Abstract
Harvesting cultivated macrophages for tissue engineering purposes by enzymatic digestion of cell adhesion molecules can potentially result in unintended activation, altered function, or behavior of these cells. Thermo-responsive polymer is a promising tool that allows for gentle macrophage detachment without artificial activation prior to subculture within engineered tissue constructs. We therefore characterized different species of thermo-responsive polymers for their suitability as cell substrate and to mediate gentle macrophage detachment by temperature shift. Primary human monocyte- and THP-1-derived macrophages were cultured on thermo-responsive polymers and characterized for phagocytosis and cytokine secretion in response to lipopolysaccharide stimulation. We found that both cell types differentially respond in dependence of culture and stimulation on thermo-responsive polymers. In contrast to THP-1 macrophages, primary monocyte–derived macrophages showed no signs of impaired viability, artificial activation, or altered functionality due to culture on thermo-responsive polymers compared to conventional cell culture. Our study demonstrates that along with commercially available UpCell carriers, two other thermo-responsive polymers based on poly(vinyl methyl ether) blends are attractive candidates for differentiation and gentle detachment of primary monocyte–derived macrophages. In summary, we observed similar functionality and viability of primary monocyte–derived macrophages cultured on thermo-responsive polymers compared to standard cell culture surfaces. While this first generation of custom-made thermo-responsive polymers does not yet outperform standard culture approaches, our results are very promising and provide the basis for exploiting the unique advantages offered by custom-made thermo-responsive polymers to further improve macrophage culture and recovery in the future, including the covalent binding of signaling molecules and the reduction of centrifugation and washing steps. Optimizing these and other benefits of thermo-responsive polymers could greatly improve the culture of macrophages for tissue engineering applications.
Keywords: Thermo-responsive polymers, monocytes, macrophages, cell detachment, cytokines
Introduction
Macrophages are essential in the homeostasis of higher vertebrates and the defense against pathogenic germs and helminthes. Their functions range from initiating, maintaining, and resolving host inflammatory responses and wound healing to presentation of microbial antigens via major histocompatibility complexes to T cells resulting in immunity of the host against the pathogen.1,2 To fulfill these functions, macrophages react sensitive to xenobiotic stimuli such as microbe-associated molecular patterns (MAMPs) or changes in the microenvironment within tissues. As part of the activation process, macrophages secrete soluble factors, such as cytokines, that is, to recruit circulating immune cells as part of the host defense response.3 Macrophages reside in most of the human tissues where they are central in the inflammatory responses upon MAMP stimulation.
However, most in vitro models neglect the function of tissue macrophages. Reasons for this include the remarkable firm attachment of mature macrophages to the cell substrate during cell culture and the low threshold of an artificial activation during cell detachment. So far, most protocols for macrophage detachment rely on enzymatic digestion of cell adhesion molecules. However, the degradation of cell surface molecules potentially alters macrophage behavior and function. Supporting this assumption, Chen et al.4 have recently shown that trypsin treatment of macrophages renders their surface marker expression level and alters cellular functionality on standard cell culture surfaces.
Furthermore, enzymatic harvesting of cells requires additional centrifugation and washing steps potentially resulting in a loss of valuable cell material. Alternatives to enzymatic harvesting procedures are therefore required to allow an appropriate transfer and embedment of differentiated non-activated macrophages into engineered tissues.
Over the last decades, many groups reported different protocols for the controlled detachment of adherent cells and cell sheets using thermo-responsive polymers (TRPs) for tissue engineering purposes.5 TRPs, such as the widely used polymers poly(N-isopropylacrylamide) (PNiPAAm) or poly(vinyl methyl ether) (PVME), exhibit a thermally stimulated volume phase transition close to the physiological cultivation temperature.6,7 A variety of technological approaches to prepare and to permanently anchor TRP coatings on suitable substrate materials were reported.8,9 Beyond the straightforward observation of cell detachment, the change in physico-chemical properties of responsive coatings10–14 as well as respective cell interactions were investigated.15,16 It was reported that the thermo-responsive detachment of cells and cell sheets including their extracellular matrix is a complex phenomenon with a number of cell-type-specific effects to be considered.17 Within this context, the crucial role of cell-type-specific balance between adhesion and thermo-responsive detachment was studied to meet the requirements of particular cells and tissues.18,19 These studies established a platform technology to fabricate thermo-responsive cell culture carriers with graded properties including physico-chemical and biomolecular parameters. Based on this, in this study, we focused on the characterization of various TRPs for culture and detachment of macrophages. We systematically investigated the effects of four recently established PVME-based TRP cell culture carriers18,19 and compared the results of macrophage functionality before and after cell detachment with the commercially available PNiPAAm-based cell culture carrier UpCell™ (UC; Nunc™, Thermo Fisher Scientific, Waltham, MA, USA). In particular, we used macrophages differentiated from the myeloid cell line THP-1 as well as macrophages differentiated from isolated human primary monocytes.20–23 Both cell types were characterized for cell viability after cell detachment. Metabolic activity, phagocytic capacity as well as the expression and secretion of pro- and anti-inflammatory cytokines in response to lipopolysaccharide (LPS) stimulation were investigated before detachment from TRP specimens.
Materials and methods
This study does not include experiments using animals. The ethics committee of the Jena University Hospital approved the study (3939-12/13).
Cell culture carriers
PVME-based TRP coatings were prepared on Νunc™ Thermanox™ coverslips with a diameter of 13 or 25 mm (Thermo Fisher Scientific) as follows: coverslips were treated with air plasma (Harrick Plasma Cleaner PDC-002, 1 min) to improve the wetting behavior for the following coating procedures. Subsequently, different blends of PVME as the main component (TCI Europe, Zwijndrecht, Belgium), the alternating copolymer of PVME and maleic acid (PVMEMA, Sigma-Aldrich, Munich, Germany), and poly(N-isopropylacrylamide) (PNiPAAm, Sigma-Aldrich) were prepared by spin coating (solution 2% (w/w) or 10% (w/w) in methanol, 2000 rpm, 1500 rpm s−1, 30 s) on hydrophilized Τhermanox™ surfaces. Simultaneous cross-linking and immobilization9 was carried out with 150 keV electron beam irradiation using the low-energy electron facility ADU (Advanced Electron Beams, Wilmington, MA, USA) under nitrogen atmosphere at room temperature. Samples were irradiated with an absorbed dose of 258 or 774 kGy. The dose was applied stepwise in order to reduce the temperature increase during electron beam treatment. Samples were rinsed in de-ionized water and ethanol to remove any unbound material. Finally, maleic acid groups were converted into anhydride moieties in a thermal annealing step (90°C, overnight) to allow for covalent protein binding.
The variety of PVME-based TRP carriers used in this study is summarized in Table 1. Structure formulae of applied blend components are shown in Figure 1. Additional information on physico-chemical characterization of TRP coatings by spectroscopic ellipsometry, nanoindentation, and X-ray photoelectron spectroscopy is given in Teichmann et al.18,19
Table 1.
Preparation parameters for thermo-responsive cell culture carriers used for this study.
| Identifier | Blend composition (wt %) | Solution (wt %) | Irradiation dose (kGy) |
|---|---|---|---|
| A1 | PVME50-PNiPAAm40-PVMEMA10 | 2 | 774 |
| A2 | PVME50-PNiPAAm40-PVMEMA10 | 10 | 774 |
| B1 | PVME90-PVMEMA10 | 10 | 774 |
| B2 | PVME99-PVMEMA1 | 10 | 258 |
Identifiers are used throughout the text.
Figure 1.
Structure formulae of applied blend components.
The selected blend compositions represent favorite cases identified in previous studies. Briefly, the preparation parameters correspond to the actual surface properties of the cell culture carrier as follows: the ratio of the TRPs PVME and PNiPAAm determines cell adhesion and detachment properties. The content of PVMEMA determines the density of reactive anhydride moieties for covalent protein binding from the cell culture medium. A high/low concentration of the solution during spin coating leads to a high/low dry thickness of the obtained polymer layer (a few 10 nm vs a few 100 nm). A high/low irradiation dose during electron beam cross-linking leads to low/high degree of swelling below the transition temperature, which corresponds to a high/low elastic modulus of the swollen material.18,19
TRP blends composed of PVME and PNiPAAm were compared to commercially available PNiPAAm-based UCs and uncoated Τhermanox™ cell culture carriers (CTRL). All cell culture carriers were initially sterilized with 70% ethanol for 30 min before cell seeding. Results of cell culture evaluation were normalized to cell density and culture area.
Primary monocytes and THP-1 cultivation and differentiation
Primary monocytes were isolated from whole blood of healthy human individuals as previously reported.24,25 Monocytes were collected from one donor per experiment. We normalized the obtained data for each experiment to the corresponding CTRL condition (see figure captions), due to well-known donor-specific inter-individual variates in monocyte biology and responsiveness to TRP coatings. Subjects were informed about the aim of the study and gave written informed consent. Cell isolation and culture were performed in a sterile, endotoxin-low environment. Primary monocytes were seeded on the above-mentioned cell culture carriers and were differentiated to primary monocyte–derived macrophages for 6 days in X-VIVO15 medium containing 10% autologous serum and 10 ng/mL granulocyte-macrophage colony-stimulating factor. THP-1 cells were obtained from German Collection of Microorganisms and Cell Cultures (DSMZ, Braunschweig, Germany) and cultured in Roswell Park Memorial Institute (RPMI) 1640 supplemented with 10% fetal calf serum (FCS) (Life Technologies, Karlsruhe, Germany). After seeding on cell culture carriers, THP-1 were differentiated for 6 days to macrophages by addition of 10 ng/mL 12-O-tetradecanoylphorbol-13-acetate (TPA) in RPMI 1640 supplemented with 10% FCS. 1.5 × 105 THP-1 cells/cm2 or 1.0 × 105 primary monocytes/cm2 were seeded on cell culture carriers.
Growth, detachment, and quantification of macrophages
Adherent macrophages cultured on PVME-based TRPs A1, A2, B1, B2, or CTRL were transferred after 6 days of differentiation into a new six-well plate (Greiner BioOne, Frickenhausen, Germany) containing 37°C warm X-VIVO15 medium and were used for indicated assays. Macrophages on UC dishes were rinsed with 37°C warm X-VIVO15 medium and were used for the indicated assays.
Prior to cell detachment, carriers were rinsed once with 37°C warm medium on a 37°C warm heating plate HP-3 (Kunz Instruments, Nynashamn, Sweden). Subsequently, for detachment and flow cytometry analysis (vitality assay and phagocytosis assay), TRP carriers with differentiated macrophages were transferred into a new six-well plate (for UC, cells were left in the dish) filled with 25°C warm X-VIVO15 medium and were incubated for 60 min at 25°C. Macrophages on CTRL were detached by incubation with 0.25% trypsin for 10 min at 37°C. Detached cells were collected by centrifugation at 300 × g for 5 min at room temperature and resuspended in 1× annexin V binding buffer or phosphate-buffered saline (PBS)/0.1% bovine serum albumin (BSA)/2 mM EDTA for subsequent viability and cell content analysis using flow cytometry.
Quantification was performed by flow cytometry and cell debris was excluded from counting by adjustment of forward and sideward scatter. Cell counts of macrophages detached from thermo-responsive carriers were normalized to cell counts obtained from CTRL (uncoated Thermanox™ cell culture carriers).
Evaluation of macrophage viability using MTT
Differentiated adherent macrophages on TRPs and CTRL were transferred into a new well plate and washed once with the respective culture medium at 37°C. For the MTT assay, cells were incubated for 4 h at 37°C in 500 µL of 37°C warmed medium containing 50 µL of 3-(4,5-methylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, VWR, Darmstadt, Germany) solution (2 mg/mL in PBS, pH 7.4). Subsequently, supernatants were discarded and the remaining formazan crystals formed from MTT were dissolved in 1 mL isopropanol. Then, the plate was shaken for 10 min and centrifuged (400 × g, 5 min). Finally, the isopropanol/formazan solution was aliquoted (100 µL) into a 96-well plate. The absorption of dissolved formazan was measured at 570 nm using a FLUOstar omega microplate reader (BMG Labtech, Germany) and MARS data analysis software version 2.41.
Viability assay
Cell viability assay was performed using Annexin V PE and 7-amino-actinomycin D (7-AAD) staining. Fluorescence intensity was measured on a FACSCanto II within 1 h according to manufacturer’s recommendations. Gating for detecting viable, apoptotic, and dead cells is shown in Supplementary Figure 1. Data were analyzed using FlowJo version 7.6.5. (TreeStar, Ashland, OR, USA).
Phagocytosis assay
Differentiated and attached macrophages on TRPs and CTRL were incubated 24 h with opsonized pHrodo™ Escherichia coli BioParticles® (Invitrogen, Karlsruhe, Germany) according to manufacturer’s protocol. Bioparticles-to-monocyte ratio was adjusted to 2:1. Macrophages were detached by a temperature shift to 25°C for 60 min (TRPs) or by trypsin treatment for 10 min at 37°C (CTRL). Subsequently, detached cells were collected by centrifugation at 300 × g for 5 min at room temperature and cells were resuspended in PBS/2 mM EDTA/0.1% BSA. Particles taken up by phagocytosis were measured by flow cytometry. Gating strategy for phagocytosis quantification is shown in Supplementary Figure 2. Extracellular pHrodo™-Bioparticles® adhering to cell surface did not contribute to quantification (lack of signal).
Ribonucleic acid isolation and complementary deoxyribonucleic acid synthesis
Differentiated adherent macrophages on TRPs and CTRL were stimulated with 100 ng/mL LPSs (E. coli strain E0111:B4; Sigma-Aldrich) or left untreated for 24 h. Supernatants were cleared by centrifugation at 300 × g at 4°C for 10 min and were transferred into a new vial and subsequently stored at −80°C until cytometric bead array (CBA) analysis. Adherent macrophages were lysed using Trizol (Invitrogen) and total RNA was isolated with the Qiagen RNeasy Mini kit (Qiagen, Hilden, Germany) including on-column DNase I digestion (Qiagen).26 Complementary DNA (cDNA) was prepared with Revert Aid First Strand cDNA Synthesis Kit (Fermentas, St. Leon-Rot, Germany), 5 µg of total RNA, and 500 ng/µL oligo-dT primers (Table 4).27
Table 4.
Trends observed with different assays for THP-1 macrophages on TRPs and CTRL.
| Read-out | TRP |
||||
|---|---|---|---|---|---|
| A1 | A2 | B1 | B2 | UC | |
| Detachment | = | = | = | = | = |
| Vitality | < | < | < | < | = |
| Phagocytosis | = | > | > | = | = |
| TNF secretion untreated | < | < | < | < | < |
| TNF secretion + LPS | < | < | < | < | < |
| IL-1β secretion untreated | < | = | = | < | = |
| IL-1β secretion + LPS | < | < | < | < | = |
| IL-10 secretion untreated | < | = | = | < | = |
| IL-10 secretion + LPS | < | < | < | < | = |
TRP: thermo-responsive polymer; TNF: tumor necrosis factor; LPS: lipopolysaccharide; IL: interleukin; =: similar to CTRL; <: lower than CTRL; >: higher than CTRL.
CTRL was set as standard.
Quantitative real-time polymerase chain reaction
Quantitative real-time polymerase chain reaction (PCR) was performed on a LightCycler 480 (Roche Diagnostics, Mannheim, Germany) using QuantiTect SYBR® Green PCR kit (Qiagen).27 Primers (Table 4) were purchased from Invitrogen. PCR runs included a 15 min pre-incubation at 95°C, followed by a 40-cycle two-step PCR consisting of a denaturing phase at 94°C for 15 s and a combined annealing and extension phase at 60°C for 30 s. Results were analyzed using LightCycler software version 1.5.0.39. The sequences of PCR primers used in this study are given in Table 2.
Table 2.
PCR primers used in this study.
| mRNA | mRNA name | GenBank accession no. | Forward primer | Reverse primer | Amplicon size (bp) |
|---|---|---|---|---|---|
| TNF | Tumor necrosis factor | NM_000594.1 | TCTTCTCGAACCCCGAGTGAC | GGTACAGGCCCTCTGATGGC | 159 |
| IL-1β | Interleukin 1, beta | NM_000576 | TCCCTGCCCACAGACCTTC | GTGCATCGTGCACATAAGCCT | 115 |
| RPL37A | Ribosomal protein L37a | NM_000998 | ATTGAAATCAGCCAGCACGC | AGGAACCACAGTGCCAGATCC | 94 |
| IL-10 | Interleukin 10 | NM_000572.2 | CCTGTGAAAACAAGAGCAAGGC | TCACTCATGGCTTTGTAGATGCC | 88 |
PCR: polymerase chain reaction; mRNA: messenger RNA; TNF: tumor necrosis factor; IL: interleukin.
In each case, forward and reverse primers are located in different exons.
Cytometric bead array
Secreted interleukin (IL)-10, IL-1β, and tumor necrosis factor (TNF) were measured by CBA FlexSets (BD Biosciences, Heidelberg, Germany) and analyzed with the FCAP Array software version 2.0.2 (Soft Flow, Pecs, Hungary).
Statistical analysis
All results are reported as mean with standard deviation (SD). Vitality and cytokine expression and secretion assays were analyzed by multiple comparison using two-way analysis of variance (ANOVA) with Tukey’s multiple testing correction. Comparison of cytokine secretion between the two macrophage types was analyzed by two-way ANOVA with Sidak’s multiple comparisons test. Statistical analysis of cell quantification and phagocytosis efficiency was done with two-tailed, paired Student’s t-test. All statistical tests were performed using GraphPad Prism 6 software (Graphpad Software, La Jolla, CA, USA). Statistical significances between CTRL and TRPs are indicated by *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001. Statistical significances between primary monocyte–derived macrophages and THP-1 macrophages are indicated by #p < 0.05 and ##p < 0.01.
Results
Culture and detachment
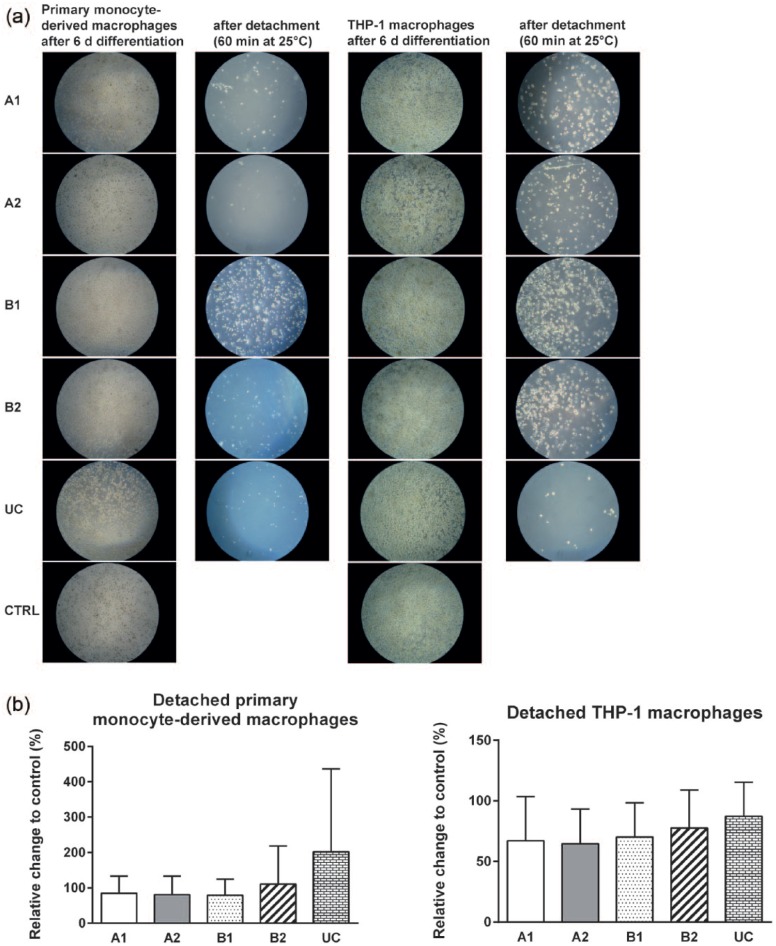
Adherence and growth of primary human macrophages (derived from primary monocytes and THP-1 monocytes) on A1, A2, B1, B2, UC, and CTRL were evaluated by microscopic images (Figure 2(a)). Both monocyte cell types adhered and differentiated to macrophages within 6 days. No morphological differences were observed between culture of adherent macrophages on TRPs and CTRL (Figure 2(a)). Macrophages were subsequently detached from TRPs by decreasing the temperature to 25°C for 60 min. We observed a higher number of both monocyte-derived and THP-1 macrophages remaining on cell culture carriers coated with TRP B1 compared to TRPs A1, A2, B2, and UC. Furthermore, after temperature reduction, a minor amount of THP-1 macrophages still adhere to PVME-based TRPs but not to UC surfaces (Figure 2(a)). However, with respect to harvested cell numbers, we found no significant differences between macrophages detached from TRPs and from CTRL, where cells were detached by trypsin treatment (Figure 2(b)).
Figure 2.
Culture of human primary monocyte–derived macrophages and THP-1 macrophages on TRPs and CTRL and their detachment. (a) Representative microscopic images of adherent macrophages differentiated for 6 days on TRPs and CTRL are shown in the first (primary monocyte–derived macrophages) and third (THP-1) columns. Representative microscopic images of the TRPs after detaching macrophages for 60 min at 25°C are shown in the second (primary monocyte–derived macrophages) and fourth (THP-1) columns. Magnification: 40×. (b) Quantification of detached macrophages from TRPs by temperature reduction compared to trypsin-treated macrophages from CTRL. The means and standard deviations of three independent experiments are shown.
Viability
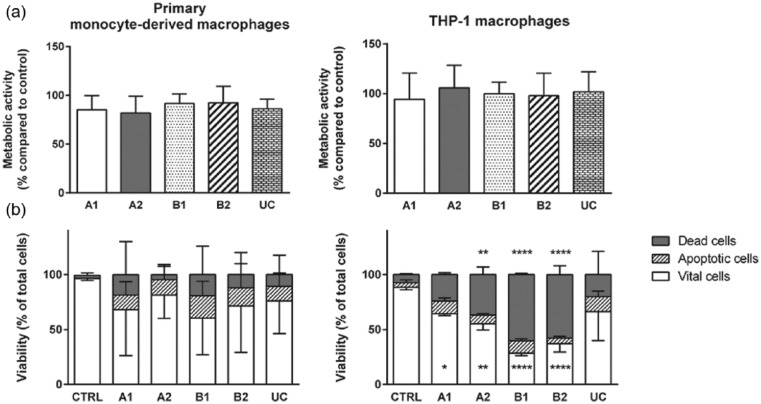
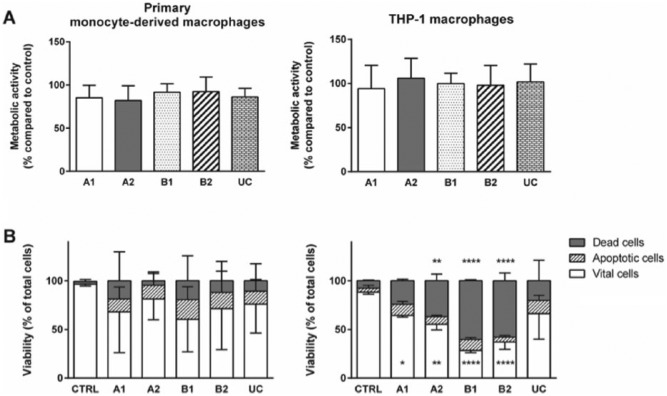
Cell viability and metabolic activity were measured using the MTT assay. We observed no differences in the metabolic activity of attached macrophages cultured on TRPs and CTRL, indicating that adhesive cells were not altered in their metabolic capacity by the cell substrate (Figure 3(a)). Furthermore, after cell detachment from TRPs and from CTRL, the cell viability and induction of cell death were assayed by Annexin V and 7-AAD staining (Figure 3(b)). For human primary monocyte–derived macrophages, we found no differences after TRP detachment compared to trypsin-treated cells from CTRL. However, viability of THP-1 macrophages was significantly reduced after detachment from PVME-based TRPs compared to CTRL. In addition, THP-1 macrophages detached from A2, B1, and B2 exhibited an increased number of dead cells, which was not observed after cell detachment from UC (Figure 3(b)).
Figure 3.
Metabolic activity and vitality state of macrophages attached to TRPs and CTRL (a) and detached (b) from TRPs and CTRL. (a) MTT assay of primary monocyte–derived macrophages (left panel) and THP-1 macrophages (right panel). Data obtained were set into relation to data from macrophages on CTRL. (b) Viability analysis of detached primary monocyte–derived macrophages (left panel) and THP-1 macrophages (right panel) were performed by flow cytometry. Cells were stained with 7-AAD (dead cells) and Annexin V PE (apoptotic cells) allowing discrimination of vital, apoptotic, and dead cells. For better view, error bars are only shown in one direction. For MTT and vitality assays, the means and standard deviations of three independent experiments are shown. (b) Asterisks inside the bar represent significant changes in vital cells detached from TRPs compared to detached cells from CTRL. Asterisks above the bars represent significant changes of dead cells from TRPs compared to CTRL.
Phagocytosis
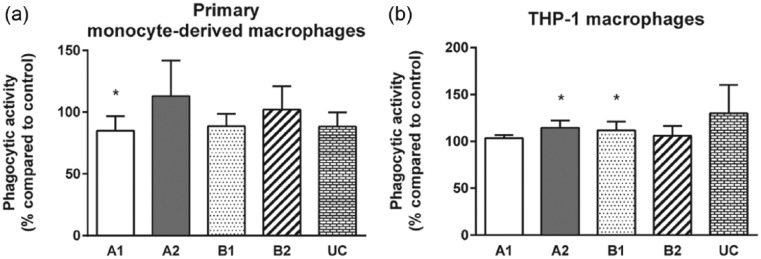
Phagocytosis of microbial components and endogenous cell debris is a major function of differentiated macrophages in vivo. Phagocytosis capability was therefore analyzed by quantification of pHrodo™ E. coli bioparticles internalized within 24 h by adherent macrophages cultured on TRPs and on CTRL (Figure 4 and Supplementary Figure 3). Although no differences to CTRL were measured for attached primary monocyte–derived macrophages cultured on TRPs A2, B2, and UC, macrophages cultured on TRP A1 displayed a significant decrease in their phagocytosis activity (mean ± SD: 85% ± 12%) compared to CTRL (Figure 4(a)).
Figure 4.
Effect of TRPs on phagocytic activity of adherent macrophages. Phagocytosis of opsonized pHrodoTM Escherichia coli BioParticles® for 24 h by (a) primary monocyte–derived macrophages and (b) THP-1 macrophages is shown for the indicated TRPs compared to CTRL. Relation of MFI of phagocytosed fluorescent pHrodo particles in macrophages on TRPs to MFI of phagocytosed fluorescent pHrodo particles in macrophages on the control surface is shown. The means and standard deviations of four independent experiments are shown.
For adherent THP-1 macrophages, statistically significant differences were detected for TRPs A2 and B1 when compared to CTRL. On these TRPs, the efficiency of macrophage phagocytosis was marginally increased (A2: 114% ± 8%; B1: 112% ± 9%; Figure 4(b)).
Cytokine expression and secretion
Macrophages can become easily activated by contact to MAMPs but also xenobiotic substrates such as biomaterials used in tissue engineering which is not desirable.28,29 In addition, it is necessary to maintain the reactivity of macrophages to MAMPs, for example, LPS, to achieve responsive macrophages for tissue engineering applications. Activation of macrophages is thereby associated with the expression and release of various cytokines including pro-inflammatory cytokines TNF, IL-1β, as well as the anti-inflammatory cytokine IL-10.3 We therefore measured the mRNA expression and the release of these three cytokines by adherent macrophages cultured on the respective TRPs. Furthermore, the inducibility of macrophage immune response upon LPS stimulation was characterized.
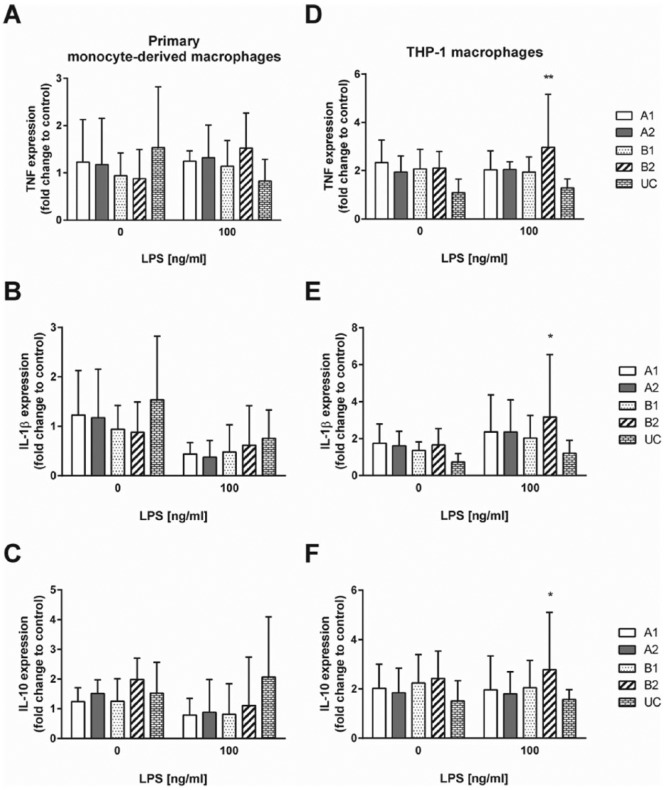
Attached human primary monocyte–derived macrophages displayed no significant differences in TNF, IL-1β, and IL-10 mRNA expression on all investigated TRPs compared to CTRL, irrespective of whether they were left untreated or challenged with LPS for 24 h (Supplementary Figure 4(a)–(c)). In contrast, adherent THP-1 macrophages showed significantly higher TNF, IL-1β, and IL-10 mRNA expression on TRP B2 (Supplementary Figure 4(d)–(f)).
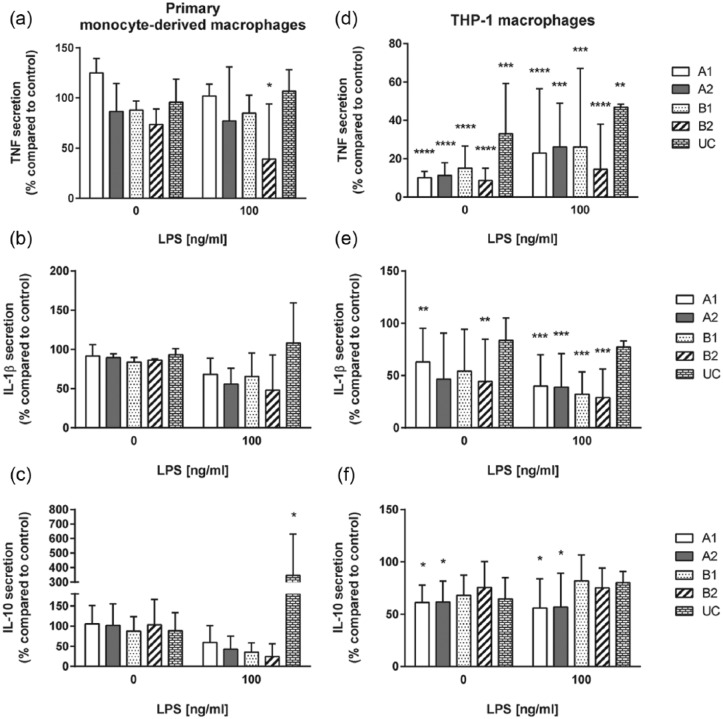
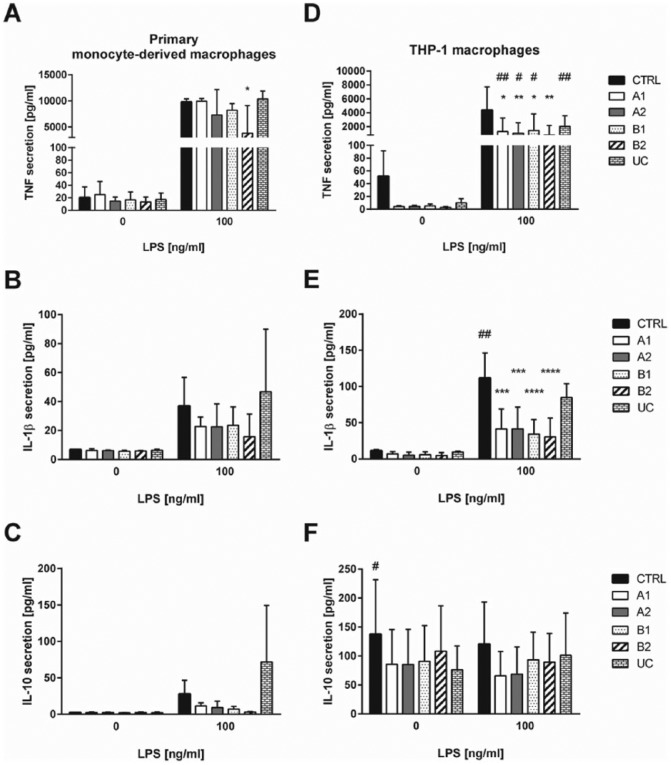
Significant differences for cytokine secretion were observed for adherent primary monocyte–derived macrophages and THP-1 macrophages (Figure 5 and Supplementary Figure 5). Primary monocyte–derived macrophages showed similar secretion levels of cytokines when cultured on TRPs and CTRL. However, after LPS treatment, adherent macrophages cultured on B2 secreted a significantly reduced amount of TNF. Furthermore, attached macrophages cultured on UC exhibited a significantly increased IL-10 secretion upon LPS challenge (Figure 5(a) and (c) and Supplementary Figure 5(a)).
Figure 5.
Cytokine secretion of adherent macrophages grown on TRPs and CTRL surface. Secretion of TNF, IL-1β, and IL-10 by unstimulated and 100 ng/mL LPS stimulated primary monocyte–derived macrophages is shown in (a)–(c). Secretion of TNF, IL-1b, and IL-10 by unstimulated and 100 ng/mL LPS stimulated THP-1 macrophages is shown in (d)–(f). Stimulation was performed for 24 h. Data from attached macrophages on TRPs were set into relation to data from macrophages adhered to CTRL. The mean and standard deviation of three independent experiments are shown.
Adherent THP-1 macrophages displayed a significantly reduced secretion of TNF compared to adherent primary monocyte–derived macrophages on TRP A1, A2, B1, and UC (Supplementary Figure 5(d)). THP-1 macrophages further secreted significantly reduced cytokine levels of TNF and IL-1β on all PVME-based TRPs compared to CTRL, either left untreated or LPS challenged (Figure 5(d) and (e)). In addition, THP-1 macrophages cultured on UC showed a significantly reduced TNF secretion (Figure 5(d)). For IL-10 secretion, we only found minor differences depending on TRP substrate or LPS stimulation. Secretion of IL-10 by THP-1 macrophages was reduced on TRP A1 and A2 (Figure 5(f)). Furthermore, THP-1 did not show any induction of IL-10 secretion after LPS treatment, which is in line with previous reports.30 All observations are summarized in Tables 3 and 4.
Table 3.
Trends observed with different assays for human primary monocyte–derived macrophages on TRPs and CTRL.
| Read-out | TRP |
||||
|---|---|---|---|---|---|
| A1 | A2 | B1 | B2 | UC | |
| Detachment | = | = | = | = | = |
| Vitality | = | = | = | = | = |
| Phagocytosis | < | = | = | = | = |
| TNF secretion untreated | = | = | = | = | = |
| TNF secretion + LPS | = | = | = | < | = |
| IL-1β secretion untreated | = | = | = | = | = |
| IL-1β secretion + LPS | = | = | = | = | = |
| IL-10 secretion untreated | = | = | = | = | = |
| IL-10 secretion + LPS | = | = | = | = | > |
TRP: thermo-responsive polymer; TNF: tumor necrosis factor; LPS: lipopolysaccharide; IL: interleukin; =: similar to CTRL; <: lower than CTRL; >: higher than CTRL.
CTRL was set as standard.
Discussion
Today’s standard method to detach macrophages relies on enzymatic digestion of surface molecules. However, it is known that enzymatic digestion could harm macrophages marker expression and functionality. Chen et al. recently observed significant impact of enzymatic cell detachment by trypsin treatment on macrophage functionality. They detected reduced macrophage expression of CD14, CD163 and CD206, decreased uptake of hemoglobin A0/haptoglobin, and dextran after macrophages detachment.4 Furthermore, it was shown that partial cleavage of CD14 by enzymatic digestion correlates with the loss of the functional response to LPS.31,32 This could potentially introduce bias in studies involving macrophages intended for functional studies. The use of recently established TRPs could allow to circumvent these drawbacks. Therefore, we evaluated in this study whether custom-made TRPs could be useful as non-activating cell substrates and alternative detachment approach of macrophages.
Adhesive THP-1 macrophages on TRPs already exhibited a significant decline in cytokine secretion compared to culture on uncoated surfaces already in the absence of LPS. As cytokine mRNA expression of macrophages on TRPs was not significantly reduced compared to macrophages grown on uncoated surfaces, the reduced cytokine secretion observed could be a result of a diminished protein stability or an impaired cytokine exocytosis. However, this has to be proven in follow-up studies. In contrast, macrophages derived from primary monocytes and cultured on TRPs were fully responsive to LPS. Furthermore, significant differences in secreted TNF levels between primary monocyte–derived macrophages and THP-1 macrophages were observed. Schildberger et al.30 had demonstrated that THP-1 monocytes exhibit significantly reduced TNF secretion compared to primary monocytes upon LPS challenge. This impairment was also seen for THP-1 macrophages cultured on uncoated surfaces compared to macrophages derived from primary monocytes.
The effects of TRPs on cytokine secretion by murine macrophages were studied by others.33,34 Fan et al. used the cell line RAW264.7 cultured on PNiPAAm polymers in different compositions. They observed an increase in IL-1β secretion when growing cells for 48 h without additional stimulation. Bygd et al.34 have demonstrated that functionalized PNiPAAm particles with different chemical modifications induce specific cytokine secretion of TNF, IL-10, and arginase. These results, however, are difficult to extrapolate to our study, as differences might be related to variations in the chemical composition of the polymers, species-related differences of macrophages, and a shortened cell incubation time.
Both macrophage types adhesive on TRPs and CTRL efficiently phagocytose microbial particles. We demonstrated that macrophages cultured on PNiPAAm or PVME-based polymers were not altered in their phagocytic capacity. Phagocytosis of THP-1 macrophages on UC surface was also investigated by Maeß et al.35 Although different particles and different time points were used, both studies show no effect of the UC surface on macrophage phagocytosis when compared to control.
The characterized thermo-responsive carriers allowed an appropriate initial adhesion of macrophages and a decrease in temperature for 1 h to 25°C resulted in the expected detachment of almost all adherent macrophages. The number of detached macrophages obtained from TRPs and uncoated surfaces were comparable. Similar results were found by Collier et al.,36 where monocyte adhesion and macrophage detachment were studied on different PNiPAAm-coated surfaces and compared to tissue culture polystyrene substrates. Collier et al. measured cell viability by determination of the number of adhesive monocytes upon subculture. Here, we have given additional insight into the effect of the thermo-responsive carriers by analyzing the metabolic activity of attached macrophages including a quantification of the percentage of vital, apoptotic, and dead cells after detachment from TRPs. We demonstrated that THP-1 macrophages had a higher percentage of dead cells after detachment from TRPs A1, A2, B1, and B2 compared to enzymatically harvested cells. In contrast, we found no differences in viability for human primary monocyte–derived macrophages detached from TRPs and uncoated surfaces.
THP-1 macrophage differentiation is induced by TPA stimulation. However, THP-1 macrophages dedifferentiate again into a monocytic cell types when TPA is detracted from the cell culture medium.37 In addition, a prolonged culture of THP-1 macrophages with the diacylglycerol analogue TPA will likely lead to physiological alterations in co-cultured cells. Due to this limitation in macrophage differentiation of THP-1 and the known impairment of cytokine secretion ability upon LPS stimulation, this cell line might not be an optimal model to study inflammatory responses in bioengineered tissues.
Conclusion
In conclusion, our study reveals that along with commercially available UCs, the TRPs A2 (PVME50-PNiPAAm40-PVMEMA10) and B1 (PVME90-PVMEMA10) appear to be attractive candidates for differentiation of primary monocyte–derived macrophages. Macrophages were shown to adhere to these polymers without additional activation and show similar cellular functionality like macrophages on conventional cell culture surfaces. These macrophages are vital, show no signs of an unintended activation, and maintain their responsiveness to inflammatory triggers when cultured on TRPs.
Although there is no obvious advantage for the functionality of primary monocyte–derived macrophages on TRPs compared to standard cell culture surface, the custom-made TRPs exhibit two important benefits: First, TRPs as cell substrate permits the reduction of centrifugation and washing steps, thereby minimizing the risk of cell loss. Second, and most important, these TRPs owe maleic acid groups that can be converted into reactive anhydride moieties allowing covalent protein binding. In the future, it could be important to polarize macrophages depending on the favored organ model or application. Macrophages orchestrate acute inflammation, host defense, and resolution of inflammation where they undergo marked phenotypic and functional changes toward pro-inflammatory M1 and anti-inflammatory M2 subtypes.38 In this simplified model, M1 and M2 are hallmarks of two opposite activation stages within a continuum and rapid transitions among each other. Custom-made TRPs allow the coupling of a set of bioactive substances, that is, chemokines or small molecule agonists could be bound to the anhydride moieties before seeding monocytes. These functionalized polymers would allow the induction and collection of macrophages in an already pre-defined differentiation and polarization state for subsequent tissue integration.
This characteristic feature is unique to our novel TRP specimens, which have been thoroughly characterized for its impact on macrophage physiology.
Supplementary Material
Acknowledgments
The authors thank Stefan Gramm and Juliane Teichmann (formerly at Leibniz Institute of Polymer Research Dresden) for providing PVME-based cell culture carriers. We are grateful to Maria Franke and Margot Voigt for their excellent technical assistance. K.R., M.W., N.K., and M.R. performed experiments, analyzed, and interpreted data. M.W. and S.L. wrote parts of the manuscript and interpreted data. K.R., M.N., and A.S.M. designed the study and wrote the manuscript. A.S.M. supervised the study. All authors read and approved the final manuscript.
Supplementary Information
Supplementary Figure 1.
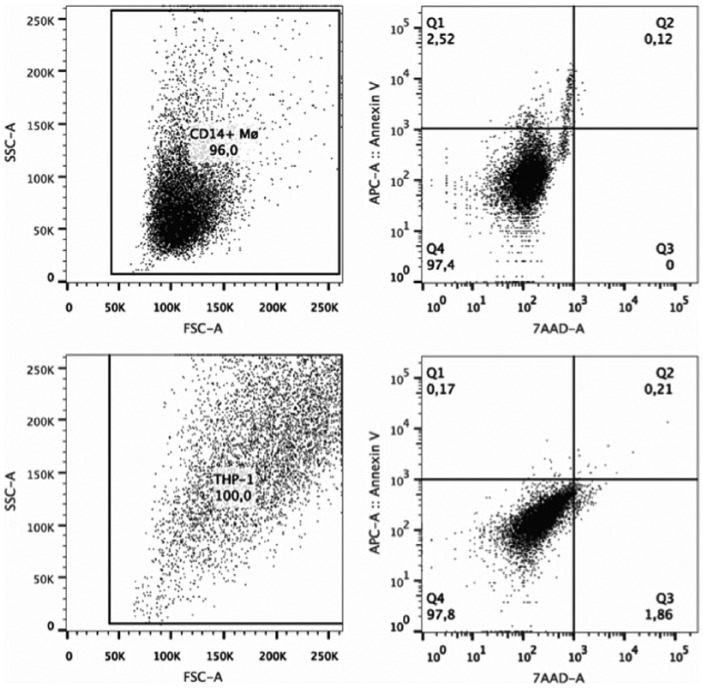
Gating for macrophage viability assay. Upper panel: primary monocyte–derived macrophages, lower panel: THP-1 macrophages. Representative analyses of macrophages grown on CTRL and detached by trypsin are shown. Regions Q1: apoptotic cells; Q2/Q3: dead cells; Q4: viable cells.
Supplementary Figure 2.
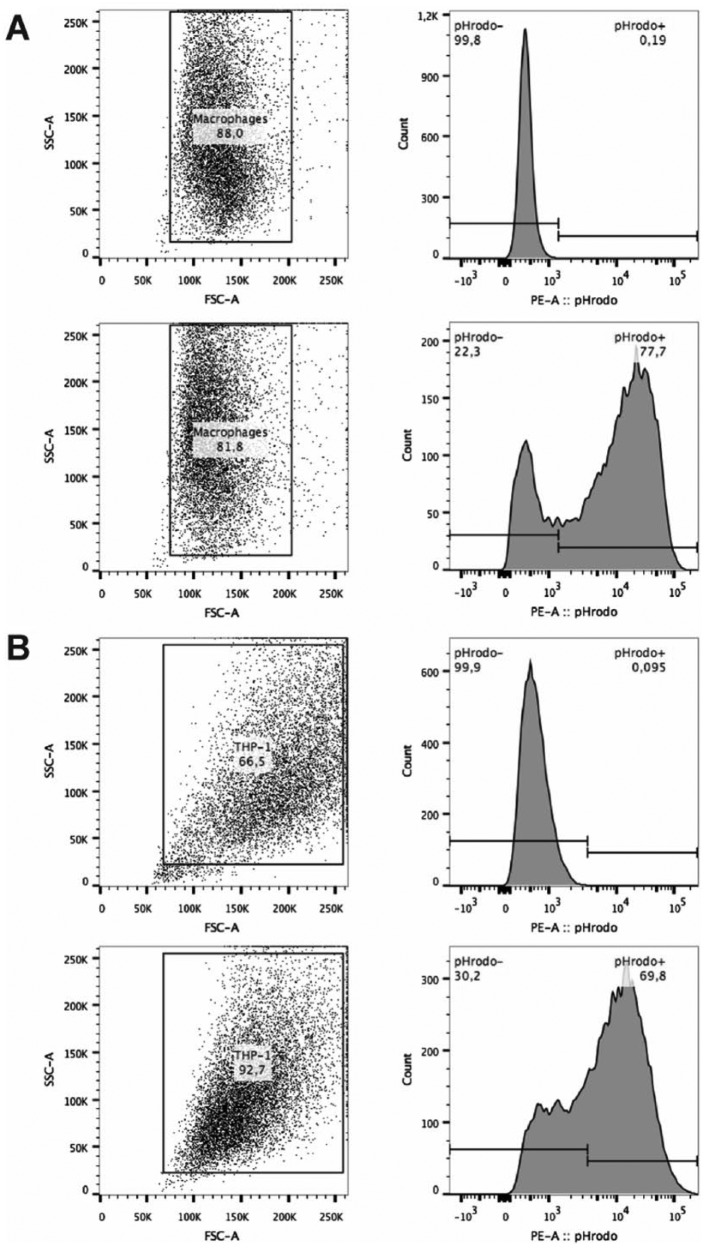
Gating strategy for flow cytometry analysis of phagocytosis of macrophages: Two upper panels: primary monocyte–derived macrophages, two lower panels: THP-1 macrophages. Representative analyses of macrophages grown on CTRL, incubated with or without opsonized pHrodoTM BioParticles for 24 h and detached by trypsin are shown. Gating for macrophage populations and their representative histogram plot for pHrodoTM MFI is shown.
Supplementary Figure 3.
Phagocytosis of macrophages on TRPs and CTRL. Macrophages were incubated for 24 h with opsonized pHrodoTM Escherichia coli BioParticles and subsequently detached by temperature reduction or trypsin treatment and analyzed by flow cytometry. Percentages of pHrodo positive primary monocyte–derived macrophages and THP-1 macrophages are shown in (A) and (B), respectively. (C) and (D) show MFI of intracellular pHrodo in primary monocyte–derived macrophages and THP-1 macrophages, respectively. A–D: The mean and standard deviation of four independent experiments are shown.
Supplementary Figure 4.
Cytokine expression of macrophages grown on TRPs and CTRL. After 6 days of differentiation adherent macrophages were stimulated with 100 ng/mL LPS or left untreated for 24 h. Expression levels in primary monocyte–derived macrophages (A) to (C) for TNF (A), IL-1β (B), and IL-10 (C) grown on TRPs compared to CTRL are shown. Expression levels in THP-1 macrophages (D) to (F) for TNF (D), IL-1β (E), and IL-10 (F) grown on TRPs compared to CTRL are shown. The means and standard deviations of four independent experiments are shown.
Supplementary Figure 5.
Cytokine secretion of macrophages grown on TRPs and CTRL. Six days differentiated and adherent macrophages were stimulated with 100 ng/mL LPS or left untreated for 24 h. Secretion levels (pg/mL) of primary monocyte–derived macrophages (A) to (C) for TNF (A), IL-1β (B), and IL-10 (C) grown on TRPs and CTRL are shown. Secretion levels (pg/mL) of THP-1 macrophages (D) to (F) for TNF (D), IL-1β (E), and IL-10 (F) grown on TRPs and CTRL are shown. The means and standard deviations of three independent experiments are shown.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Galli SJ, Borregaard N, Wynn TA. Phenotypic and functional plasticity of cells of innate immunity: macrophages, mast cells and neutrophils. Nat Immunol 2011; 12: 1035–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol 2011; 11: 723–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Davies LC, Jenkins SJ, Allen JE, et al. Tissue-resident macrophages. Nat Immunol 2013; 14: 986–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen S, So EC, Strome SE, et al. Impact of detachment methods on M2 macrophage phenotype and function. J Immunol Methods 2015; 426: 56–61. [DOI] [PubMed] [Google Scholar]
- 5. Yang J, Yamato M, Nishida K, et al. Cell delivery in regenerative medicine: the cell sheet engineering approach. J Control Release 2006; 116: 193–203. [DOI] [PubMed] [Google Scholar]
- 6. Schild HG. Poly(N-isopropylacrylamide): experiment, theory and application. Prog Polym Sci 1992; 17: 163–249. [Google Scholar]
- 7. Schafer-Soenen H, Moerkerke R, Berghmans H, et al. Zero and off-zero critical concentrations in systems containing polydisperse polymers† with very high molar masses. 2. The system water-poly(vinyl methyl ether). Macromolecules 1997; 30: 410–416. [Google Scholar]
- 8. Da Silva RMP, Mano JF, Reis RL. Smart thermoresponsive coatings and surfaces for tissue engineering: switching cell-material boundaries. Trends Biotechnol 2007; 25: 577–583. [DOI] [PubMed] [Google Scholar]
- 9. Gramm S, Teichmann J, Nitschke M, et al. Electron beam immobilization of functionalized poly(vinyl methyl ether) thin films on polymer surfaces—towards stimuli responsive coatings for biomedical purposes. Express Polym Lett 2011; 5: 970–976. [Google Scholar]
- 10. Schmaljohann D, Beyerlein D, Nitschke M, et al. Thermo-reversible swelling of thin hydrogel films immobilized by low-pressure plasma. Langmuir 2004; 20: 10107–10114. [DOI] [PubMed] [Google Scholar]
- 11. Mendez S, Andrzejewski BP, Canavan HE, et al. Understanding the force-vs-distance profiles of terminally attached poly(N-isopropyl acrylamide) thin films. Langmuir 2009; 25: 10624–10632. [DOI] [PubMed] [Google Scholar]
- 12. Cordeiro AL, Zimmermann R, Gramm S, et al. Temperature dependent physicochemical properties of poly(N-isopropylacrylamide-co-N-(1-phenylethyl) acrylamide) thin films. Soft Matter 2009; 5: 1367–1377. [Google Scholar]
- 13. Kooij ES, Sui X, Hempenius MA, et al. Probing the thermal collapse of poly(N-isopropylacrylamide) grafts by quantitative in situ ellipsometry. J Phys Chem B 2012; 116: 9261–9268. [DOI] [PubMed] [Google Scholar]
- 14. Ishida N, Biggs S. Effect of grafting density on phase transition behavior for poly(N-isopropylacrylamide) brushes in aqueous solutions studied by AFM and QCM-D. Macromolecules 2010; 43: 7269–7276. [Google Scholar]
- 15. Uhlig K, Wischerhoff E, Lutz J-F, et al. Monitoring cell detachment on PEG-based thermoresponsive surfaces using TIRF microscopy. Soft Matter 2010; 6: 4262–4267. [Google Scholar]
- 16. Fukumori K, Akiyama Y, Kumashiro Y, et al. Characterization of ultra-thin temperature-responsive polymer layer and its polymer thickness dependency on cell attachment/detachment properties. Macromol Biosci 2010; 10: 1117–1129. [DOI] [PubMed] [Google Scholar]
- 17. Canavan HE, Cheng X, Graham DJ, et al. Cell sheet detachment affects the extracellular matrix: a surface science study comparing thermal liftoff, enzymatic, and mechanical methods. J Biomed Mater Res A 2005; 75: 1–13. [DOI] [PubMed] [Google Scholar]
- 18. Teichmann J, Nitschke M, Pette D, et al. Thermo-responsive cell culture carriers based on poly(vinyl methyl ether)—the effect of biomolecular ligands to balance cell adhesion and stimulated detachment. Sci Technol Adv Mater 2015; 16: 045003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Teichmann J, Valtink M, Gramm S, et al. Human corneal endothelial cell sheets for transplantation: thermo-responsive cell culture carriers to meet cell-specific requirements. Acta Biomater 2013; 9: 5031–5039. [DOI] [PubMed] [Google Scholar]
- 20. Qin Z. The use of THP-1 cells as a model for mimicking the function and regulation of monocytes and macrophages in the vasculature. Atherosclerosis 2012; 221: 2–11. [DOI] [PubMed] [Google Scholar]
- 21. Chanput W, Mes JJ, Wichers HJ. THP-1 cell line: an in vitro cell model for immune modulation approach. Int Immunopharmacol 2014; 23: 37–45. [DOI] [PubMed] [Google Scholar]
- 22. Lacey DC, Achuthan A, Fleetwood J, et al. Defining GM-CSF- and macrophage-CSF-dependent macrophage responses by in vitro models. J Immunol 2012; 188: 5752–5765. [DOI] [PubMed] [Google Scholar]
- 23. Erbel C, Rupp G, Helmes CM, et al. An in vitro model to study heterogeneity of human macrophage differentiation and polarization. J Vis Exp 2013; 76: e50332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mosig S, Rennert K, Büttner P, et al. Monocytes of patients with familial hypercholesterolemia show alterations in cholesterol metabolism. BMC Med Genomics 2008; 1: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mosig S, Rennert K, Krause S, et al. Different functions of monocyte subsets in familial hypercholesterolemia: potential function of CD14+ CD16+ monocytes in detoxification of oxidized LDL. FASEB J 2009; 23: 866–874. [DOI] [PubMed] [Google Scholar]
- 26. Stolle K, Schnoor M, Fuellen G, et al. Cloning, genomic organization, and tissue-specific expression of the RASL11B gene. Biochim Biophys Acta 2007; 1769: 514–524. [DOI] [PubMed] [Google Scholar]
- 27. Stolle K, Schnoor M, Fuellen G, et al. Cloning, cellular localization, genomic organization, and tissue-specific expression of the TGFbeta1-inducible SMAP-5 gene. Gene 2005; 351: 119–130. [DOI] [PubMed] [Google Scholar]
- 28. Xia Z, Triffitt JT. A review on macrophage responses to biomaterials. Biomed Mater 2006; 1: R1–R9. [DOI] [PubMed] [Google Scholar]
- 29. Rostam HM, Singh S, Vrana NE, et al. Impact of surface chemistry and topography on the function of antigen presenting cells. Biomater Sci 2015; 3: 424–441. [DOI] [PubMed] [Google Scholar]
- 30. Schildberger A, Rossmanith E, Eichhorn T, et al. Monocytes, peripheral blood mononuclear cells, and THP-1 cells exhibit different cytokine expression patterns following stimulation with lipopolysaccharide. Mediators Inflamm 2013; 2013: 697972-1–697972-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Smith PD, Smythies LE, Mosteller-Barnum M, et al. Intestinal macrophages lack CD14 and CD89 and consequently are down-regulated for LPS- and IgA-mediated activities. J Immunol 2001; 167: 2651–2656. [DOI] [PubMed] [Google Scholar]
- 32. Le-Barillec K, Si-Tahar M, Balloy V, et al. Proteolysis of monocyte CD14 by human leukocyte elastase inhibits lipopolysaccharide-mediated cell activation. J Clin Invest 1999; 103: 1039–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fan X, Nosov M, Carroll W, et al. Macrophages behavior on different NIPAm-based thermoresponsive substrates. J Biomed Mater Res A 2014; 102: 2901–2910. [DOI] [PubMed] [Google Scholar]
- 34. Bygd HC, Forsmark KD, Bratlie KM. Altering in vivo macrophage responses with modified polymer properties. Biomaterials 2015; 56: 187–197. [DOI] [PubMed] [Google Scholar]
- 35. Maeß MB, Keller AA, Rennert K, et al. Optimization of the transfection of human THP-1 macrophages by application of Nunc UpCell technology. Anal Biochem 2015; 479: 40–42. [DOI] [PubMed] [Google Scholar]
- 36. Collier TO, Anderson JM, Kikuchi A, et al. Adhesion behavior of monocytes, macrophages, and foreign body giant cells on poly(N-isopropylacrylamide) temperature-responsive surfaces. J Biomed Mater Res A 2002; 59: 136–143. [DOI] [PubMed] [Google Scholar]
- 37. Spano A, Barni S, Sciola L. PMA withdrawal in PMA-treated monocytic THP-1 cells and subsequent retinoic acid stimulation, modulate induction of apoptosis and appearance of dendritic cells. Cell Prolif 2013; 46: 328–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Glass CK, Natoli G. Molecular control of activation and priming in macrophages. Nat Immunol 2015; 17: 26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.