Abstract
Introduction
Moisturizers play a prominent role in the management of atopic dermatitis by improving the impaired skin barrier function and enhancing skin hydration. Their efficacy was evaluated in a recently published Cochrane Review ‘Emollients and moisturizers for eczema’.
Objective
In the present review, we summarize the performance and safety of Cetaphil® and Excipial® moisturizing products.
Methods
This review was carried out in compliance with standard Cochrane methodological procedures, which means independent study selection, data extraction, assessment of risk of bias, and analyses by two review authors. The quality of evidence for the predefined outcomes was rated with the GRADE approach. The prespecified outcomes of the review included participant assessments, satisfaction, adverse events, investigator assessments, prevention of flares, change in use of topical active treatment, skin barrier function and quality of life.
Results
Four randomized controlled studies examining these moisturizers were included in the previously published Cochrane Review. For the performance and tolerability of these moisturizers, there was very low to moderate quality evidence for the prespecified outcomes.
Conclusion
The results from these four studies are in line with those of the Cochrane Review that moisturizers themselves have beneficial effects, and that combining moisturizers with active topical treatment produced better results when compared to active topical treatment alone.
Electronic supplementary material
The online version of this article (doi:10.1007/s13555-017-0184-3) contains supplementary material, which is available to authorized users.
Keywords: Atopic dermatitis, Evidence-based dermatology, GRADE approach, Moisturizers
Introduction
Atopic dermatitis, also known as atopic eczema or just eczema, is a chronic inflammatory skin disease that is characterized by decreased skin barrier function, (very) dry skin and inflammatory lesions which cause intense itch leading to scratching [1]. The etiology of atopic dermatitis continues to attract research interest, and, although it is not yet fully understood, most probably it has a multifactorial origin (e.g., genetic, environmental and immunological) [2]. In the absence of specific laboratory or histological findings [3], the diagnosis of atopic dermatitis is based on clinical signs and symptoms, by using, e.g., the criteria of Hanifin and Rajka or the UK Working Party’s diagnostic criteria for atopic dermatitis [4, 5]. Atopic dermatitis has a lifetime prevalence of 10–20% in developed countries [3]. The prevalence rates in developing countries are more difficult to estimate due to the use of different outcome measures and diagnostic criteria but seem to increase in certain parts of Africa and eastern Asia [6]. Since 60% of the diagnoses are made in the 1st year of life and 85% before age 5, prevalence is highest in children [3, 7]. A recent meta-analysis showed that 80% of children with the disease have outgrown it within 8 years of onset and this percentage reaches 95% at 20 years after onset [8]. This meta-analysis also reported that the risk factors for persistence of the disease are twofold: late-onset and greater disease severity. The severity of the disease can vary quite markedly, with data indicating that 80% of affected children have a mild form, and 20% a moderate to severe form [8]. Atopic dermatitis is further characterized by intermittent periods of milder symptoms, which are interspersed with sudden relapses or flare-ups (exacerbations) [3].
Treatment of atopic dermatitis consists of the avoidance of triggers that may exacerbate the disease (e.g., allergens, irritants), of restoring skin barrier function with moisturizers and by decreasing inflammation through the use of topical corticosteroids or topical immunomodulators [9]. In more severe cases, systemic treatment with immunomodulators or phototherapy might have to be considered [9]. The characteristic flare-ups which can occur in atopic dermatitis make the prevention of flares and exacerbations one of the key aims of long-term control [1].
Impaired Skin Barrier
The impairment of the skin barrier in atopic skin, both lesional and non-lesional, continues to be a topic of interest [10–17]. The two mechanisms for this impairment are discussed further here. Dysfunction of the corneocytes in the stratum corneum results in a decrease in production of the protein filaggrin. Filaggrin itself is broken down into amino acids (e.g., arginine) and smaller molecules such as urea, organic acids (e.g., lactic acid), sugars and electrolytes, which together form the natural moisturizing factor (NMF) [10, 12, 13]. NMF is the skin’s natural humectant and is essential for keeping the stratum corneum properly hydrated, which is necessary for all the biochemical processes that take place in the skin [10]. The lamellar bodies within the epithelial cells of the skin deliver other ingredients for the intercellular membrane of the stratum corneum, such as free fatty acids, cholesterol and ceramides (50% of the total lipid weight concerns ceramides) [10, 13, 14]. In people with atopic dermatitis, this production is dysregulated, causing a different composition of the various ceramides and a lack of, e.g., ceramide-1 and ceramide-3, which in turn leads to an increase in transepidermal water loss (TEWL) [10, 11]. In view of this impairment of skin barrier function, moisturizing of the skin is considered an essential part of the treatment regimen for people with atopic dermatitis [1]. There is thus a rationality to use moisturizers with ingredients that mimic the composition of the intercellular membrane by using, for instance, humectants, emollients and lipids, or other lacking substances, and to use occlusives to reduce or further prevent TEWL.
Efficacy of Moisturizers
Most studies evaluating the efficacy of moisturizers on dry skin or the improvement of skin disorders have an open label design and often don’t include a control group. Studies assessing moisturizers cannot be fairly compared with studies conducted to demonstrate the efficacy and safety of drugs, for which methodologically robust and randomized controlled trials are required to obtain approval by the drug registration authorities (e.g., Food and Drug Administration or European Medicines Agency). Moisturizers are most often sold over the counter without prescription, and therefore the development of these moisturizers tends to focus more on tolerance and status of skin condition (young or old skin, dry skin, sensitive skin or inflamed skin), rather than on improvement of atopic dermatitis per se as a stand-alone treatment. The consequences are, as has been reported in a meta-analysis on moisturizers in atopic dermatitis and related skin disorders, that studies evaluating the efficacy of moisturizers often do not meet the high standards with regards to methodology, e.g., of appropriate study size, adequately randomized and blinded, and using standardized outcome measures [18]. The efficacy and safety of emollients and moisturizers in atopic dermatitis has recently been evaluated in a Cochrane Review titled ‘Emollients and moisturizers for eczema’ [19]. This review reported that ample use of moisturizers reduces the rate of flares, prolongs the time to flare and enhances the efficacy of topical active treatment. This current review focuses on three of the moisturizers evaluated: Cetaphil® Moisturizing Cream (CMC), Cetaphil® RestoraDerm®Moisturizer (CRM) and Excipial® U lipo lotion (EUL). These products, contain certain ingredients that may restore barrier function albeit each in a different way, such as humectants, lipids and/or ceramides (or their precursors). Four randomized controlled studies which evaluated these products were included in the Cochrane Review [20–23].
Methods
The protocol and subsequent review on which this sub-analysis is based, were previously published in the Cochrane Library [19, 24]. This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors. The following databases were searched up to December 2015: the Cochrane Skin Group Specialized Register, CENTRAL (2015, Issue 11), MEDLINE (from 1946), EMBASE (from 1974), LILACS (from 1982), GREAT database, five ongoing trials registers (ISRCTN, ClinicalTrials.gov, the Australian New Zealand Clinical Trial Registry, WHO International Clinical Trials Registry and the EU Clinical Trials Register) and references of the included studies (see, for search strategy of MEDLINE Appendix 1, Electronic Supplemental material). Only randomized controlled trials (RCT) evaluating the efficacy and safety of moisturizers in people with atopic dermatitis, eczema, or atopic eczema were eligible for inclusion. Two reviewers (E.J.v.Z. and Z.F.) independently reviewed all studies from the searches. This manuscript provides a more in-depth evaluation of the specific RCTs which addressed CMC, CRM and EUL moisturizers.
Outcome Measures
Our primary outcome measures were (1) participant-assessed change in disease severity, (2) participant’s satisfaction with the moisturizer and (3) the proportion of participants with an adverse event. Secondary outcome measures were investigator-assessed change in disease severity, prevention of flares, change in use of active topical treatment, changes in epidermal barrier function and change in quality of life.
Data Extraction and Synthesis
Trial details of eligible studies were extracted independently by two review authors using pre-piloted data extraction forms (E.J.v.Z. and Z.F.). The risk of bias assessments were made using the Cochrane domain-based evaluation tool as described in Chapter 8, Sect. 8.5, in the Cochrane Handbook for Systematic Reviews of Interventions [25]. Mean differences (MD) were calculated for continuous outcomes and for dichotomous data we calculated risk ratios (RR). All outcomes were reported with their associated 95% confidence intervals (CI). We used the I 2 statistic in meta-analyses to assess heterogeneity [25]. The quality (or certainty) of the evidence for the prespecified outcomes was rated using the GRADE approach [26]. Further details on the data analysis are reported in the full Cochrane Systematic Review [19].
Results
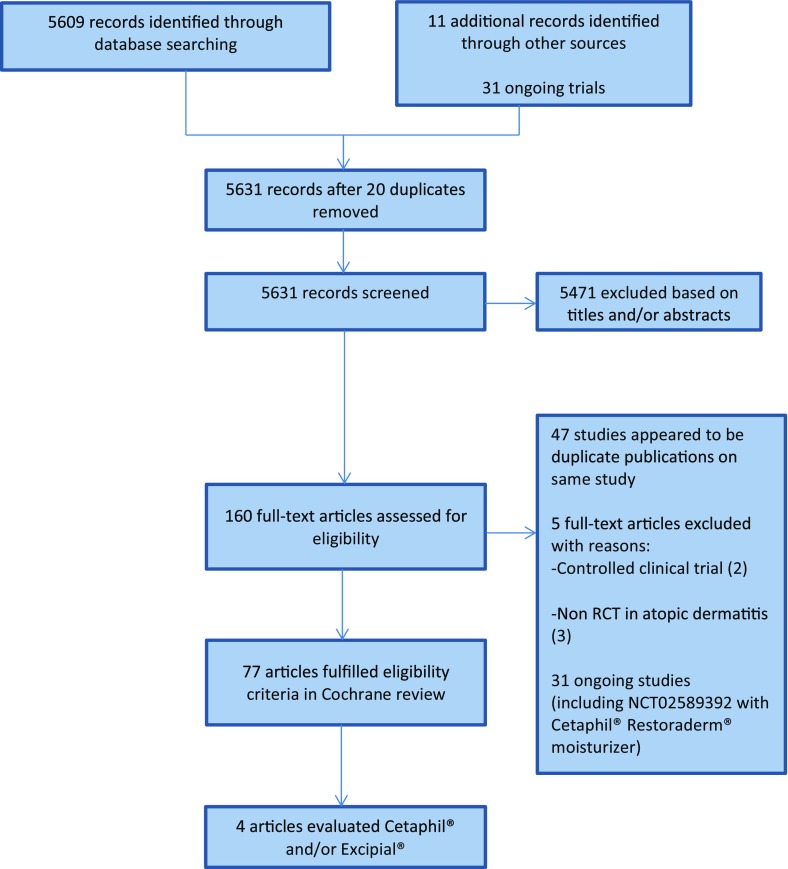
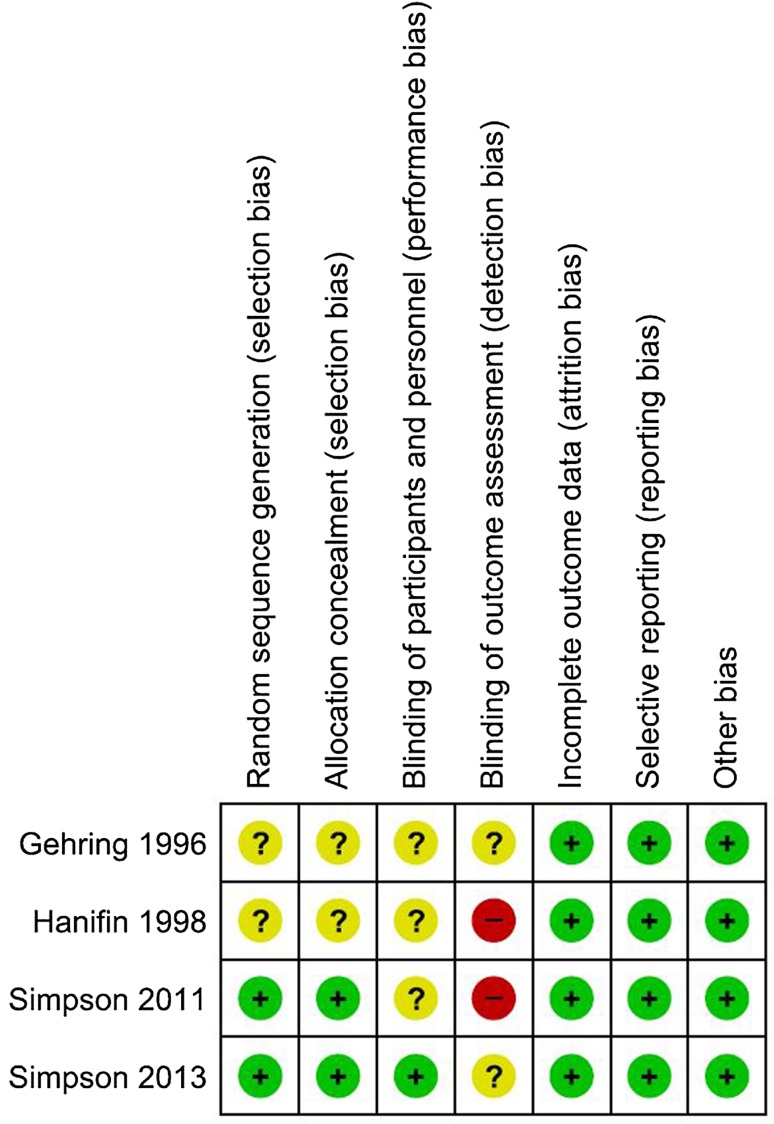
Full details of the process of study selection are provided in Fig. 1. Four studies which examined a total of 296 adult patients were included (Table 1). Two studies [20, 23] were assessed as at unclear risk of bias, and two studies [21, 22] as at high risk of bias. Lack of blinding of the patients was the principal reason that the studies were judged as high risk of bias (Fig. 2).
Fig. 1.
Flow diagram
Table 1.
Characteristics of the included studies and results
| Study ID design and location | Participants: gender/age/eczema status | Intervention and comparator | Outcomes as reported | Conclusions |
|---|---|---|---|---|
|
Gehring [20] Double-blind Single-center Germany |
69 (39 female/24 male/6 gender unreported) Eczema Mean age 27 years |
1 week A: EUL b.i.d. (31) B: Hydrocortisone acetate 1% in EUL b.i.d. (32) EUL contains 4% urea |
Participant-assessed change in roughness (1–10, higher better): group A 2.19 (1.31) vs group B 2.60 (0.98) Investigator-assessed redness (1–4, lower better): group A −0.84 (0.66) vs group B −1.00 (0.52) Investigator-assessed roughness (1–4, lower better): group A −0.97 (0.59) vs group B −1.06 (0.46) Change in TEWL: group A −8.2 g/m2/h vs group B −8 g/m2/h |
Six losses to follow-up (8.7%), unclear from which group |
|
Hanifin [21] Investigator-blind Multicenter US Within-participant |
80 (51 female/29 male) Mild to moderate eczema Mean age 24.4 years |
3 weeks A: desonide 0.05% lotion b.i.d. plus CMC on one side B: desonide 0.05% lotion b.i.d. on contralateral side |
Treatment preference: side A 96% vs side B 4% Adverse events: side A 10 vs. side B 11 after 1 week and 0 vs 2 after 3 weeks Marked to excellent improvement: side A 70% vs. side B 55% |
Combination of topical active treatment with a moisturiser is more effective than topical active treatment alone |
|
Simpson [22] Investigator-blind Multicenter US Within-participant |
127 (gender unreported) Mild to moderate eczema Mean age not reported |
4 weeks A: routine use of topical corticosteroids plus CRM on one side B: routine use of topical corticosteroids on contralateral side |
Treatment satisfaction: 84%–96.7% felt that addition of moisturiser resulted in better effect Change in EASI: side A −1.28 (1.94) vs. group B −1.01 (1.50) Change in skin capacitance: side A 5.4 vs. side B 3 |
Combination of topical active treatment with a moisturiser is more effective than topical active treatment alone |
|
Simpson [23] Investigator-blinded Single-center Germany Within-participant |
20 (16 female/4 male) Controlled atopic dermatitis and dry skin Mean age 40.9 years |
27 days A: CRM b.i.d. on one leg B: no moisturiser on contralateral leg |
Adverse events: none on either leg Change on dryness scale (0–4): side A −1.15 (0.41) vs. side B −0.91 (0.58) Change in TEWL: side A −1.59 g/m2/h vs. side B −0.42 g/m2/h (1.13) Change in skin capacitance: side A 16.91 (6.3) vs. side B 3.3 (3.86) |
There was a statistically significant difference in favor of CRM for all these outcomes |
b.i.d. twice daily, CMC Cetaphil® moisturising cream, CRM Cetaphil Restoraderm® moisturizer, EASI Eczema Area Severity Index, EUL Excipial® U lipo lotion, HR hazard ratio, TEWL transepidermal water loss
Fig. 2.
Risk of bias summary
In the study of Gehring and Gloor, EUL containing 4% urea twice daily was compared to hydrocortisone acetate 1% in EUL in 69 participants over a period of 1 week [20]. Disease severity was assessed by the participants as roughness of the skin on a visual analogue scale (VAS) from 1 to 10, with higher being better. VAS scores increased from baseline after 1 week by 2.19 [1.31 standard deviation (SD)] in the 31 patients treated with EUL and 2.60 (0.98 SD) in the 32 patients that applied hydrocortisone acetate 1% in EUL with a MD of −0.41 (95% CI −0.98 to 0.16; P = 0.16). Our primary outcomes participant satisfaction and adverse events were not evaluated. Investigators assessed redness on a 1–4 scale (lower score being less red). The changes in redness after 1 week were −0.84 (0.66 SD) in the EUL group and −1.00 (0.52 SD) in the hydrocortisone acetate 1% in EUL group (MD 0.16, 95% CI −0.13 to 0.45; P = 0.29). Roughness was also assessed on a scale from 1 to 4 and showed changes of −0.97 (0.59 SD) and −1.06 (0.45 SD), respectively, with a MD of 0.09 (95% CI −0.18 to 0.36; P = 0.52). The other secondary outcomes were not assessed. The quality of the evidence was low to moderate for the addressed outcomes (see Table 2).
Table 2.
Summary of findings table study of Gehring and Gloor [20]
| EUL twice daily compared to hydrocortisone acetate 1% in EUL twice daily for atopic dermatitis |
|---|
| Patient or population: atopic dermatitis |
| Intervention: EUL twice daily |
| Comparison: hydrocortisone acetate 1% in EUL twice daily |
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) |
No. of participants (studies) |
Quality of the evidence (GRADE) |
Comments | |
|---|---|---|---|---|---|---|
| Risk with hydrocortisone acetate 1% in EUL twice daily | Risk with EUL twice daily | |||||
|
Change from baseline in disease severity as assessed by the participants (roughness of the skin) Assessed with: Visual Analogue Scale (VAS) Scale from: 1 to 10 (higher better) Follow-up: mean 1 week |
The mean change from baseline in disease severity as assessed by the patients was 2.60 (0.98) | The mean change from baseline in disease severity as assessed by the patients in the intervention group was 0.41 lower (0.98 lower to 0.16 higher) | – | 63 (1 RCT)a | LOWb,c | P = 0.16 No difference between the two treatment groups after 1 week |
| Participant satisfaction with the moisturiser—not measured | – | – | – | – | – | The study did not address this outcome |
| Number of participants reporting an adverse event—not measured | – | – | – | – | – | The study did not address this outcome |
|
Change from baseline in disease severity as assessed by the investigators Assessed with: Likert scale Scale from: 1 to 4 (lower better) Follow-up: mean 1 week |
The mean change from baseline in disease severity as assessed by the investigators was −1 (0.52) | The mean change from baseline in disease severity as assessed by the investigators in the intervention group was 0.16 higher (0.13 lower to 0.45 higher) | – | 63 (1 RCT)a | LOWc,d | P = 0.29. There was no difference according to the investigators between the two treatment arms |
| Number of participants experiencing a flare—not measured | – | – | – | – | – | The study did not address this outcome |
| Change in use of active topical treatment—not measured | – | – | – | – | – | The study did not address this outcome |
|
Change in skin barrier function Assessed with: transepidermal water loss Follow-up: mean 1 week |
The mean change in skin barrier function was 8 g/m2/h | The mean change in skin barrier function in the intervention group was 0.2 g/m2/h lower | – | 63 (1 RCT)a | MODERATEf | Data had to be estimated from figure |
| Change in health-related quality of life—not measured | – | – | – | – | – | The study did not address this outcome |
GRADE Working Group grades of evidence
High quality: We are very confident that the true effect lies close to that of the estimate of the effect
Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different
Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect
Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect
CI confidence interval, EUL Excipial® U lipo lotion, MD mean difference
* The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI)
aGehring [20]
bDowngraded one level for serious indirectness, ‘roughness’ of the skin is not the same as ‘disease severity’
cDowngraded one level for serious imprecision, low sample size and confidence interval includes appreciable harm (0.75) and no difference (1)
dDowngraded one level for serious indirectness, ‘redness’ of the skin is not the same as disease severity
eDowngraded one level for serious imprecision, low sample size and confidence interval includes no difference (1), and appreciable harm (1.25)
fDowngraded one level for serious imprecision, low sample size, data had to be estimated from figure
A study by Simpson et al. had a within participant design in which CRM twice daily was compared to ‘no moisturizer’ on the contralateral leg of 20 patients over a period of 27 days [23]. Two of our primary outcomes, disease severity as assessed by the participants and their satisfaction with the moisturizer, were not evaluated. Adverse events were evaluated and there were none reported to the treatment. In this study, the investigators used a dryness scale (0–4, higher score being worse) to assess disease severity. The reductions reported at the end of 27 days were 1.15 (0.41 SD) on the legs of the 20 patients treated with CRM and 0.91 (0.58 SD) on the non-treated contralateral legs with a mean of the paired differences of −0.24 (95% CI −0.42 to −0.06). In addition, both TEWL (measured with an evaporimeter) and skin hydration (measured with a corneometer) were used to investigate changes in skin barrier function. The reduction in TEWL was 1.59 g/m2/h (0.97 SD) on the CRM treated legs and 0.42 g/m2/h (1.13 SD) on the contralateral legs with a mean of the paired differences of −1.17 g/m2/h (95% CI −1.52 to −0.82). However, both of these reductions can be regarded as relatively minimal. Skin hydration improved by 16.91 units (6.31 SD) on the CRM treated legs and by 3.3 (3.86 SD) on the non-treated contralateral legs (mean of the paired differences 13.61, 95% CI 11.60–15.60). There was a statistically significant difference in favor of CRM for all of these specific investigator-assessed outcomes. None of the other secondary outcomes (prevention of flares, change in active topical treatment and quality of life) were assessed in this study. The quality of the evidence was rated low to very low for the prespecified outcomes that were addressed (see Table 3).
Table 3.
Summary of findings table study of Simpson [23]
| CRM compared to no moisturizer for eczema |
|---|
| Patient or population: eczema |
| Intervention: CRM |
| Comparison: no moisturizer |
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | ||
|---|---|---|---|---|---|---|---|
| Risk with no moisturizer | Risk with CRM | ||||||
| Change from baseline in disease severity as assessed by the participants—not measured | – | – | – | – | – | The study did not address this outcome | |
| Participant satisfaction with the moisturiser—not measured | – | – | – | – | – | The study did not address this outcome | |
|
Number of participants reporting an adverse event Follow-up: mean 27 days |
20 (1 RCT)a | VERY LOWb,c | There were no adverse events reported on either leg (within-participant design) | ||||
|
Change from baseline in disease severity as assessed by the investigators Assessed with: dryness scale Scale from 0 to 4 (higher worse) Follow-up: mean 27 days |
The reductions were 1.15 (0.41 SD) on the legs of the 20 patients treated with CRM and 0.91 (0.58) on the contralateral legs (no moisturizer) with a mean of the paired differences of −0.24 (95% CI −0.42 to −0.06) | 20 (1 RCT)a | LOWc | Study with a within-participant design | |||
| Number of participants experiencing a flare—not measured | – | – | – | – | – | The study did not address this outcome | |
| Change in use of active topical treatment—not measured | – | – | – | – | – | The study did not address this outcome | |
|
Change in skin barrier function Assessed with: transepidermal water loss and corneometry Follow-up: mean 27 days |
The reduction in TEWL was 1.59 g/m2/h (0.97 SD) on the CRM treated legs and 0.42 g/m2/h (1.13 SD) on the contralateral legs with a mean of the paired differences of −1.17 g/m2/h (95% CI −1.52 to −0.82). Both reductions can be regarded as small. Skin hydration improved by 16.91 units (6.31 SD) on the CRM treated legs and by 3.3 (3.86 SD) on the non-treated contralateral legs (mean of the paired differences 13.61, 95% CI 11.60–15.60) | 20 (1 RCT)a | LOWc | Study with a within-participant design | |||
| Change in health-related quality of life—not measured | – | – | – | – | – | The study did not address this outcome | |
GRADE Working Group grades of evidence
High quality: We are very confident that the true effect lies close to that of the estimate of the effect
Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different
Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect
Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect
CI confidence interval, CRM Cetaphil®Restoraderm® Body Moisturizer
* The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI)
aSimpson [23]
bDowngraded one level for serious detection bias, participants were not blinded
cDowngraded two levels for very serious imprecision, low sample size
The two studies at high risk of bias (due to lack of blinding of the participants) evaluated topical corticosteroids plus moisturizer versus topical corticosteroid alone [21, 22].
In Hanifin et al. desonide 0.05% lotion twice daily in combination with the use of moisturizing cream three times daily (CMC) was compared over a period of 3 weeks to desonide 0.05% lotion twice daily, in 80 participants in a within-participant study design [21]. In the within-participant design study of Simpson et al., routine use of topical corticosteroids combined with twice daily CRM was compared to routine use of topical corticosteroids alone without the use of any moisturizer [22]. This study examined these comparisons in 123 patients over a 4-week period [22]. Participant-assessed disease severity was not assessed in either of the two studies. However, although participant satisfaction was measured in both, it did not involve the more direct measurement of our outcome of ‘satisfaction’. Thus, in Hanifin et al. [21], it was measured as ‘preference’, and in Simpson et al. [22] as ‘perception of the product’. In Hanifin et al. [21], the combined therapy of desonide 0.05% lotion plus moisturizing cream was preferred by 96% of the 78 participants and the remaining 4% preferred the desonide 0.05% lotion without the use of any moisturizer. In the other study [22], between 84.3% and 96.7% of the 123 participants reported that adding CRM to topical corticosteroids “reduces inflammation, relieves dry and itchy skin, provides long-lasting hydration, leaves skin protected and maintains healthy skin” [22]. Adverse events were only reported in one of the two studies [21]. After the 1st week of the study, 10 of the 80 participants reported burning and stinging on the side treated with desonide 0.05% and moisturizer, compared to 11 reports on the side treated with desonide 0.05% lotion alone. However, after 3 weeks, no adverse events were reported for the combined treatment, but two participants still reported burning and stinging on the side treated with desonide 0.05% lotion alone [21].
The investigators in Hanifin et al. assessed disease severity as ‘global assessment of improvement’ [21]. Based on a per-protocol analysis of 78 participants and their assessments, 70% of the participants were markedly improved to ‘clear’ on the body side treated with desonide 0.05% lotion with moisturizer used three times a day, versus 55% on the side that was treated with only desonide 0.05% lotion (investigators reported a P value of <0.01).
The investigators in Simpson et al. used the Eczema Area and Severity Index (EASI; score 0–72, higher is worse) [22]. The reductions in EASI were small on both sides and did not meet the minimal important difference (MID) of 6.6 [27]. On the side treated with desonide 0.05% lotion and moisturizer the reduction was 1.28 (1.94 SD) and on the desonide 0.05% lotion ‘only’ treated side 1.0 (1.50 SD), with a mean of the paired differences of −0.27 (95% CI −0.52 to −0.02), which although statistically significant is not clinically important.
Only Simpson et al. investigated skin barrier function using corneometry [22]. On the side treated with topical corticosteroids combined with moisturizer, skin hydration increased by 5.4 arbitrary units compared to 3 arbitrary units on the side treated with topical corticosteroids alone, both of which were considered small improvements. The other secondary outcomes (prevention of flares, change in topical active treatments and quality of life) were not assessed in these two studies. The quality of evidence was rated low to moderate for the addressed outcomes (see Table 4).
Table 4.
| Topical corticosteroids + CMC or CRM compared to topical corticosteroids alone for eczema |
|---|
| Patient or population: eczema |
| Intervention: topical corticosteroids + CMC or CRM |
| Comparison: topical corticosteroids alone |
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | ||
|---|---|---|---|---|---|---|---|
| Risk with topical corticosteroids alone | Risk with topical corticosteroids + CMC or CRM | ||||||
| Change from baseline in disease severity as assessed by the participants—not measured | – | – | – | – | – | The studies did not address this outcome | |
|
Participant satisfaction with the moisturiser Follow-up: range 3–4 weeks |
In Hanifin et al. [21], the combined therapy of desonide 0.05% lotion plus moisturizing cream was preferred in 96% of the 78 participants and only 4% preferred the desonide 0.05% lotion without the use of any moisturizer. In the other study [22], between 84.3% and 96.7% of the 123 participants reported that adding CRM to topical corticosteroids “reduces inflammation, relieves dry and itchy skin, provides long lasting hydration, leaves skin protected and maintains healthy skin” | 201 (2 RCTs)a | LOWb,c,d | Both studies had a within-participant design. In both studies the addition of the moisturizer increased the effect of topical corticosteroids | |||
|
Number of participants reporting an adverse event Follow-up: mean 3 weeks |
After 1 week study duration, 10 of the 80 participants reported burning and stinging on the side were both desonide 0.05% as well as moisturizer was applied compared to 11 reports on the side only treated with desonide 0.05% lotion. However, after 3 weeks no adverse events were mentioned for the combined treatment, but two participants still reported burning and stinging on the side treated with only desonide 0.05% lotion | 80 (1 RCT)e | LOWb,f | ||||
|
Change from baseline in disease severity as assessed by the investigators Follow-up: range 3–4 weeks |
Hanifin et al. [21] assessed as ‘global assessment of improvement’. Of the 78 participants 70% were markedly improved to clear on the body side treated with desonide 0.05% lotion with moisturizer used three times a day versus 55% on the side that was treated with only desonide 0.05% lotion (investigators reported a P value of <0.01). In Simpson et al. [22], EASI was used (score 0–72, higher is worse). On the side treated with both desonide 0.05% lotion and moisturiser the reduction was 1.28 (1.94 SD) and on the desonide 0.05% lotion ‘only’ treated side 1.0 (1.50 SD) with a mean of the paired differences of −0.27 (95% CI −0.52 to −0.02) | 201 (2 RCTs)a | MODERATEf | Both studies have a within-participant design In Simpson 2011; the reductions in EASI were small on both sides and not meeting the minimal important difference of 6.6 [27] | |||
| Number of participants experiencing a flare—not measured | – | – | – | – | – | The studies did not address this outcome | |
| Change in use of active topical treatment—not measured | – | – | – | – | – | The studies did not address this outcome | |
|
Change in skin barrier function Assessed with: corneometry Follow-up: mean 4 weeks |
On the side treated with topical corticosteroids in combination with moisturizer skin hydration increased by 5.4 arbitrary units and on the side treated with only topical corticosteroids by 3 arbitrary units | 123 (1 RCT)g | LOWh | The study has a within-participant design. Both improvements are small, no SDs were provided | |||
| Change in health-related quality of life—not measured | – | – | – | – | – | The studies did not address this outcome | |
GRADE Working Group grades of evidence
High quality: We are very confident that the true effect lies close to that of the estimate of the effect
Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different
Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect
Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect
CI confidence interval, CMC Cetaphil® Moisturising cream, CRM Cetaphil® RestoraDerm® moisturiser
* The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI)
bDowngraded one level for serious risk of detection bias, participants were not blinded
cDowngraded one level for serious indirectness, in both studies a surrogate outcome was measured
dWe did not downgrade for imprecision, as well already downgraded for risk of bias and indirectness, and further downgrading was not felt appropriate
eHanifin [21]
fDowngraded one level for serious imprecision, low sample size
gSimpson [22]
hDowngraded two levels for very serious imprecision, low sample size and we did not downgrade for anything else
Discussion
The duration of the studies did not last beyond 4 weeks, and three of the four studies addressed the efficacy of moisturizers combined with topical corticosteroids in atopic dermatitis [20–22]. One study was conducted in people with controlled atopic dermatitis [23]. However, none of the studies were designed as maintenance studies to evaluate the efficacy of moisturizers in preventing flares.
One randomized controlled trial, which was conducted 20 years ago, evaluated EUL, a moisturizer containing 4% urea and 36% lipids [20]. As only the 1st week of this 2-week study was randomized, we could only include the 1st week’s data. Based on these data, we can conclude that adding hydrocortisone acetate 1% to the EUL did not make a difference in terms of efficacy compared to EUL used alone.
Urea is normally present in healthy skin as part of the natural moisturizing factor (NMF) in the stratum corneum [14]. Urea is a humectant with water attracting properties from dermis into epidermis and aids in holding water in the stratum corneum [14, 28, 29]. In atopic skin epidermal barrier function is impaired, TEWL is increased and the ability to retain water in the skin is decreased [14, 29]. Urea-containing moisturizers enhance hydration, but also appear to improve skin barrier function and antimicrobial defense [30]. In concentrations of 10% and higher, urea works as a keratolytic agent and therefore urea-containing moisturizers work well on both dry and scaly skin.
Based on the published Cochrane Review, there was low to moderate quality evidence for the effect of urea-containing moisturizers, and these could reduce the risk of flare by one-third when compared to the use of no moisturizer or compared to its vehicle. A long-term study conducted over a period of 180 days also demonstrated that urea 5%-containing cream could, after the atopic dermatitis had been (almost) cleared with topical corticosteroids, reduce the number of patients having a flare during the 6-months follow-up, as well as increase the time to flare when compared to a moisturizer without urea [31]. Over the last 20 years, the urea-containing product line (which includes EUL) has expanded its development to “hydrate to relieve, protect, and repair the most dry and frustrating skin” [32].
Whereas it is unlikely that many more randomized controlled trials with urea-containing moisturizers will be conducted, the benefits of urea appear to be already well acknowledged by most physicians as well as patients, even in the absence of robustly designed and conducted studies. The most important reasons for downgrading the quality of the evidence for urea-containing moisturizers in the Cochrane Review were low sample sizes, making the effect estimate less precise (due to wide confidence interval) and risk of bias (e.g., lack of blinding) [19]. The absence of high-quality evidence is more directly related to the poor methodological quality and low number of existing studies than to the efficacy of the urea-containing moisturizers.
The other three included studies evaluated CRM and CMC [21–23]. The study of Simpson et al. demonstrated that CRM performed better on all assessed outcomes than no moisturizer, albeit based on very low- to low-quality evidence [23]. This product has been especially developed for atopic skin, and contains occlusives, emollients and humectants to restore and maintain barrier function and prevent transepidermal water loss [10]. Additional inclusion of NMFs and pseudoceramides among others within this product have been shown to be capable of augmenting the water-binding and -holding properties of the stratum corneum [10, 13]. The recently published Cochrane Review emphasized that most moisturizers showed some beneficial effects; however, the extent of the benefits varied among the included studies [19]. Nonetheless, it was clear that the use of moisturizers prolonged time to flare, reduced the number of flares, and, when moisturizers were abundantly and frequently applied, also reduced the need for topical active treatment. Another important conclusion of the Cochrane Review was that there was moderate-quality evidence that adding a moisturizer to topical active treatment was more effective than topical active treatment alone [19]. This was confirmed by two of the studies in this report which evaluated CRM in combination with active treatment and CMC [21, 22].
Conclusions
The four randomized controlled studies included in this review on EUL, CMC and CRM show that they have to a certain extent beneficial effects for their use in atopic dermatitis, including as an add-on to augment topical active treatment, although the quality of the evidence was very low to moderate for the prespecified outcomes.
The conclusions reached in these studies are in concordance with those drawn in the Cochrane Review, and essentially reinforce the rationality and benefits of moisturizer therapy. Gaps in the evidence included a lack of clarity as to which moisturizers are preferred for the different parts of the body and any indication of how personal preferences and external factors (e.g., weather, seasons) influenced the choice of moisturizer. Most importantly, there was a noticeable lack of assessment of the comparative effectiveness of the moisturizers, e.g., as to which are most appropriate for the actual disease status (acute or chronic) and severity (mild, moderate or severe). Therefore, clinical decision-making on the choice of moisturizer should be based not only on the available evidence but should also take into account the experiences and preferences of the individual suffering from atopic dermatitis, as well as the direct costs for the patients.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Prof Zbys Fedorowicz had contract support from Galderma for participating in the development of this manuscript to include article processing charges. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Disclosures
Esther J van Zuuren, Zbys Fedorowicz and Bernd WM Arents have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
Data Availability
All data generated or analyzed during this study are included in this published article.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/B598F060114B35B2.
References
- 1.Wollenberg A, Oranje A, Deleuran M, Simon D, Szalai Z, Kunz B, et al. ETFAD/EADV Eczema task force 2015 position paper on diagnosis and treatment of atopic dermatitis in adult and paediatric patients. J Eur Acad Dermatol Venereol. 2016;30:729–747. doi: 10.1111/jdv.13599. [DOI] [PubMed] [Google Scholar]
- 2.Peng W, Novak N. Pathogenesis of atopic dermatitis. Clin Exp Allergy. 2015;45:566–574. doi: 10.1111/cea.12495. [DOI] [PubMed] [Google Scholar]
- 3.Weidinger S, Novak N. Atopic dermatitis. Lancet. 2016;387:1109–1122. doi: 10.1016/S0140-6736(15)00149-X. [DOI] [PubMed] [Google Scholar]
- 4.Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Dermatol Venereol. 1980;92(Suppl.):44–47. [Google Scholar]
- 5.Williams HC, Burney PG, Hay RJ, Archer CB, Shipley MJ, Hunter JJ, et al. The UK working party’s diagnostic criteria for atopic dermatitis. I. Derivation of a minimum set of discriminators for atopic dermatitis. Br J Dermatol. 1994;131:383–396. doi: 10.1111/j.1365-2133.1994.tb08530.x. [DOI] [PubMed] [Google Scholar]
- 6.Deckers IA, McLean S, Linssen S, Mommers M, van Schayk CP, Sheikh A. Investigating international time trends in the incidence and prevalence of atopic eczema 1990–2010: a systematic review of epidemiological studies. PLoS ONE. 2012;7:e39803. doi: 10.1371/journal.pone.0039803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bieber T. Atopic dermatitis. N Engl J Med. 2008;358:1483–1494. doi: 10.1056/NEJMra074081. [DOI] [PubMed] [Google Scholar]
- 8.Kim JP, Chao LX, Simpson EL, Silverberg JI. Persistence of atopic dermatitis (AD): a systematic review and meta-analysis. J Am Acad Dermatol. 2016;75:681–687. doi: 10.1016/j.jaad.2016.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nankervis H, Thomas KS, Delamere FM, Barbarot S, Rogers NK, Williams HC. Scoping systematic review of treatments for eczema. Southampt (UK) NIHR J Libr. 2016;4:1–480. [PubMed] [Google Scholar]
- 10.Del Rosso JQ. Repair and maintenance of the epidermal barrier in patients diagnosed with atopic dermatitis: an evaluation of the components of a body wash-moisturizer skin care regimen directed at management of atopic skin. J Clin Aesthet Dermatol. 2011;4:45–55. [PMC free article] [PubMed] [Google Scholar]
- 11.Janssens M, van Smeden J, Gooris GS, Bras W, Portale G, Caspers PJ, et al. Increase in short-chain ceramides correlates with an altered lipid organization and decreased barrier function in atopic eczema patients. J Lipid Res. 2012;53:2755–2766. doi: 10.1194/jlr.P030338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fowler J. Understanding the role of natural moisturizing factor in skin hydration. Pract Dermatol. 2012;9:36–40. [Google Scholar]
- 13.Del Rosso JQ, Kircik KH. The integration of physiologically-targeted skin care in the management of atopic dermatitis: focus on the use of a cleanser and moisturizer system incorporating a ceramide precursor, filaggrin degradation products, and specific “skin-barrier-friendly” excipients. J Drugs Dermatol. 2013;12:s85–s91. [PubMed] [Google Scholar]
- 14.Moncrief G, Cork M, Lawton S, Kokiet S, Daly C, Clark C. Use of emollients in dry-skin conditions: consensus statement. Clin Exp Dermatol. 2013;38:231–238. doi: 10.1111/ced.12104. [DOI] [PubMed] [Google Scholar]
- 15.Angelova-Fisher I, Dapic I, Hoek AK, Jakasa I, Fischer TW, Zillikens D, et al. Skin barrier integrity and natural moisturising factor levels after cumulative dermal exposure to alkaline agents in atopic dermatitis. Acta Derm Venereol. 2014;94:640–644. doi: 10.2340/00015555-1815. [DOI] [PubMed] [Google Scholar]
- 16.Riethmuller C, McAleer MA, Koppes SA, Abdayem R, Franz J, Haftek M, et al. Filaggrin breakdown products determine corneocyte conformation in patient with atopic dermatitis. J Allergy Clin Immunol. 2015;136:1573–1580. doi: 10.1016/j.jaci.2015.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halling-Overgaard AS, Kezic S, Jakasa, Engebretsen KA, Maibach H, Thyssen JP. Skin absorption through atopic dermatitis skin: a systematic review. Br J Dermatol. 2016. (epub ahead of print). [DOI] [PubMed]
- 18.Lindh JD, Bradley M. Clinical effectiveness of moisturizers in atopic dermatitis and related disorders: a systematic review. Am J Clin Dermatol. 2015;16:341–359. doi: 10.1007/s40257-015-0146-4. [DOI] [PubMed] [Google Scholar]
- 19.van Zuuren EJ, Fedorowicz Z, Christensen R, Lavrijsen A, Arents BWM. Emollients and moisturisers for eczema. Cochrane Database Syst Rev. 2017;2:CD02119. doi: 10.1002/14651858.CD012119.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gehring W, Gloor M. Treatment of the atopic dermatitis with a water-in-oil emulsion with or without the addition of hydrocortisone-results of a controlled double-blind randomized study using clinical evaluation and bioengineering methods. Z Hautkr. 1996;71:554–560. [Google Scholar]
- 21.Hanifin JM, Hebert AA, Mays SR, Paller AS, Sherertz EF, Wagner AM, et al. Effects of a low-potency corticosteroid lotion plus a moisturizing regimen in the treatment of atopic dermatitis. Curr Ther Res Clin Exp. 1998;59:227–233. doi: 10.1016/S0011-393X(98)85076-5. [DOI] [Google Scholar]
- 22.Simpson E, Dutronc Y. A new body moisturiser increases skin hydration and improves atopic dermatitis symptoms among children and adults. J Drugs Dermatol. 2011;10:744–749. [PubMed] [Google Scholar]
- 23.Simpson E, Böhling A, Bielfeldt S, Bosc C, Kerrouche N. Improvement of skin barrier function in atopic dermatitis patients with a new moisturizer containing a ceramide precursor. J Dermatol Treat. 2013;24:122–125. doi: 10.3109/09546634.2012.713461. [DOI] [PubMed] [Google Scholar]
- 24.van Zuuren EJ, Fedorowicz Z, Lavrijsen A, Christensen R, Arents B. Emollients and moisturisers for eczema. Cochrane Database Syst Rev. 2016;3:CD012119. doi: 10.1002/14651858.CD012119.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions. Version 5.1.0 (updated March 2011). http://www.cochrane-handbook.org. Accessed 19 March 2017.
- 26.Schünemann H, Brożek J, Guyatt G, Oxman A. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. http://www.guidelinedevelopment.org/handbook. Accessed 19 March 2017.
- 27.Schram ME, Spuls PI, Leeflang MM, Lindeboom R, Bos JD, Schmitt J. EASI, (objective) SCORAD and POEM for atopic eczema: responsiveness and minimal clinically important difference. Allergy. 2012;67:99–106. doi: 10.1111/j.1398-9995.2011.02719.x. [DOI] [PubMed] [Google Scholar]
- 28.Sirikudta W, Kulthanan K, Varothai S, Nuchkull P. Moisturizers for patients with atopic dermatitis: an overview. J Allergy Ther. 2013;4:1–7. doi: 10.4172/2155-6121.1000143. [DOI] [Google Scholar]
- 29.Mack Correa MC, Nebus J. Management of patients with atopic dermatitis: the role of emollient therapy. Dermatol Res Pract. 2012;2012:836931. doi: 10.1155/2012/836931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grether-Beck S, Felsner I, Brenden H, Krutmann J. Urea uptake enhances barrier function and antimicrobial defense in humans by regulating epidermal gene expression. J Invest Dermatol. 2012;132:1561–1572. doi: 10.1038/jid.2012.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Åkerström U, Reitamo S, Langeland T, Berg M, Rustad L, Korhonen L. Comparison of moisturizing creams for the prevention of atopic dermatitis relapse: a randomized double-blind controlled multicentre clinical trial. Acta Derm Venereol. 2015;95:587–592. doi: 10.2340/00015555-2051. [DOI] [PubMed] [Google Scholar]
- 32.Excipial®. http://www.excipial.ca/en. Accessed 19 March 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article.