Abstract
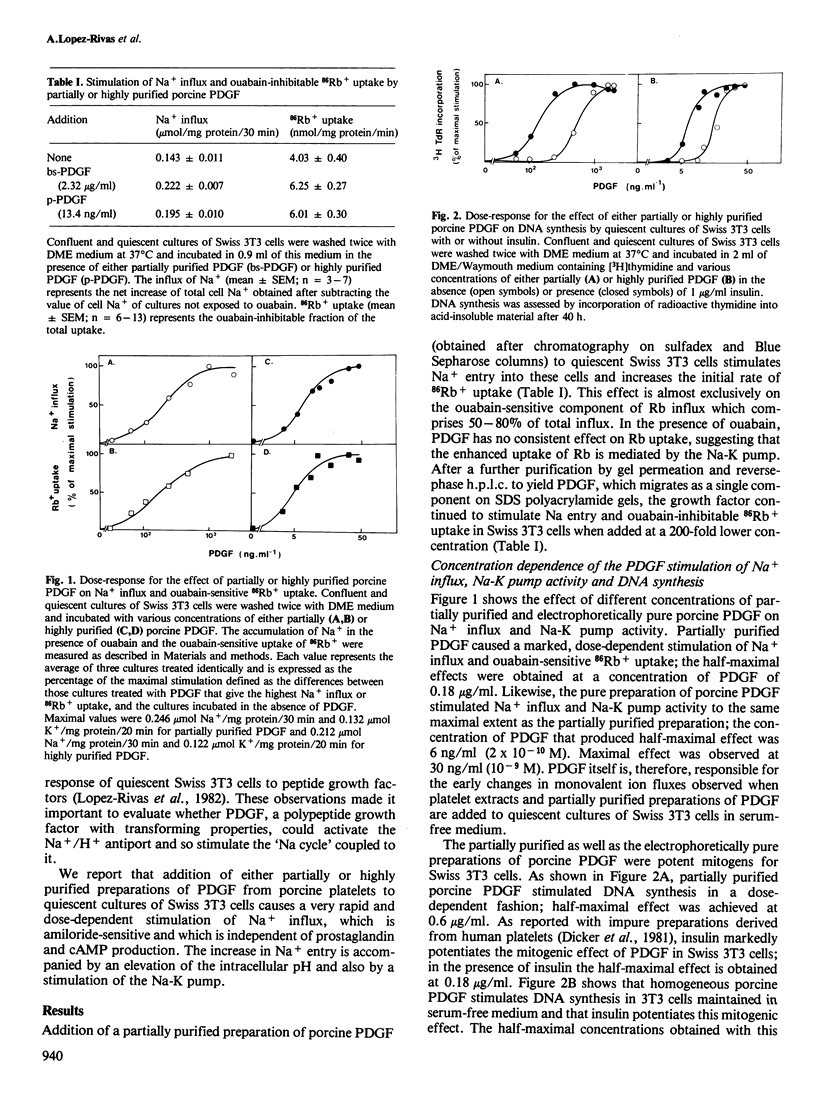
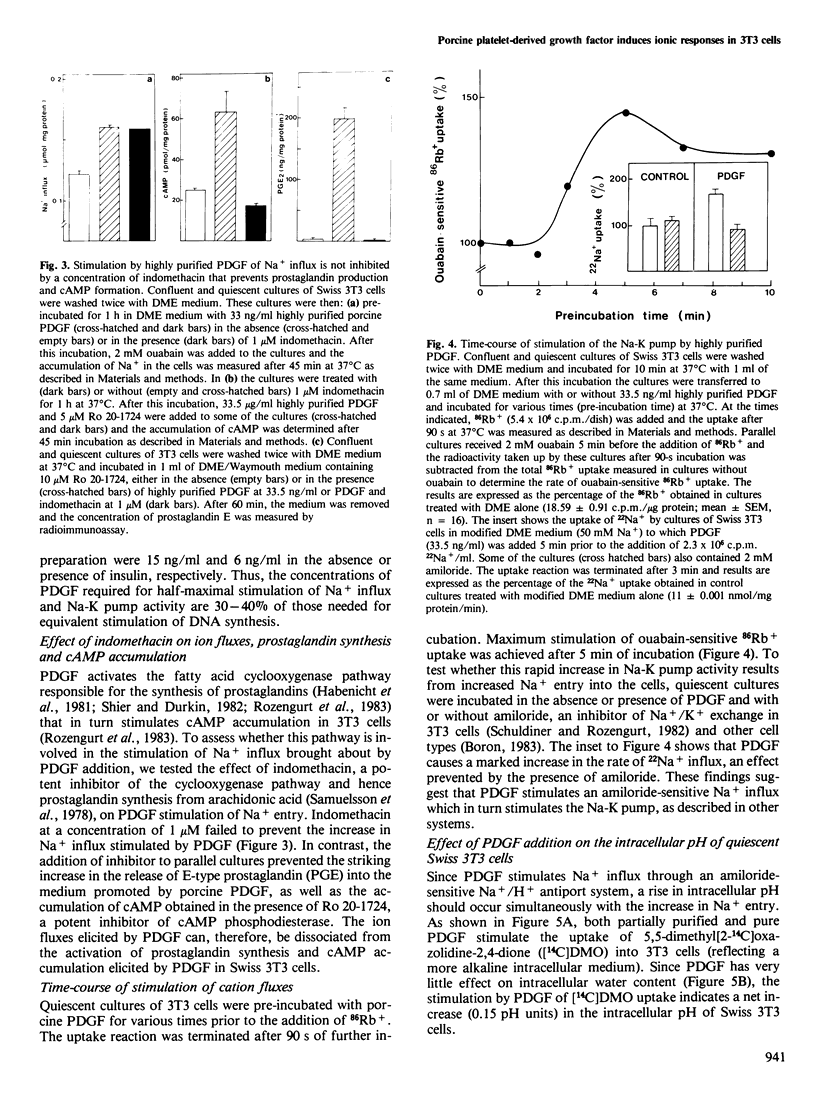
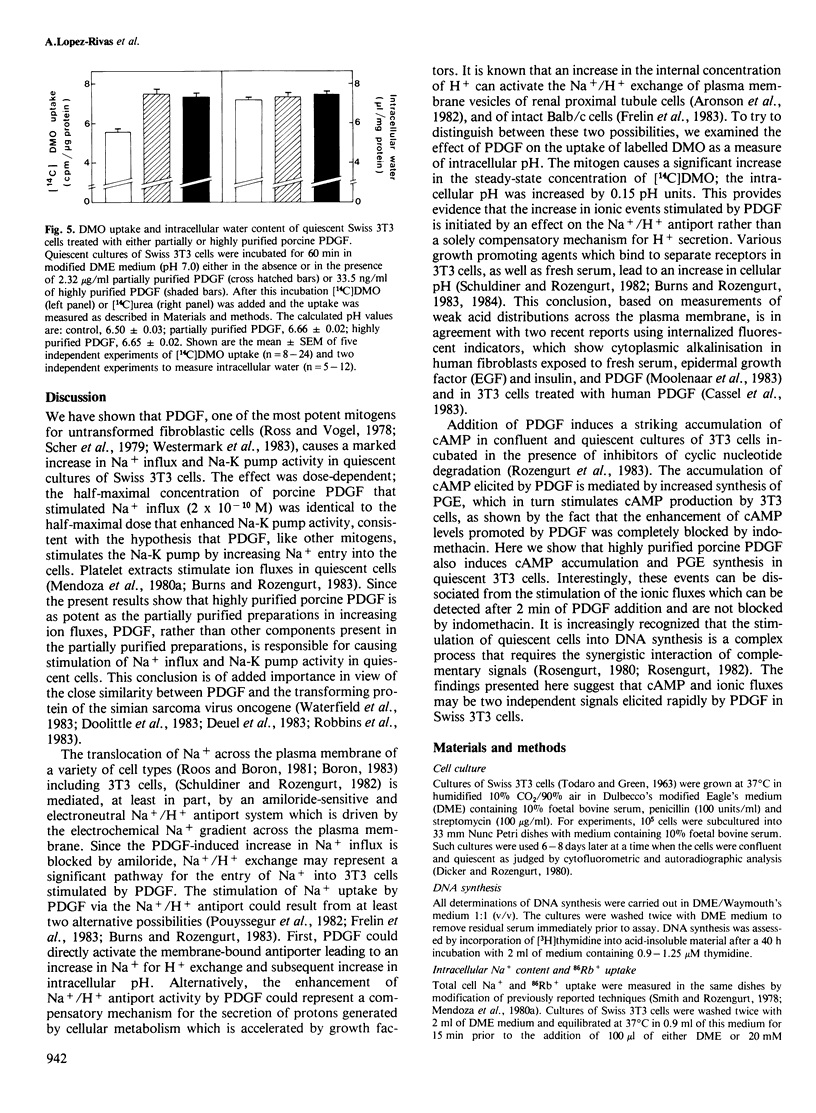
Addition of porcine platelet-derived growth factor (PDGF) to quiescent cultures of Swiss 3T3 cells caused a marked, dose-dependent stimulation of Na+ influx and Na-K pump-mediated 86Rb+ uptake. Porcine PDGF (a single component in SDS polyacrylamide gels) stimulated ion fluxes to the same maximal extent as partially purified preparations, and exhibited half-maximal effect at 6 ng/ml (2 X 10(-10) M). Maximal effect was achieved at 30 ng/ml (10(-9) M). In the presence of insulin, PDGF elicited mitogenesis at comparable concentrations. PDGF stimulated ion uptake in a time-dependent fashion; maximal effect was obtained after 5 min of exposure to the growth factor. PDGF stimulates Na+ influx via an amiloride-sensitive pathway, suggesting that PDGF enhances the activity of a Na+/H+ antiport system. In accordance with this possibility, the mitogen caused an increase of intracellular pH by 0.15 pH units, as judged by the steady-state distribution of labelled 5,5-dimethyloxazolidine-2,4-dione (DMO). Porcine PDGF stimulated E-type prostaglandin synthesis and cAMP accumulation but these events could be dissociated from the stimulation of the ionic fluxes, which was detected within minutes and was not blocked by indomethacin. It is suggested that PDGF elicits multiple signals to stimulate cell proliferation in 3T3 cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antoniades H. N. Human platelet-derived growth factor (PDGF): purification of PDGF-I and PDGF-II and separation of their reduced subunits. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7314–7317. doi: 10.1073/pnas.78.12.7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniades H. N., Scher C. D., Stiles C. D. Purification of human platelet-derived growth factor. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1809–1813. doi: 10.1073/pnas.76.4.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson P. S., Nee J., Suhm M. A. Modifier role of internal H+ in activating the Na+-H+ exchanger in renal microvillus membrane vesicles. Nature. 1982 Sep 9;299(5879):161–163. doi: 10.1038/299161a0. [DOI] [PubMed] [Google Scholar]
- Boron W. F. Transport of H+ and of ionic weak acids and bases. J Membr Biol. 1983;72(1-2):1–16. doi: 10.1007/BF01870311. [DOI] [PubMed] [Google Scholar]
- Bowen-Pope D. F., Ross R. Platelet-derived growth factor. II. Specific binding to cultured cells. J Biol Chem. 1982 May 10;257(9):5161–5171. [PubMed] [Google Scholar]
- Burns C. P., Rozengurt E. Extracellular Na+ and initiation of DNA synthesis: role of intracellular pH and K+. J Cell Biol. 1984 Mar;98(3):1082–1089. doi: 10.1083/jcb.98.3.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns C. P., Rozengurt E. Serum, platelet-derived growth factor, vasopressin and phorbol esters increase intracellular pH in Swiss 3T3 cells. Biochem Biophys Res Commun. 1983 Nov 15;116(3):931–938. doi: 10.1016/s0006-291x(83)80231-9. [DOI] [PubMed] [Google Scholar]
- Cassel D., Rothenberg P., Zhuang Y. X., Deuel T. F., Glaser L. Platelet-derived growth factor stimulates Na+/H+ exchange and induces cytoplasmic alkalinization in NR6 cells. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6224–6228. doi: 10.1073/pnas.80.20.6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins M. K., Sinnett-Smith J. W., Rozengurt E. Platelet-derived growth factor treatment decreases the affinity of the epidermal growth factor receptors of Swiss 3T3 cells. J Biol Chem. 1983 Oct 10;258(19):11689–11693. [PubMed] [Google Scholar]
- Cooper J. A., Bowen-Pope D. F., Raines E., Ross R., Hunter T. Similar effects of platelet-derived growth factor and epidermal growth factor on the phosphorylation of tyrosine in cellular proteins. Cell. 1982 Nov;31(1):263–273. doi: 10.1016/0092-8674(82)90426-3. [DOI] [PubMed] [Google Scholar]
- Deuel T. F., Huang J. S., Huang S. S., Stroobant P., Waterfield M. D. Expression of a platelet-derived growth factor-like protein in simian sarcoma virus transformed cells. Science. 1983 Sep 30;221(4618):1348–1350. doi: 10.1126/science.6310754. [DOI] [PubMed] [Google Scholar]
- Deuel T. F., Huang J. S., Proffitt R. T., Baenziger J. U., Chang D., Kennedy B. B. Human platelet-derived growth factor. Purification and resolution into two active protein fractions. J Biol Chem. 1981 Sep 10;256(17):8896–8899. [PubMed] [Google Scholar]
- Devare S. G., Reddy E. P., Law J. D., Robbins K. C., Aaronson S. A. Nucleotide sequence of the simian sarcoma virus genome: demonstration that its acquired cellular sequences encode the transforming gene product p28sis. Proc Natl Acad Sci U S A. 1983 Feb;80(3):731–735. doi: 10.1073/pnas.80.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicker P., Pohjanpelto P., Pettican P., Rozengurt E. Similarities between fibroblast-derived growth factor and platelet-derived growth factor. Exp Cell Res. 1981 Sep;135(1):221–227. doi: 10.1016/0014-4827(81)90314-1. [DOI] [PubMed] [Google Scholar]
- Dicker P., Rozengurt E. Phorbol ester stimulation of Na influx and Na-K pump activity in Swiss 3T3 cells. Biochem Biophys Res Commun. 1981 May 15;100(1):433–441. doi: 10.1016/s0006-291x(81)80115-5. [DOI] [PubMed] [Google Scholar]
- Dicker P., Rozengurt E. Phorbol esters and vasopressin stimulate DNA synthesis by a common mechanism. Nature. 1980 Oct 16;287(5783):607–612. doi: 10.1038/287607a0. [DOI] [PubMed] [Google Scholar]
- Doolittle R. F., Hunkapiller M. W., Hood L. E., Devare S. G., Robbins K. C., Aaronson S. A., Antoniades H. N. Simian sarcoma virus onc gene, v-sis, is derived from the gene (or genes) encoding a platelet-derived growth factor. Science. 1983 Jul 15;221(4607):275–277. doi: 10.1126/science.6304883. [DOI] [PubMed] [Google Scholar]
- Ek B., Heldin C. H. Characterization of a tyrosine-specific kinase activity in human fibroblast membranes stimulated by platelet-derived growth factor. J Biol Chem. 1982 Sep 10;257(17):10486–10492. [PubMed] [Google Scholar]
- Ek B., Westermark B., Wasteson A., Heldin C. H. Stimulation of tyrosine-specific phosphorylation by platelet-derived growth factor. Nature. 1982 Feb 4;295(5848):419–420. doi: 10.1038/295419a0. [DOI] [PubMed] [Google Scholar]
- Frelin C., Vigne P., Lazdunski M. The amiloride-sensitive Na+/H+ antiport in 3T3 fibroblasts. J Biol Chem. 1983 May 25;258(10):6272–6276. [PubMed] [Google Scholar]
- Gerrard J. M., Phillips D. R., Rao G. H., Plow E. F., Walz D. A., Ross R., Harker L. A., White J. G. Biochemical studies of two patients with the gray platelet syndrome. Selective deficiency of platelet alpha granules. J Clin Invest. 1980 Jul;66(1):102–109. doi: 10.1172/JCI109823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habenicht A. J., Glomset J. A., King W. C., Nist C., Mitchell C. D., Ross R. Early changes in phosphatidylinositol and arachidonic acid metabolism in quiescent swiss 3T3 cells stimulated to divide by platelet-derived growth factor. J Biol Chem. 1981 Dec 10;256(23):12329–12335. [PubMed] [Google Scholar]
- Heldin C. H., Westermark B., Wasteson A. Platelet-derived growth factor. Isolation by a large-scale procedure and analysis of subunit composition. Biochem J. 1981 Mar 1;193(3):907–913. doi: 10.1042/bj1930907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin C. H., Westermark B., Wasteson A. Platelet-derived growth factor: purification and partial characterization. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3722–3726. doi: 10.1073/pnas.76.8.3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin C. H., Westermark B., Wasteson A. Specific receptors for platelet-derived growth factor on cells derived from connective tissue and glia. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3664–3668. doi: 10.1073/pnas.78.6.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J. S., Huang S. S., Kennedy B., Deuel T. F. Platelet-derived growth factor. Specific binding to target cells. J Biol Chem. 1982 Jul 25;257(14):8130–8136. [PubMed] [Google Scholar]
- Koch K. S., Leffert H. L. Increased sodium ion influx is necessary to initiate rat hepatocyte proliferation. Cell. 1979 Sep;18(1):153–163. doi: 10.1016/0092-8674(79)90364-7. [DOI] [PubMed] [Google Scholar]
- Kohler N., Lipton A. Platelets as a source of fibroblast growth-promoting activity. Exp Cell Res. 1974 Aug;87(2):297–301. doi: 10.1016/0014-4827(74)90484-4. [DOI] [PubMed] [Google Scholar]
- Lopez-Rivas A., Adelberg E. A., Rozengurt E. Intracellular K+ and the mitogenic response of 3T3 cells to peptide factors in serum-free medium. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6275–6279. doi: 10.1073/pnas.79.20.6275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza S. A., Wigglesworth N. M., Pohjanpelto P., Rozengurt E. Na entry and Na-K pump activity in murine, hamster, and human cells--effect of monensin, serum, platelet extract, and viral transformation. J Cell Physiol. 1980 Apr;103(1):17–27. doi: 10.1002/jcp.1041030104. [DOI] [PubMed] [Google Scholar]
- Mendoza S. A., Wigglesworth N. M., Rozengurt E. Vasopressin rapidly stimulates Na entry and Na-K pump activity in quiescent cultures of mouse 3T3 cells. J Cell Physiol. 1980 Oct;105(1):153–162. doi: 10.1002/jcp.1041050117. [DOI] [PubMed] [Google Scholar]
- Miletich J. P., Broze G. J., Jr, Majerus P. W. The synthesis of sulfated dextran beads for isolation of human plasma coagulation factors II, IX, and X. Anal Biochem. 1980 Jul 1;105(2):304–310. doi: 10.1016/0003-2697(80)90462-5. [DOI] [PubMed] [Google Scholar]
- Moolenaar W. H., Boonstra J., van der Saag P. T., de Laat S. W. Sodium/proton exchange in mouse neuroblastoma cells. J Biol Chem. 1981 Dec 25;256(24):12883–12887. [PubMed] [Google Scholar]
- Moolenaar W. H., Tsien R. Y., van der Saag P. T., de Laat S. W. Na+/H+ exchange and cytoplasmic pH in the action of growth factors in human fibroblasts. Nature. 1983 Aug 18;304(5927):645–648. doi: 10.1038/304645a0. [DOI] [PubMed] [Google Scholar]
- Moolenaar W. H., Yarden Y., de Laat S. W., Schlessinger J. Epidermal growth factor induces electrically silent Na+ influx in human fibroblasts. J Biol Chem. 1982 Jul 25;257(14):8502–8506. [PubMed] [Google Scholar]
- Nishimura J., Deuel T. F. Platelet-derived growth factor stimulates the phosphorylation of ribosomal protein S6. FEBS Lett. 1983 May 30;156(1):130–134. doi: 10.1016/0014-5793(83)80263-4. [DOI] [PubMed] [Google Scholar]
- Nishimura J., Deuel T. F. Stimulation of protein phosphorylation in Swiss mouse 3T3 cells by human platelet derived growth factor. Biochem Biophys Res Commun. 1981 Nov 16;103(1):355–361. doi: 10.1016/0006-291x(81)91700-9. [DOI] [PubMed] [Google Scholar]
- Nishimura J., Huang J. S., Deuel T. F. Platelet-derived growth factor stimulates tyrosine-specific protein kinase activity in Swiss mouse 3T3 cell membranes. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4303–4307. doi: 10.1073/pnas.79.14.4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen A. J., 3rd, Geyer R. P., Antoniades H. N. Human platelet-derived growth factor stimulates amino acid transport and protein synthesis by human diploid fibroblasts in plasma-free media. Proc Natl Acad Sci U S A. 1982 May;79(10):3203–3207. doi: 10.1073/pnas.79.10.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen N. E., Villereal M. L. Lys-bradykinin stimulates Na+ influx and DNA synthesis in cultured human fibroblasts. Cell. 1983 Mar;32(3):979–985. doi: 10.1016/0092-8674(83)90082-x. [DOI] [PubMed] [Google Scholar]
- Pardee A. B., Dubrow R., Hamlin J. L., Kletzien R. F. Animal cell cycle. Annu Rev Biochem. 1978;47:715–750. doi: 10.1146/annurev.bi.47.070178.003435. [DOI] [PubMed] [Google Scholar]
- Pike L. J., Bowen-Pope D. F., Ross R., Krebs E. G. Characterization of platelet-derived growth factor-stimulated phosphorylation in cell membranes. J Biol Chem. 1983 Aug 10;258(15):9383–9390. [PubMed] [Google Scholar]
- Pledger W. J., Hart C. A., Locatell K. L., Scher C. D. Platelet-derived growth factor-modulated proteins: constitutive synthesis by a transformed cell line. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4358–4362. doi: 10.1073/pnas.78.7.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouysségur J., Chambard J. C., Franchi A., Paris S., Van Obberghen-Schilling E. Growth factor activation of an amiloride-sensitive Na+/H+ exchange system in quiescent fibroblasts: coupling to ribosomal protein S6 phosphorylation. Proc Natl Acad Sci U S A. 1982 Jul;79(13):3935–3939. doi: 10.1073/pnas.79.13.3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raines E. W., Ross R. Platelet-derived growth factor. I. High yield purification and evidence for multiple forms. J Biol Chem. 1982 May 10;257(9):5154–5160. [PubMed] [Google Scholar]
- Robbins K. C., Antoniades H. N., Devare S. G., Hunkapiller M. W., Aaronson S. A. Structural and immunological similarities between simian sarcoma virus gene product(s) and human platelet-derived growth factor. Nature. 1983 Oct 13;305(5935):605–608. doi: 10.1038/305605a0. [DOI] [PubMed] [Google Scholar]
- Ross R., Glomset J., Kariya B., Harker L. A platelet-dependent serum factor that stimulates the proliferation of arterial smooth muscle cells in vitro. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1207–1210. doi: 10.1073/pnas.71.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R., Vogel A. The platelet-derived growth factor. Cell. 1978 Jun;14(2):203–210. doi: 10.1016/0092-8674(78)90107-1. [DOI] [PubMed] [Google Scholar]
- Rothenberg P., Reuss L., Glaser L. Serum and epidermal growth factor transiently depolarize quiescent BSC-1 epithelial cells. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7783–7787. doi: 10.1073/pnas.79.24.7783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E., Heppel L. A. Serum rapidly stimulates ouabain-sensitive 86-RB+ influx in quiescent 3T3 cells. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4492–4495. doi: 10.1073/pnas.72.11.4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E., Legg A., Strang G., Courtenay-Luck N. Cyclic AMP: a mitogenic signal for Swiss 3T3 cells. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4392–4396. doi: 10.1073/pnas.78.7.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E. Stimulation of DNA synthesis in quiescent cultured cells: exogenous agents, internal signals, and early events. Curr Top Cell Regul. 1980;17:59–88. doi: 10.1016/b978-0-12-152817-1.50007-9. [DOI] [PubMed] [Google Scholar]
- Rozengurt E. Stimulation of Na influx, Na-K pump activity and DNA synthesis in quiescent cultured cells. Adv Enzyme Regul. 1980;19:61–85. doi: 10.1016/0065-2571(81)90009-1. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Stroobant P., Waterfield M. D., Deuel T. F., Keehan M. Platelet-derived growth factor elicits cyclic AMP accumulation in Swiss 3T3 cells: role of prostaglandin production. Cell. 1983 Aug;34(1):265–272. doi: 10.1016/0092-8674(83)90157-5. [DOI] [PubMed] [Google Scholar]
- Samuelsson B., Goldyne M., Granström E., Hamberg M., Hammarström S., Malmsten C. Prostaglandins and thromboxanes. Annu Rev Biochem. 1978;47:997–1029. doi: 10.1146/annurev.bi.47.070178.005025. [DOI] [PubMed] [Google Scholar]
- Scher C. D., Shepard R. C., Antoniades H. N., Stiles C. D. Platelet-derived growth factor and the regulation of the mammalian fibroblast cell cycle. Biochim Biophys Acta. 1979 Aug 10;560(2):217–241. doi: 10.1016/0304-419x(79)90020-9. [DOI] [PubMed] [Google Scholar]
- Schuldiner S., Rozengurt E. Na+/H+ antiport in Swiss 3T3 cells: mitogenic stimulation leads to cytoplasmic alkalinization. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7778–7782. doi: 10.1073/pnas.79.24.7778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shier W. T., Durkin J. P. Role of stimulation of arachidonic acid release in the proliferative response of 3T3 mouse fibroblasts to platelet-derived growth factor. J Cell Physiol. 1982 Aug;112(2):171–181. doi: 10.1002/jcp.1041120204. [DOI] [PubMed] [Google Scholar]
- Smith J. B., Rozengurt E. Serum stimulates the Na+,K+ pump in quiescent fibroblasts by increasing Na+ entry. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5560–5564. doi: 10.1073/pnas.75.11.5560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TODARO G. J., GREEN H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol. 1963 May;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tupper J. T., Zorgniotti F., Mills B. Potassium transport and content during G1 and S phase following serum stimulation of 3T3 cells. J Cell Physiol. 1977 Jun;91(3):429–440. doi: 10.1002/jcp.1040910313. [DOI] [PubMed] [Google Scholar]
- Vogel A., Raines E., Kariya B., Rivest M. J., Ross R. Coordinate control of 3T3 cell proliferation by platelet-derived growth factor and plasma components. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2810–2814. doi: 10.1073/pnas.75.6.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WADDELL W. J., BUTLER T. C. Calculation of intracellular pH from the distribution of 5,5-dimethyl-2,4-oxazolidinedione (DMO); application to skeletal muscle of the dog. J Clin Invest. 1959 May;38(5):720–729. doi: 10.1172/JCI103852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterfield M. D., Scrace G. T., Whittle N., Stroobant P., Johnsson A., Wasteson A., Westermark B., Heldin C. H., Huang J. S., Deuel T. F. Platelet-derived growth factor is structurally related to the putative transforming protein p28sis of simian sarcoma virus. Nature. 1983 Jul 7;304(5921):35–39. doi: 10.1038/304035a0. [DOI] [PubMed] [Google Scholar]


