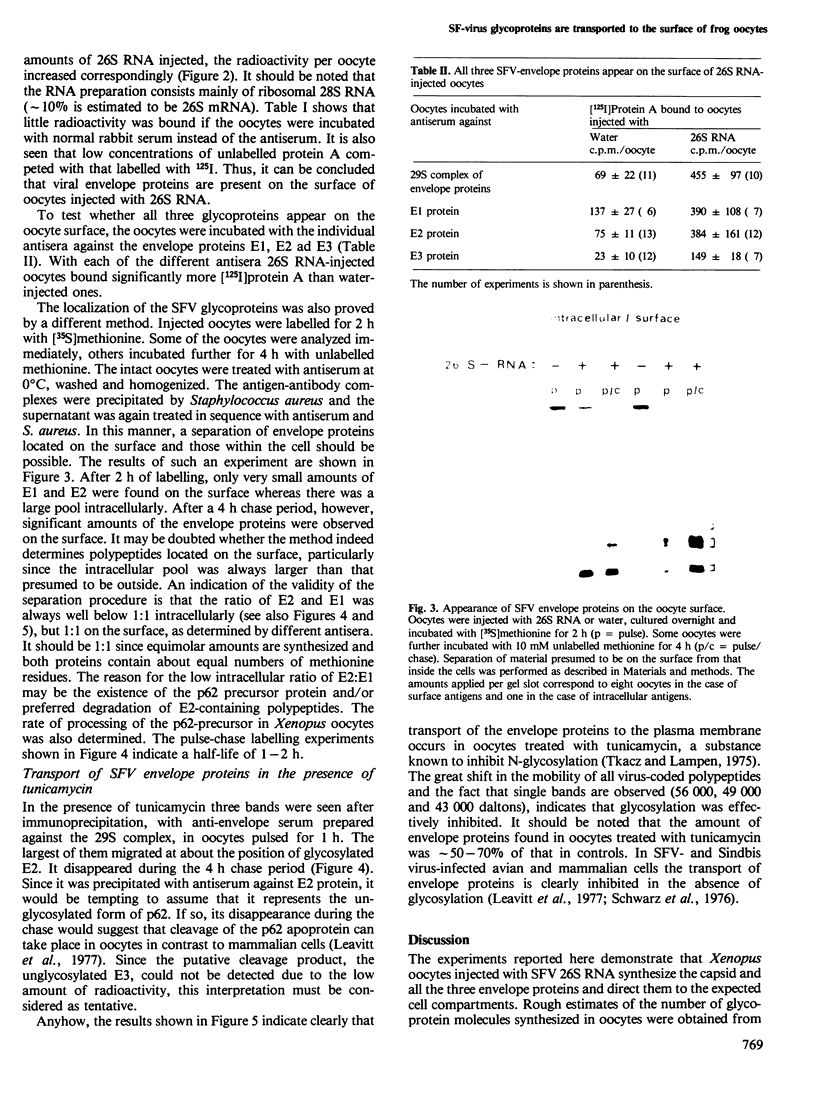
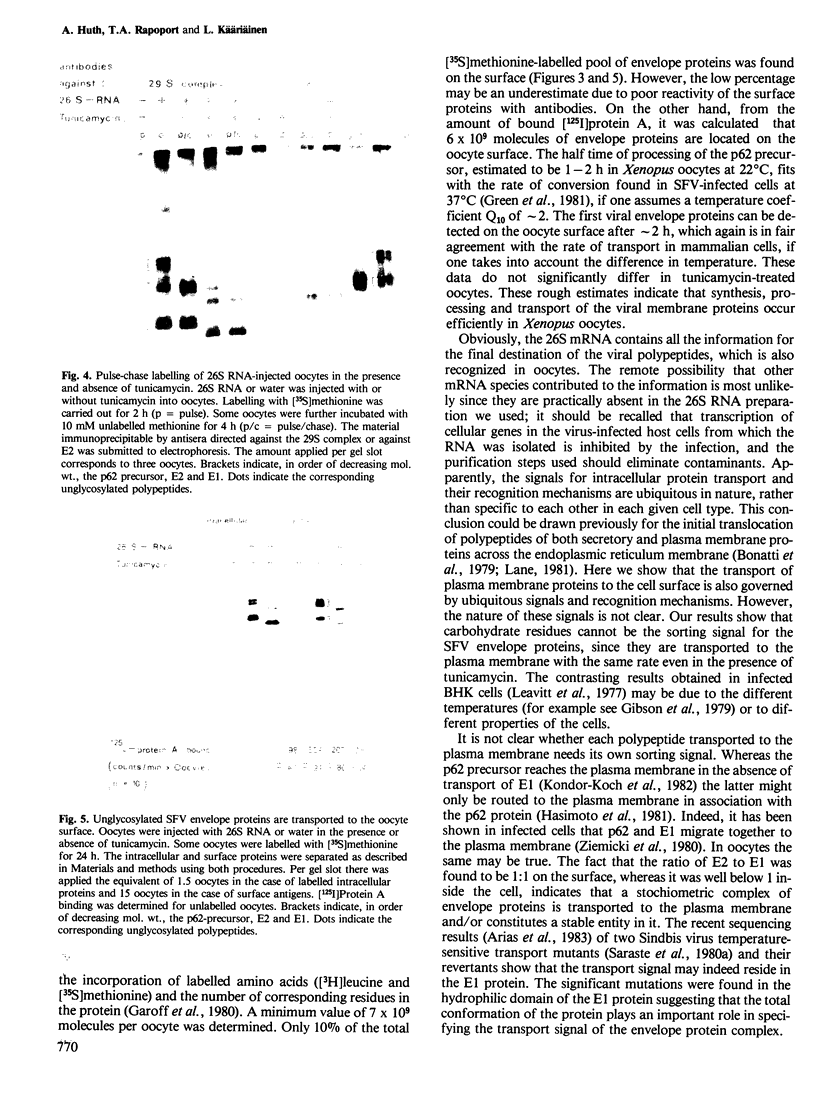
The mRNA coding for the structural proteins of Semliki Forest virus, the 26S RNA, was injected into Xenopus oocytes. Synthesis of the capsid protein and the three envelope glycoproteins E1, E2 and E2 was observed. The proteins, which are normally incorporated into the plasma membrane of infected cells, are transported to the surface of the oocytes. The transport of the membrane proteins takes place in the presence of tunicamycin. The results show that the proteins foreign to the oocyte reach their destination in the plasma membrane. Consequently, the mRNA contains the information for the transport and proteolytic cleavage of the polypeptides.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arias C., Bell J. R., Lenches E. M., Strauss E. G., Strauss J. H. Sequence analysis of two mutants of Sindbis virus defective in the intracellular transport of their glycoproteins. J Mol Biol. 1983 Jul 25;168(1):87–102. doi: 10.1016/s0022-2836(83)80324-6. [DOI] [PubMed] [Google Scholar]
- Barnard E. A., Miledi R., Sumikawa K. Translation of exogenous messenger RNA coding for nicotinic acetylcholine receptors produces functional receptors in Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1982 May 22;215(1199):241–246. doi: 10.1098/rspb.1982.0040. [DOI] [PubMed] [Google Scholar]
- Bonatti S., Cancedda R., Blobel G. Membrane biogenesis. In vitro cleavage, core glycosylation, and integration into microsomal membranes of sindbis virus glycoproteins. J Cell Biol. 1979 Jan;80(1):219–224. doi: 10.1083/jcb.80.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bonner W. M. Protein migration into nuclei. II. Frog oocyte nuclei accumulate a class of microinjected oocyte nuclear proteins and exclude a class of microinjected oocyte cytoplasmic proteins. J Cell Biol. 1975 Feb;64(2):431–437. doi: 10.1083/jcb.64.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman A., Lane C. D., Craig R., Boulton A., Mohun T., Morser J. The influence of topology and glycosylation on the fate of heterologous secretory proteins made in Xenopus oocytes. Eur J Biochem. 1981 Jan;113(2):339–348. doi: 10.1111/j.1432-1033.1981.tb05072.x. [DOI] [PubMed] [Google Scholar]
- Colman A., Morser J. Export of proteins from oocytes of Xenopus laevis. Cell. 1979 Jul;17(3):517–526. doi: 10.1016/0092-8674(79)90260-5. [DOI] [PubMed] [Google Scholar]
- Feldherr C. M., Ogburn J. A. Mechanism for the selection of nuclear polypeptides in Xenopus oocytes. II. Two-dimensional gel analysis. J Cell Biol. 1980 Dec;87(3 Pt 1):589–593. doi: 10.1083/jcb.87.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garoff H., Frischauf A. M., Simons K., Lehrach H., Delius H. Nucleotide sequence of cdna coding for Semliki Forest virus membrane glycoproteins. Nature. 1980 Nov 20;288(5788):236–241. doi: 10.1038/288236a0. [DOI] [PubMed] [Google Scholar]
- Garoff H., Simons K., Dobberstein B. Assembly of the Semliki Forest virus membrane glycoproteins in the membrane of the endoplasmic reticulum in vitro. J Mol Biol. 1978 Oct 5;124(4):587–600. doi: 10.1016/0022-2836(78)90173-0. [DOI] [PubMed] [Google Scholar]
- Garoff H., Söderlund H. The amphiphilic membrane glycoproteins of Semliki Forest virus are attached to the lipid bilayer by their COOH-terminal ends. J Mol Biol. 1978 Sep 25;124(3):535–549. doi: 10.1016/0022-2836(78)90186-9. [DOI] [PubMed] [Google Scholar]
- Gibson R., Schlesinger S., Kornfeld S. The nonglycosylated glycoprotein of vesicular stomatitis virus is temperature-sensitive and undergoes intracellular aggregation at elevated temperatures. J Biol Chem. 1979 May 10;254(9):3600–3607. [PubMed] [Google Scholar]
- Glanville N., Morser J., Uomala P., Käri5AAINEN L. Simultaneous translation of structural and nonstructural proteins from Semliki-forest-virus RNA in two eukaryotic systems in vitro. Eur J Biochem. 1976 Apr 15;64(1):167–175. doi: 10.1111/j.1432-1033.1976.tb10285.x. [DOI] [PubMed] [Google Scholar]
- Green J., Griffiths G., Louvard D., Quinn P., Warren G. Passage of viral membrane proteins through the Golgi complex. J Mol Biol. 1981 Nov 15;152(4):663–698. doi: 10.1016/0022-2836(81)90122-4. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B. Changes in somatic cell nuclei inserted into growing and maturing amphibian oocytes. J Embryol Exp Morphol. 1968 Nov;20(3):401–414. [PubMed] [Google Scholar]
- Gurdon J. B. Nuclear transplantation and the control of gene activity in animal development. Proc R Soc Lond B Biol Sci. 1970 Dec 1;176(1044):303–314. doi: 10.1098/rspb.1970.0050. [DOI] [PubMed] [Google Scholar]
- Hashimoto K., Erdei S., Keränen S., Saraste J., Käriäinen L. Evidence for a separate signal sequence for the carboxy-terminal envelope glycoprotein E1 of Semliki forest virus. J Virol. 1981 Apr;38(1):34–40. doi: 10.1128/jvi.38.1.34-40.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt L. A., Etchison J. R., Summers D. F. Oligosaccharide chains are trimmed during synthesis of the envelope glycoprotein of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1978 Feb;75(2):754–758. doi: 10.1073/pnas.75.2.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. J., Waite M. R., Bose H. R. Cleavage of a viral envelope precursor during the morphogenesis of Sindbis virus. J Virol. 1974 Apr;13(4):809–817. doi: 10.1128/jvi.13.4.809-817.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keränen S., Käriäinen L. Proteins synthesized by Semliki Forest virus and its 16 temperature-sensitive mutants. J Virol. 1975 Aug;16(2):388–396. doi: 10.1128/jvi.16.2.388-396.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondor-Koch C., Riedel H., Söderberg K., Garoff H. Expression of the structural proteins of Semliki Forest virus from cloned cDNA microinjected into the nucleus of baby hamster kidney cells. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4525–4529. doi: 10.1073/pnas.79.15.4525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Käriäinen L., Hashimoto K., Saraste J., Virtanen I., Penttinen K. Monensin and FCCP inhibit the intracellular transport of alphavirus membrane glycoproteins. J Cell Biol. 1980 Dec;87(3 Pt 1):783–791. doi: 10.1083/jcb.87.3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Käriäinen L., Söderlund H. Structure and replication of alpha-viruses. Curr Top Microbiol Immunol. 1978;82:15–69. doi: 10.1007/978-3-642-46388-4_2. [DOI] [PubMed] [Google Scholar]
- Lane C. D., Champion J., Haiml L., Kreil G. The sequestration, processing and retention of honey-bee promelittin made in amphibian oocytes. Eur J Biochem. 1981 Jan;113(2):273–281. doi: 10.1111/j.1432-1033.1981.tb05063.x. [DOI] [PubMed] [Google Scholar]
- Lane C. D., Colman A., Mohun T., Morser J., Champion J., Kourides I., Craig R., Higgins S., James T. C., Applebaum S. W. The Xenopus oocyte as a surrogate secretory system. The specificity of protein export. Eur J Biochem. 1980 Oct;111(1):225–235. doi: 10.1111/j.1432-1033.1980.tb06097.x. [DOI] [PubMed] [Google Scholar]
- Lane C. D. The fate of foreign proteins introduced in Xenopus oocytes. Cell. 1981 May;24(2):281–282. doi: 10.1016/0092-8674(81)90315-9. [DOI] [PubMed] [Google Scholar]
- Lane C., Shannon S., Craig R. Sequestration and turnover of guinea-pig milk proteins and chicken ovalbumin in Xenopus oocytes. Eur J Biochem. 1979 Nov;101(2):485–495. doi: 10.1111/j.1432-1033.1979.tb19743.x. [DOI] [PubMed] [Google Scholar]
- Leavitt R., Schlesinger S., Kornfeld S. Impaired intracellular migration and altered solubility of nonglycosylated glycoproteins of vesicular stomatitis virus and Sindbis virus. J Biol Chem. 1977 Dec 25;252(24):9018–9023. [PubMed] [Google Scholar]
- Lebleu B., Hubert E., Content J., De Wit L., Braude I. A., De Clercq E. Translation of mouse interferon mRNA in Xenopus laevis oocytes and in rabbit reticulocyte lysates. Biochem Biophys Res Commun. 1978 May 30;82(2):665–673. doi: 10.1016/0006-291x(78)90926-9. [DOI] [PubMed] [Google Scholar]
- Neville D. M., Jr Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971 Oct 25;246(20):6328–6334. [PubMed] [Google Scholar]
- Ohlsson R. I., Lane C. D., Guengerich F. P. Synthesis and insertion, both in vivo and in vitro, of rat-liver cytochrome P-450 and epoxide hydratase into Xenopus laevis membranes. Eur J Biochem. 1981 Apr;115(2):367–373. doi: 10.1111/j.1432-1033.1981.tb05247.x. [DOI] [PubMed] [Google Scholar]
- Pesonen M., Käriäinen L. Incomplete complex oligosaccharides in semliki forest virus envelope proteins arrested within the cell in the presence of monensin. J Mol Biol. 1982 Jun 25;158(2):213–230. doi: 10.1016/0022-2836(82)90430-2. [DOI] [PubMed] [Google Scholar]
- Pesonen M., Saraste J., Hashimoto K., Käriäinen L. Reversible defect in the glycosylation of the membrane proteins of Semliki Forest virus ts-1 mutant. Virology. 1981 Feb;109(1):165–173. doi: 10.1016/0042-6822(81)90481-5. [DOI] [PubMed] [Google Scholar]
- Rapoport T. A. Intracellular compartmentation and secretion of carp proinsulin synthesized in Xenopus oocytes. Eur J Biochem. 1981 Apr;115(3):665–669. doi: 10.1111/j.1432-1033.1981.tb06254.x. [DOI] [PubMed] [Google Scholar]
- Saraste J., von Bonsdorff C. H., Hashimoto K., Keränen S., Käriäinen L. Reversible transport defects of virus membrane glycoproteins in Sindbis virus mutant infected cells. Cell Biol Int Rep. 1980 Mar;4(3):279–286. doi: 10.1016/0309-1651(80)90060-0. [DOI] [PubMed] [Google Scholar]
- Saraste J., von Bonsdorff C. H., Hashimoto K., Käriäinen L., Keränen S. Semliki forest virus mutants with temperature-sensitive transport defect of envelope proteins. Virology. 1980 Jan 30;100(2):229–245. doi: 10.1016/0042-6822(80)90516-4. [DOI] [PubMed] [Google Scholar]
- Schlesinger S., Schlesinger M. J. Formation of Sindbis virus proteins: identification of a precursor for one of the envelope proteins. J Virol. 1972 Nov;10(5):925–932. doi: 10.1128/jvi.10.5.925-932.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz R. T., Rohrschneider J. M., Schmidt M. F. Suppression of glycoprotein formation of Semliki Forest, influenza, and avian sarcoma virus by tunicamycin. J Virol. 1976 Sep;19(3):782–791. doi: 10.1128/jvi.19.3.782-791.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton B. M. Immediate glycosylation of Sindbis virus membrane proteins. Cell. 1977 Apr;10(4):659–668. doi: 10.1016/0092-8674(77)90099-x. [DOI] [PubMed] [Google Scholar]
- Soreq H., Miskin R. Secreted proteins in the medium of microinjected Xenopus oocytes are degraded by oocyte proteases. FEBS Lett. 1981 Jun 15;128(2):305–310. doi: 10.1016/0014-5793(81)80104-4. [DOI] [PubMed] [Google Scholar]
- Soreq H., Parvari R., Silman I. Biosynthesis and secretion of catalytically active acetylcholinesterase in Xenopus oocytes microinjected with mRNA from rat brain and from Torpedo electric organ. Proc Natl Acad Sci U S A. 1982 Feb;79(3):830–834. doi: 10.1073/pnas.79.3.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumikawa K., Houghton M., Emtage J. S., Richards B. M., Barnard E. A. Active multi-subunit ACh receptor assembled by translation of heterologous mRNA in Xenopus oocytes. Nature. 1981 Aug 27;292(5826):862–864. doi: 10.1038/292862a0. [DOI] [PubMed] [Google Scholar]
- Tkacz J. S., Lampen O. Tunicamycin inhibition of polyisoprenyl N-acetylglucosaminyl pyrophosphate formation in calf-liver microsomes. Biochem Biophys Res Commun. 1975 Jul 8;65(1):248–257. doi: 10.1016/s0006-291x(75)80086-6. [DOI] [PubMed] [Google Scholar]
- Vänänen P. The use of red cells with fused Semliki Forest virus envelope proteins in antibody determinations by hemolysis in gel. J Virol Methods. 1982 Mar;4(2):117–126. doi: 10.1016/0166-0934(82)90081-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehavi-Willner T., Lane C. Subcellular compartmentation of albumin and globin made in oocytes under the direction of injected messenger RNA. Cell. 1977 Jul;11(3):683–693. doi: 10.1016/0092-8674(77)90085-x. [DOI] [PubMed] [Google Scholar]
- Ziemiecki A., Garoff H., Simons K. Formation of the Semliki Forest virus membrane glycoprotein complexes in the infected cell. J Gen Virol. 1980 Sep;50(1):111–123. doi: 10.1099/0022-1317-50-1-111. [DOI] [PubMed] [Google Scholar]