Abstract
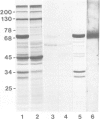
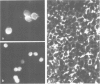
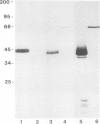
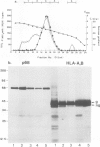
A Ca2+-binding protein of mol. wt. 68 000 ( p68 ) is a major component of a Nonidet P-40 insoluble fraction of human and pig lymphocyte plasma membrane. An affinity-purified rabbit antibody has been produced against p68 and used to study its cellular distribution. The antibody stained fixed and permeabilised human B lymphoblastoid cells, peripheral blood lymphocytes and sections of human tonsil. Whole cells, however, were not stained, indicating that the protein was not represented at the cell surface. This assignment was consistent with the detection of p68 in immunoprecipitates from biosynthetically- but not surface-labelled cells. It is concluded that p68 is located on the cytoplasmic face of the plasma membrane. Subcellular fractionation experiments confirmed that p68 was largely membrane-bound in lymphocytes, although a small soluble fraction (approximately 10% of the total) was detected. Sub-fractionation of lymphocyte membranes revealed that p68 was associated not only with the plasma membrane but also with other endomembrane systems. As judged by immunoprecipitation, p68 was present in a variety of cultured cell lines of both lymphoid and non-lymphoid origin. p68 demonstrated a diffuse distribution in fixed and permeabilised fibroblasts which did not correspond to the distribution of either microfilaments or intermediate filaments. However, in detergent-extracted cells the protein was localised in a lamina-like network. A similar immunofluorescent staining pattern has recently been observed for spectrin-related proteins in the detergent-resistant cytoskeleton of fibroblasts. It is suggested that p68 is part of a sub-membranous cytoskeletal complex not only in lymphocytes but also in other cell types.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnstable C. J., Bodmer W. F., Brown G., Galfre G., Milstein C., Williams A. F., Ziegler A. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens-new tools for genetic analysis. Cell. 1978 May;14(1):9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- Barnstable C. J., Jones E. A., Crumpton M. J. Isolation, structure and genetics of HLA-A, -B, -C and -DRw (Ia) antigens. Br Med Bull. 1978 Sep;34(3):241–246. doi: 10.1093/oxfordjournals.bmb.a071504. [DOI] [PubMed] [Google Scholar]
- Ben-Ze'ev A., Duerr A., Solomon F., Penman S. The outer boundary of the cytoskeleton: a lamina derived from plasma membrane proteins. Cell. 1979 Aug;17(4):859–865. doi: 10.1016/0092-8674(79)90326-x. [DOI] [PubMed] [Google Scholar]
- Bennett V., Davis J., Fowler W. E. Brain spectrin, a membrane-associated protein related in structure and function to erythrocyte spectrin. Nature. 1982 Sep 9;299(5879):126–131. doi: 10.1038/299126a0. [DOI] [PubMed] [Google Scholar]
- Bennett V., Stenbuck P. J. Association between ankyrin and the cytoplasmic domain of band 3 isolated from the human erythrocyte membrane. J Biol Chem. 1980 Jul 10;255(13):6424–6432. [PubMed] [Google Scholar]
- Branton D., Cohen C. M., Tyler J. Interaction of cytoskeletal proteins on the human erythrocyte membrane. Cell. 1981 Apr;24(1):24–32. doi: 10.1016/0092-8674(81)90497-9. [DOI] [PubMed] [Google Scholar]
- Burridge K., Kelly T., Mangeat P. Nonerythrocyte spectrins: actin-membrane attachment proteins occurring in many cell types. J Cell Biol. 1982 Nov;95(2 Pt 1):478–486. doi: 10.1083/jcb.95.2.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtneidge S. A., Levinson A. D., Bishop J. M. The protein encoded by the transforming gene of avian sarcoma virus (pp60src) and a homologous protein in normal cells (pp60proto-src) are associated with the plasma membrane. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3783–3787. doi: 10.1073/pnas.77.7.3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crumpton M. J., Snary D. Preparation and properties of lymphocyte plasma membrane. Contemp Top Mol Immunol. 1974;3:27–56. doi: 10.1007/978-1-4684-2838-4_2. [DOI] [PubMed] [Google Scholar]
- Davies A. A., Wigglesworth N. M., Allan D., Owens R. J., Crumpton M. J. Nonidet P-40 extraction of lymphocyte plasma membrane. Characterization of the insoluble residue. Biochem J. 1984 Apr 1;219(1):301–308. doi: 10.1042/bj2190301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenckhahn D., Gröschel-Stewart U., Kendrick-Jones J., Scholey J. M. Antibody to thymus myosin: its immunological characterization and use for immunocytochemical localization of myosin in vertebrate nonmuscle cells. Eur J Cell Biol. 1983 Mar;30(1):100–111. [PubMed] [Google Scholar]
- Erikson E., Erikson R. L. Identification of a cellular protein substrate phosphorylated by the avian sarcoma virus-transforming gene product. Cell. 1980 Oct;21(3):829–836. doi: 10.1016/0092-8674(80)90446-8. [DOI] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Glenney P. Fodrin is the general spectrin-like protein found in most cells whereas spectrin and the TW protein have a restricted distribution. Cell. 1983 Sep;34(2):503–512. doi: 10.1016/0092-8674(83)90383-5. [DOI] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Glenney P., Osborn M., Weber K. An F-actin- and calmodulin-binding protein from isolated intestinal brush borders has a morphology related to spectrin. Cell. 1982 Apr;28(4):843–854. doi: 10.1016/0092-8674(82)90063-0. [DOI] [PubMed] [Google Scholar]
- Goding J. W. Conjugation of antibodies with fluorochromes: modifications to the standard methods. J Immunol Methods. 1976;13(3-4):215–226. doi: 10.1016/0022-1759(76)90068-5. [DOI] [PubMed] [Google Scholar]
- Greenberg M. E., Edelman G. M. The 34 kd pp60src substrate is located at the inner face of the plasma membrane. Cell. 1983 Jul;33(3):767–779. doi: 10.1016/0092-8674(83)90019-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazarides E. Intermediate filaments as mechanical integrators of cellular space. Nature. 1980 Jan 17;283(5744):249–256. doi: 10.1038/283249a0. [DOI] [PubMed] [Google Scholar]
- Lee J. S., Varmus H. E., Bishop J. M. Virus-specific messenger RNAs in permissive cells infected by avian sarcoma virus. J Biol Chem. 1979 Aug 25;254(16):8015–8022. [PubMed] [Google Scholar]
- Lehto V. P. 140 000 Dalton surface glycoprotein. A plasma membrane component of the detergent-resistant cytoskeletal preparations of cultured human fibroblasts. Exp Cell Res. 1983 Feb;143(2):271–286. doi: 10.1016/0014-4827(83)90052-6. [DOI] [PubMed] [Google Scholar]
- Lehto V. P., Virtanen I., Paasivuo R., Ralston R., Alitalo K. The p36 substrate of tyrosine-specific protein kinases co-localizes with non-erythrocyte alpha-spectrin antigen, p230, in surface lamina of cultured fibroblasts. EMBO J. 1983;2(10):1701–1705. doi: 10.1002/j.1460-2075.1983.tb01645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenk R., Penman S. The cytoskeletal framework and poliovirus metabolism. Cell. 1979 Feb;16(2):289–301. doi: 10.1016/0092-8674(79)90006-0. [DOI] [PubMed] [Google Scholar]
- Levine J., Willard M. Fodrin: axonally transported polypeptides associated with the internal periphery of many cells. J Cell Biol. 1981 Sep;90(3):631–642. doi: 10.1083/jcb.90.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine J., Willard M. Redistribution of fodrin (a component of the cortical cytoplasm) accompanying capping of cell surface molecules. Proc Natl Acad Sci U S A. 1983 Jan;80(1):191–195. doi: 10.1073/pnas.80.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson A. D., Courtneidge S. A., Bishop J. M. Structural and functional domains of the Rous sarcoma virus transforming protein (pp60src). Proc Natl Acad Sci U S A. 1981 Mar;78(3):1624–1628. doi: 10.1073/pnas.78.3.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loube S. R., Owen M. J., Crumpton M. J. Human class I histocompatibility antigens (HLA-A,B,C). A small proportion only is phosphorylated. Biochem J. 1983 Jan 15;210(1):79–87. doi: 10.1042/bj2100079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March S. C., Parikh I., Cuatrecasas P. A simplified method for cyanogen bromide activation of agarose for affinity chromatography. Anal Biochem. 1974 Jul;60(1):149–152. doi: 10.1016/0003-2697(74)90139-0. [DOI] [PubMed] [Google Scholar]
- McConahey P. J., Dixon F. J. Radioiodination of proteins by the use of the chloramine-T method. Methods Enzymol. 1980;70(A):210–213. doi: 10.1016/s0076-6879(80)70050-2. [DOI] [PubMed] [Google Scholar]
- Mescher M. F., Jose M. J., Balk S. P. Actin-containing matrix associated with the plasma membrane of murine tumour and lymphoid cells. Nature. 1981 Jan 15;289(5794):139–144. doi: 10.1038/289139a0. [DOI] [PubMed] [Google Scholar]
- Nelson W. J., Colaço C. A., Lazarides E. Involvement of spectrin in cell-surface receptor capping in lymphocytes. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1626–1630. doi: 10.1073/pnas.80.6.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville D. M., Jr Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971 Oct 25;246(20):6328–6334. [PubMed] [Google Scholar]
- Nigg E. A., Cooper J. A., Hunter T. Immunofluorescent localization of a 39,000-dalton substrate of tyrosine protein kinases to the cytoplasmic surface of the plasma membrane. J Cell Biol. 1983 Jun;96(6):1601–1609. doi: 10.1083/jcb.96.6.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen M. J., Kissonerghis A. M., Lodish H. F. Biosynthesis of HLA-A and HLA-B antigens in vivo. J Biol Chem. 1980 Oct 25;255(20):9678–9684. [PubMed] [Google Scholar]
- Owens R. J., Crumpton M. J. Isolation and characterization of a novel 68,000-Mr Ca2+-binding protein of lymphocyte plasma membrane. Biochem J. 1984 Apr 1;219(1):309–316. doi: 10.1042/bj2190309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens R. J., Northcote D. H. The location of arabinosyl:hydroxyproline transferase in the membrane system of potato tissue culture cells. Biochem J. 1981 Jun 1;195(3):661–667. doi: 10.1042/bj1950661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinder J. C., Gratzer W. B. Structural and dynamic states of actin in the erythrocyte. J Cell Biol. 1983 Mar;96(3):768–775. doi: 10.1083/jcb.96.3.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruss R. M., Mirsky R., Raff M. C., Thorpe R., Dowding A. J., Anderton B. H. All classes of intermediate filaments share a common antigenic determinant defined by a monoclonal antibody. Cell. 1981 Dec;27(3 Pt 2):419–428. doi: 10.1016/0092-8674(81)90383-4. [DOI] [PubMed] [Google Scholar]
- Reiser J., Wardale J. Immunological detection of specific proteins in total cell extracts by fractionation in gels and transfer to diazophenylthioether paper. Eur J Biochem. 1981 Mar;114(3):569–575. doi: 10.1111/j.1432-1033.1981.tb05182.x. [DOI] [PubMed] [Google Scholar]
- Snary D., Goodfellow P., Hayman M. J., Bodmer W. F., Crumpton M. J. Subcellular separation and molecular nature of human histocompatibility antigens (HL-A). Nature. 1974 Feb 15;247(5441):457–461. doi: 10.1038/247457a0. [DOI] [PubMed] [Google Scholar]
- Snary D., Woods F. R., Crumpton M. J. Disruption of solid tissue for plasma membrane preparation. Anal Biochem. 1976 Aug;74(2):457–465. doi: 10.1016/0003-2697(76)90226-8. [DOI] [PubMed] [Google Scholar]
- Stanley K. K., Edwards M. R., Luzio J. P. Subcellular distribution and movement of 5'-nucleotidase in rat cells. Biochem J. 1980 Jan 15;186(1):59–69. doi: 10.1042/bj1860059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh F. S., Barber B. H., Crumpton M. J. Preparation of inside-out vesicles of pig lymphocyte plasma membrane. Biochemistry. 1976 Aug 10;15(16):3557–3563. doi: 10.1021/bi00661a025. [DOI] [PubMed] [Google Scholar]
- Walsh F. S., Crumpton M. J. Orientation of cell-surface antigens in the lipid bilayer of lymphocyte plasma membrane. Nature. 1977 Sep 22;269(5626):307–311. doi: 10.1038/269307a0. [DOI] [PubMed] [Google Scholar]
- Weber K., Groeschel-Stewart U. Antibody to myosin: the specific visualization of myosin-containing filaments in nonmuscle cells. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4561–4564. doi: 10.1073/pnas.71.11.4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham M. C., Jay G., Pastan I. Localization of the ASV src gene product to the plasma membrane of transformed cells by electron microscopic immunocytochemistry. Cell. 1979 Sep;18(1):125–134. doi: 10.1016/0092-8674(79)90361-1. [DOI] [PubMed] [Google Scholar]
- Yu J., Fischman D. A., Steck T. L. Selective solubilization of proteins and phospholipids from red blood cell membranes by nonionic detergents. J Supramol Struct. 1973;1(3):233–248. doi: 10.1002/jss.400010308. [DOI] [PubMed] [Google Scholar]