Abstract
Microneedles, fabricated by nano-moulding technology show great promise in the field of drug delivery by enabling the painless self-administration of drugs in a patient-friendly manner. In this study, double-stranded salmon DNA (SDNA) was used as both a drug-delivery vehicle and structural material with a microneedle system. SDNA is non-toxic and demonstrates good mechanical robustness, mouldability, biocompatibility, bio-absorbability, and binding affinity with drug molecules for bio-functional applications. Benign fabrication conditions to protect temperature-sensitive biomolecules are used to produce SDNA structures of various sizes with a high aspect ratio (4: 1). Unlike existing dissolving microneedle structure materials, the special binding characteristics of doxorubicin hydrochloride, anti-cancer drug molecules, and SDNA demonstrate the stability of drug-molecule encapsulation via UV-absorption and photoluminescence analyses. Based on COMSOL simulation and in vitro analysis of the stratum corneum of porcine skin, the mechanical functionality of SDNA microneedles was evaluated in vitro by penetrating the stratum corneum of porcine skin. The SDNA microneedle dissolved and drug permeation was assessed using rhodamine, a drug surrogate. Owing to its many beneficial characteristics, we anticipate that the SDNA microneedle platform will serve as an effective alternative for drug delivery.
Introduction
The patterning of biological molecules, such as proteins, DNA, and cells, on surfaces has emerged as a useful mechanism to enhance the performance of bioelectronics1–3. Effective biomolecular patterns and structures are critical for producing effective microdevices. Accordingly, various fabrication techniques have been developed for biomolecular patterning to satisfy the requirements of biofunctional microdevices2, 4. In particular, microtransfer moulding has been utilized to fabricate patterns of biomolecules on designated substrates5. We hypothesized that this patterning technique could be combined with DNA to provide a useful new direction in the area of drug delivery.
Microneedles have come to the forefront owing to their great impact in the field of drug delivery. They readily and effectively enter the human body, without drug degradation, and serve as pain-free, micro-scale devices with easy operation. Traditional methods, e.g. hypodermic needles and oral drug delivery, induce pain or result in biomolecule degradation in the digestive system, respectively. Large biomolecules, like peptides, antibodies, and proteins, are strong candidates for the development of novel therapeutics, but their applications are limited, since the epidermis facilitates the permeation of molecules with a molecular weight of less than 500 Da6–11. Microneedles create small holes in the skin, through which a greater range of drug materials can be readily injected12, 13. Accordingly, microneedles have the potential to replace existing drug-delivery methods.
Three main microneedle types have been suggested: solid14–16, hollow17, 18, and dissolving microneedles19–24. Solid microneedles have associated safety issues; they can shatter in the skin, resulting in a biohazard. Hollow microneedles are limited by their complicated fabrication process and weak mechanical strength. In contrast, the tip and backing layer of dissolving microneedles dissolve, without leaving biohazardous substances during insertion, and the fabrication process is simple. Owing to these advantages, we focused on the development of a dissolving microneedle for drug delivery in this study.
However, issues remain in the implementation of dissolvable microneedles, including a lack of suitable materials to maintain their shape and limited stability under severe conditions, which restricts the kinds of drugs that can be delivered and the material costs. Overcoming these limits of transdermal delivery will enable microneedles to be utilized as a novel therapeutic mechanism6, 7.
To improve microneedle systems for drug delivery, materials that are mechanically robust25, biocompatible (i.e. do not damage protein integrity), and non-toxic (i.e. do not generate biohazardous waste) are needed. When penetrating the skin, the microneedle material strongly influences the insertion force required19, 26–28. Additionally, appropriate fabrication methods are needed to minimize damage at high temperatures.
Therefore, we utilized salmon sperm DNA (SDNA) as a microneedle application system; this material can generate mechanically strong, well-mouldable, biocompatible, bio-absorbable, and non-toxic devices. It can be extracted from the sperm of salmon using a cost-effective enzyme-isolation process. Double-stranded SDNA has the potential for use in biological, physical, and medical applications, e.g. in optoelectronic devices, biosensors, or drug delivery. DNA molecules confined on a solid surface for interactions with other molecules have received increasing interest29–37. Furthermore, enzymes or drugs could be blended with an SDNA solution because it has special binding properties that facilitate drug containment in microneedles38. These mixtures are mechanically strong and have high biocompatibility, surpassing the performance of other bio-materials used in microneedles, and remain stable in dried form for long periods13. Based on these characteristics, SDNA is an appropriate biomaterial for microneedle systems.
We expect that SDNA microneedles will be effective for drug delivery, including the delivery of anti-cancer therapeutics and vaccines. Owing to the beneficial properties of SDNA, SDNA microneedles can potentially be combined with other drug materials according to various therapeutic needs, including human growth hormone therapy39, 40, measles vaccines41, and influenza vaccines42.
In this study, we generated various three-dimensional DNA structures to verify the moulding characteristic of SDNA, ranging from a nano- to micro-scale. The suggested fabrication method is an aqueous-based, nano-moulding process that uses gentle techniques to allow the introduction of variably sized and high-aspect-ratio DNA structures and the protection of encapsulated temperature-sensitive drugs. During the fabrication process, we incorporated doxorubicin hydrochloride (DOX) into the SDNA microneedle and verified the binding characteristics. Furthermore, we performed several experiments to ensure that the SDNA structures have suitable mechanical properties for skin penetration for use as a microneedle. We built on the initial drug-delivery system using the three-dimensional DNA structure.
Results
Figure 1(a) demonstrates how the SDNA microneedles deliver anti-cancer therapeutics to the body. The DOX-doped SDNA microneedles penetrate the stratum corneum of the skin to make small holes for DOX dispersion. As the microneedle is inserted into the skin, the SDNA material is dissolved for DOX delivery. The DOX-doped SDNA solution was fabricated by magnetic stirring, as shown in Fig. 1(b). Following SDNA solution fabrication, both SDNA patterns and the microneedle could be fabricated, as demonstrated in Fig. 1(c). This method minimized damage to temperature-sensitive materials, since the mild fabrication process proceeds at ambient pressure and temperatures of less than 45 °C.
Figure 1.
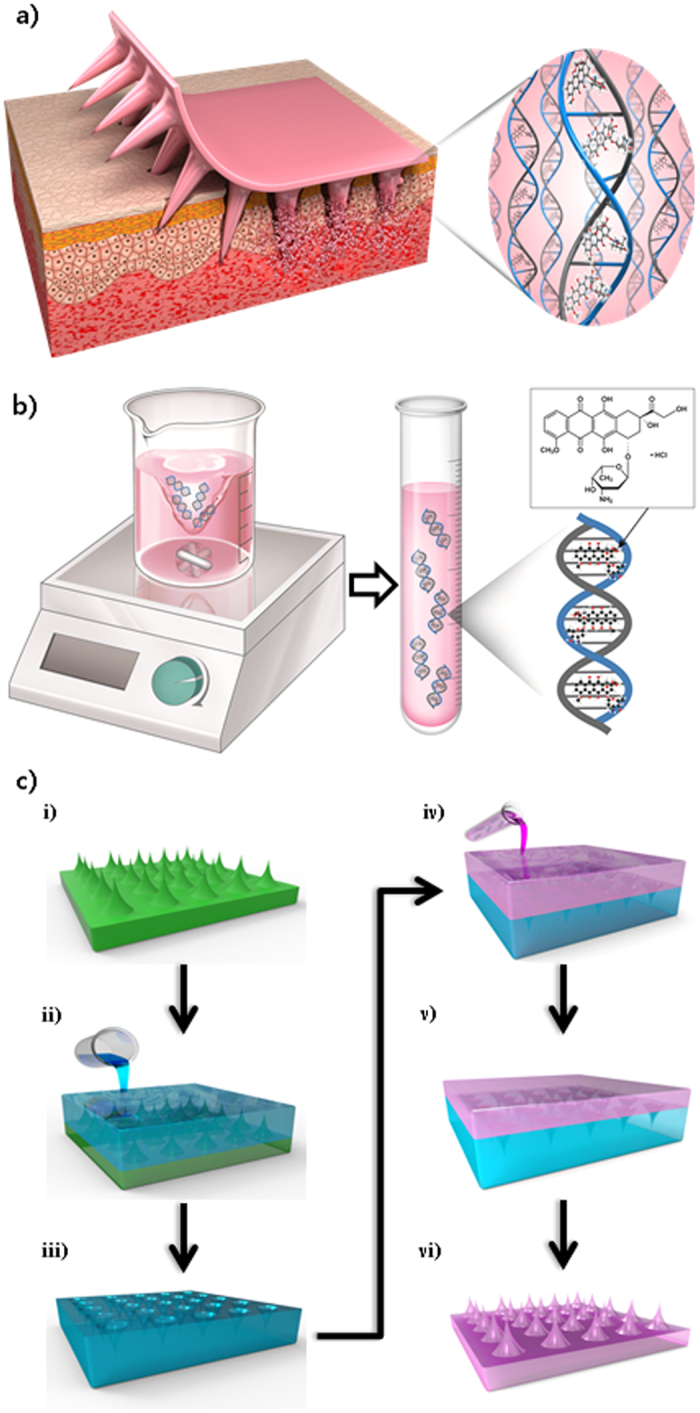
Schematic illustrations of the SDNA solution and structure fabrication, molecular structure of DOX, and DOX intercalation in SDNA. (a) Schematic diagram of drug delivery using the SDNA microneedle. (b) Generation of the SDNA solution with DOX by magnetic stirring, and a representative image of DOX intercalation into SDNA. (c) Schematic illustration of the fabrication process used to prepare an SDNA microneedle loaded with DOX. i) Microneedle array master. ii) PDMS is poured into the master to obtain the negative mould. iii) the PDMS mould is detached from the master. iv) the SDNA solution with DOX is poured into the PDMS mould. v) the SDNA solution with DOX is dried for more than 2 days. vi) the SDNA microneedle with DOX is detached from the PDMS mould.
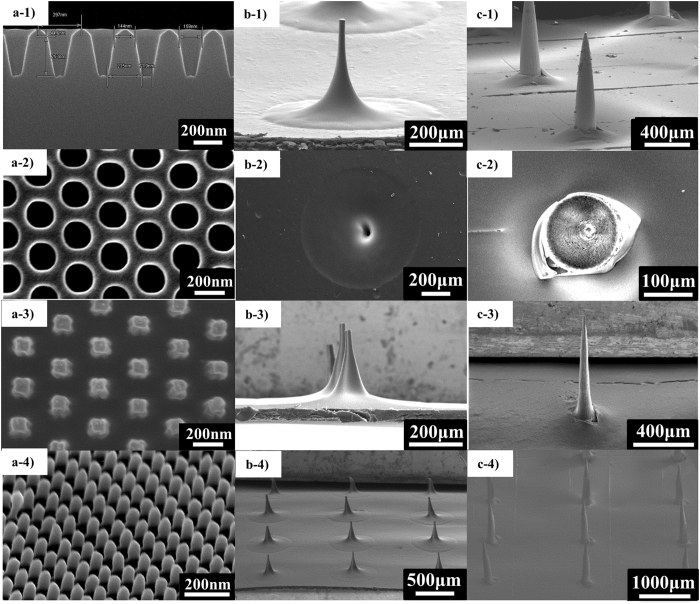
The nano-sized moulding process utilizes the excellent moulding characteristics of SDNA. First, a master structure was prepared by electron beam lithography with Si etching. Then, the master structure was moulded using ultraviolet (UV)-cured resin to create reverse moulds. This process facilitated the reproduction of numerous structures that could be reused. The UV-cured resin was chosen as a mould material owing to the rapid fabrication process and potentially high resolution of nano-scale moulding. The same process was repeated to construct the original patterns. These moulds were then utilized to prepare nano-sized structures. The SDNA solution was casted over the nano-sized moulds to fill cavities. After drying for more than 2 days at ambient pressure, without UV, at 45 °C, nano-scale DNA patterns were acquired, and these could be applied to biofunctional devices. As shown in Fig. 2a, 150-nm hole structures with a high aspect ratio (300 nm in height) could be easily obtained, indicating that SDNA has good mouldability. Based on these results, we could exploit SDNA to develop large structures with high aspect ratios for biological applications.
Figure 2.
SEM images of the cylindrical hole pattern with a diameter of 150 nm and 300-nm spacing (300 nm in height), and images of the mould and DOX-doped SDNA microneedle patches. (a-1) Cross-sectional scanning electron microscopy (SEM) image of the master structure. (a-2) High-magnification SEM image of the second replica with UV resin. (a-3) High-magnification SEM image of the SDNA pattern. (a-4) Tilted SEM image of the SDNA pattern. (b-1) SEM image of the master template, 300 μm. (b-2) SEM image of the PDMS mould for the 300-μm microneedle. (b-3) SEM image of the SDNA microneedle (300 μm). (b-4) SEM image of the 3 × 3 array of the SDNA microneedle (300 μm). (c-1) SEM image of the master template (1000 μm). (c-2) SEM image of the PDMS mould for the 1000-μm microneedle. (c-3) SEM image of the single SDNA microneedle (1000 μm). (c-4) SEM image of the 3 × 3 array of the SDNA microneedle (1000 μm).
These excellent moulding characteristics supported the use of SDNA to fabricate microneedles. We used commercial microneedles as master templates; they were 1000 and 300 µm high with a tip radius of approximately 20 µm, as shown in Figs 2b-1 and 2c-1. This master was utilized to fabricate a negative mould for microneedles with poly-dimethylsiloxane (PDMS) using a soft lithography technique (Fig. 2b-2, 2c-2)43. PDMS has a variety of useful properties, including a low surface energy, ease of use, conformal contact, low cost, and flexibility. Additionally, we altered the surface property from a hydrophobic to a hydrophilic nature by oxygen plasma treatment44. These properties of the soft elastomer material enable SDNA detachment from the microneedle with minimal damage, and the micro-sized structure can be readily reproduced45. Moreover, the porous structure helps to remove water from the elastomer46. Next, the SDNA solution was cast over the PDMS mould and solidified by drying for at least 2 days. During the process, shrinkage was observed in both the PDMS moulding and SDNA microneedles. The SDNA microneedle array was gently removed from the PDMS mould (Figs 2b-3, 4 and 2c-3, 4). We conducted numerous experiments to optimize the annealing time for fabrication. The benign temperature and aqueous-based fabrication process minimized damage to drugs and ensured a low cost so that it is competitive in the market.
Figure 3.
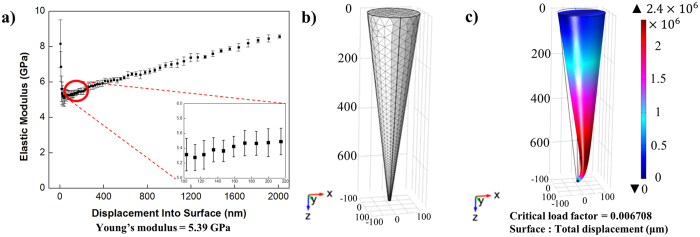
Mechanical-property simulation using COMSOL for SDNA microneedles when an axial load of 5 N was applied to the base of a single SDNA microneedle with a fixed constraint on the microneedle tip. (a) Young’s modulus of SDNA film. (b) Mesh analysis of the single SDNA microneedle. (c) Prediction of the buckling of the SDNA microneedle.
Figure 4.
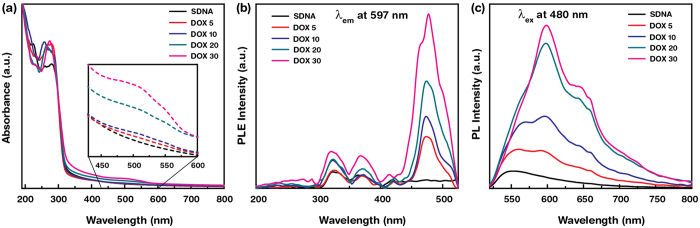
Absorption, photoluminescence excitation (PLE), and PL spectra of DOX-doped SDNA microneedles. (a) Absorption spectra of SDNA microneedles with various concentrations of DOX, i.e. 0, 5, 10, 20, and 30 μM (marked as SDNA, DOX 5, DOX 10, DOX 20, and DOX 30, respectively). (inset) For clarity, the absorption bands of DOX in SDNA microneedles in the wavelength range of 430–600 nm are magnified. (b) PLE spectra of DOX-doped SDNA microneedles at a fixed emission wavelength of λem = 597 nm. (c) PL spectra of DOX-doped SDNA microneedles at a fixed excitation wavelength, λex = 480 nm.
We used SDNA as a microneedle material for several reasons. First, based on the Young’s modulus, SDNA is mechanically stronger than other individual dissolvable microneedle materials; therefore, it can maintain its shape when penetrating the skin19. Additionally, it can rapidly dissolve in water owing to the hydrophilicity of the sugar and phosphate molecules that make up the DNA backbone. Third, it has special binding properties, including intercalation between base-pairs, steric preference in major and minor grooves, and electrostatic interactions around the phosphate backbone. Desired drug materials can exhibit groove binding owing to their geometrical compatibility with designated base sequences and can intercalate with nucleobases via hydrogen bonding and π–π stacking38, 47. Finally, the SDNA solution is aqueous at room temperature, enabling fabrication at ambient pressures and under 45 °C to protect temperature-sensitive drugs.
For microneedles to replace existing hypodermic needle technology, they should exhibit sufficient strength to penetrate the stratum corneum. Ample mechanical strength for insertion is an important property for microneedle materials. In particular, Young’s modulus is closely related to the insertion force and buckling load of the microneedle25. We measured the Young’s modulus of SDNA, which ranged from 5.22–5.56 GPa (average, 5.39 0.17 Gpa; n = 9), as shown in Fig. 3(a). The average value was larger than those of amylopectin (4.5 GPa)48, CMC (1 GPa)19, and PLA (5 GPa)49. Based on the Young’s modulus estimates, we conducted a COMSOL simulation to identify the extent to which the SDNA microneedle withstands a given axial load (Fig. 3). The critical buckling force of a microneedle can be estimated by multiplying the estimated critical load factor by the applied force. Based on the simulation, the critical buckling load for the SDNA microneedle was approximately 0.035 N, which is much larger than the force at which the needle can be inserted in the skin without buckling50. These results demonstrated that the SDNA microneedle has sufficient mechanical strength for practical applications.
UV−visible (Vis) absorption spectra were determined for DOX-doped SDNA microneedles, and the binding interactions between DOX and SDNA molecules were analysed. Figure 4(a) shows the absorption spectra as a function of wavelength for pristine and SDNA microneedles doped with various DOX concentrations (DOX; 0, 5, 10, 20, and 30 µM), referred to here as DOX 0, DOX 5, DOX 10, DOX 20, and DOX 30, respectively. The absorption spectra of DOX-doped SDNA microneedles showed characteristic absorption bands for DNA at wavelengths of ~260 nm and bands for DOX in the range of 470–550 nm. The DOX-doped SDNA microneedles showed increasing absorption above 400 nm with an increasing DOX, owing to the strong interaction between DNA and DOX via intercalation. For clear visualisation of the presence of DOX, a magnified graph is shown as an inset in Fig. 4(a). The intercalation of DOX into DNA molecules was probably due to strong π–π stacking interactions between the DNA bases and DOX molecules. Guanine and adenine are preferable for intercalation, whereas fewer interactions occur with thymine or cytosine51, 52.
Additionally, the photoluminescence (PL) characteristics of DOX-doped SDNA microneedles with respect to the DOX were analysed to determine the energy-transfer mechanism between DOX and SDNA microneedles and to provide support for DOX doping. PL requires proper excitation energy to transfer an electron from the singlet ground state to the excited state upon absorbing the energy of a photon. The inset of Fig. 4(b) indicates the PL excitation of the DOX-doped SDNA microneedles at a fixed emission wavelength (595 nm). An appreciable characteristic excitation peak was observed at ~480 nm and this excitation wavelength. PL emerges from the release of a photon upon relaxation of the electron from the triplet excited state. Because the absorption band of DNA molecules is in the range of 250 to 280 nm, energy transfer occurred by internal conversion within the excited singlet state, then from the excited singlet state to the triplet state via intersystem crossing, and finally to the emissive state47, 53. Figure 4(c) reveals the PL spectra of DOX-doped SDNA microneedles as a function of the DOX at a fixed excitation wavelength of 480 nm. These emission spectra showed three strong broad characteristic emission peaks for DOX at ~562 ~597, and ~650 nm, which were not observed for pristine SDNA microneedles. We noticed a change in the PL intensity as a function of the DOX, which revealed the degree of DOX binding to DNA molecules54. In addition, the emission peak area and full width at half maximum also increased as the DOX increased. This enhancement in PL (according to the DOX) was indicative of the degree to which DOX bound the DNA nanostructure in designated and improper regions, reflecting the size and shape of the DOX molecules. Significant changes in PL intensity for DOX-doped SDNA microneedles clearly revealed that DOX was loaded into the SDNA microneedles.
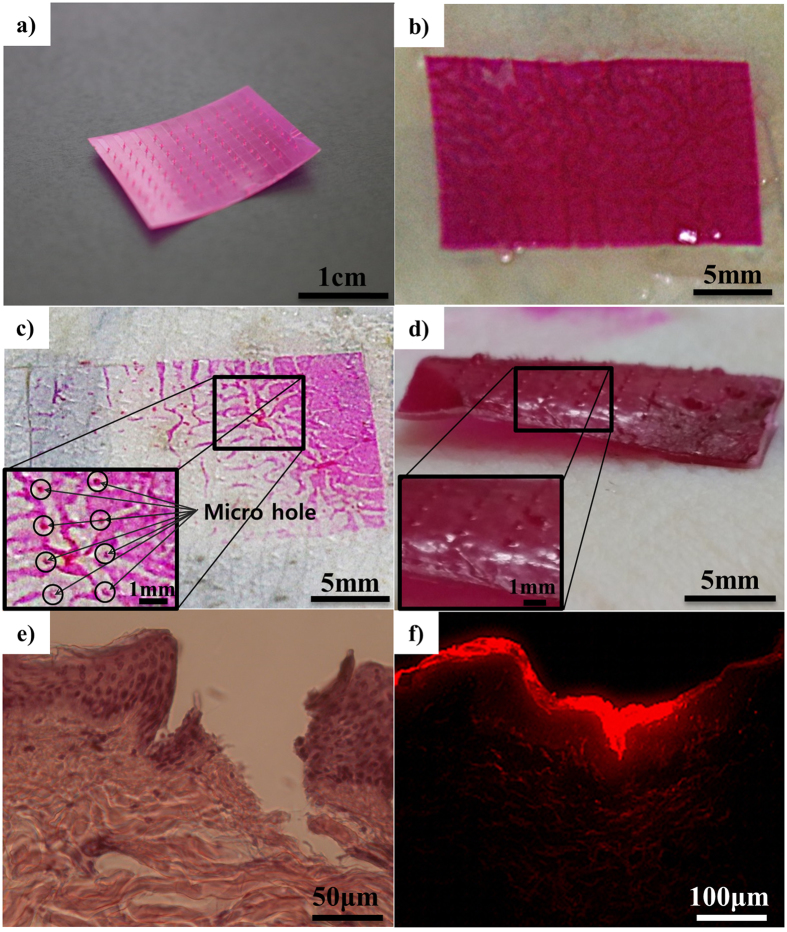
To evaluate the practical application of SDNA microneedles (and confirm that they had sufficient mechanical strength and dissolvability), we inserted SDNA microneedles into porcine cadaver skin. Figure 5(a) shows a flexible SDNA microneedle patch loaded with DOX and fluorescent dye. Figure 5(b) shows a macroscopic image of the SDNA microneedle attached to the porcine skin. After a 10-min attachment to the porcine skin, the microneedles were detached from porcine skin and the insertion was traced by incorporating red dyes into the SDNA microneedle for visualisation (Fig. 5(c)). More than half of the microneedle dissolved within 10 min after insertion into porcine skin, as shown on Fig. 5(d). To visualise the mechanical functionality, the location of epidermal breach was verified by histology (Fig. 5(e)). The drug surrogate dispersed from the insertion site, supporting the application of the system for drug delivery (Fig. 5(f)). Based on the histological results, the depth of insertion was generally shorter than the height of the microneedles. This can be explained by the elastic nature of the skin and the geometry of the microneedles themselves, hindering their injection. Regardless of their mechanical characteristics, microneedles could be easily deformed at the surface of the skin during insertion. However, the SDNA microneedles had enough mechanical functionality to successfully penetrate the stratum corneum, and they dissolved completely, indicating their solubility (Supplementary Fig. S4)16.
Figure 5.
Microscopy images of SDNA microneedle insertion into porcine skin. (a) Image of a curled SDNA microneedle showing flexibility. (b) Application of the SDNA microneedle patch to porcine skin. (c) Microscopy image of porcine skin after removal of the microneedle patch. (d) Illustrative image of the dissolution of SDNA microneedle arrays after a 10-min insertion and subsequent removal from porcine skin. (e) Cross-sectional image of hematoxylin and eosin-stained skin at a SDNA microneedle-insertion site. (f) Fluorescence microscopy image of an SDNA microneedle with a drug surrogate.
Discussion
Our results indicated that SDNA functions as both a drug-delivery vehicle and structural material for effective dissolving microneedles. SDNA microneedles represent a pragmatic and novel approach to directly deliver various drugs into the body with a non-toxic, bio-absorbable, and biocompatible structure material. This system could replace existing drug-delivery methods owing to its convenience, safety (as established by the Japan Foundation of Food Research Laboratory at Chitose for SDNA), and ease of use for delivering biomolecules into the human body. Owing to the good mouldability of SDNA, structures of various sizes ranging from the nano- to micro-scale at a 4:1 aspect ratio could be used to develop the microneedle structure.
Furthermore, the solution-based moulding process takes advantage of the special binding characteristics of SDNA, which enable strong chemical interactions to intercalate desired drugs, e.g. DOX, within the DNA structure. The unique intercalation characteristic of DNA molecules allows them to function as stable drug-delivery vehicles due to strong π–π stacking interactions.
Additionally, the SDNA microneedles demonstrated adequate mechanical functionality; the critical buckling load for the single SDNA microneedle was approximately 0.035 N. Using an in vitro model, the efficacy of the microneedle for the delivery of a drug surrogate over the stratum corneum of pigs was confirmed.
This novel DNA drug-delivery platform will be further developed for applications with various diseases; it overcomes the disadvantages of existing technologies for the delivery of a wide range of biomolecules, including vaccines, proteins, and peptides.
Methods
SDNA solution preparation
SDNA (1 g) was dissolved in 100 mL of de-ionized water to prepare the SDNA solution (Chitose Institute of Science and Technology, Hokkaido, Japan); the final concentration of the SDNA solution was 1.0 wt%. Magnetic stirring at approximately 1000 rpm for 10 h at room temperature was required to acquire a homogeneous mixture of SDNA and DOX or rhodamine47.
SDNA doping with DOX
A suitable amount of DOX, i.e. 0, 10, or 30 μM, was pipetted into the 1.0 wt% SDNA solution. Then, the DOX-doped SDNA solution was prepared by vortexing for 5 min and incubation for 1 day at room temperature to acquire a homogeneous mixture47.
Fabrication of a nano-sized mould
The first step in fabrication was the generation of a nano-patterned silicon master template, which was fabricated by electron beam lithography with Si etching. The dimensions of the arrays were 150 nm with a height of 300 nm (300 nm spacing). Subsequently, a UV-resin mould was used to fabricate the negative mould. UV resin was deposited on the surface of the master template and coated with a polyethylene phthalate (PET) film. Then, the back of the PET film was rolled with a hand roller to fill the cavities with UV resin. After 3 min of UV curing, the PET film was detached from the master template. The process was repeated to obtain the original mould.
Microneedle mould fabrication
A commercial microneedle patch, the Acropass Microneedle Patch (Seoul, South Korea) and a commercial Derma Stamp (Larcobaleno, Seoul, South Korea) were utilized as a master stamp for the microneedle patch arrays. The microneedle patch arrays had heights of 300 μm (base width of 200 μm and tip radius of 10 μm) and 1000 μm (base width of 200 μm and tip radius of 10 μm). Subsequently, the female PDMS (Sylgard 184; Dow Corning, Midland, MI, USA) moulds were poured over the microneedle arrays to obtain a reverse polymer mould. The PDMS mould was manufactured by mixing the elastomer and curing agent at a ratio of 10: 1 by weight. The PDMS mould was then vacuum-treated for 1 h to remove air bubbles and cured at below 70 °C for 2 h. Subsequently, the PDMS mould was gently detached from the master stamp.
SDNA pattern fabrication
The SDNA solution was cast over the UV-resin mould, which was attached to the substrate on a 2-inch Petri dish and dried in an oven (Thermostable ON-105; DAIHAN Scientific, Seoul, South Korea) at 45 °C for 2 days. After it solidified, the SDNA pattern was separated from the UV-resin mould.
SDNA microneedle fabrication
Rhodamine B (>98% pure; Acros-Organics, Geel, Belgium), a water-soluble red fluorescent dye, was added at a concentration of 0.01 wt% to 1.0 wt% SDNA solution. Oxygen plasma treatment was conducted to modify the surface of the PDMS mould. The mixture was cast over the PDMS mould and dried in an oven (ThermoStable ON-105; DAIHAN Scientific) for 2 days. After it solidified, the SDNA microneedle patch was separated from the PDMS mould.
Statistical analysis
Statistical comparisons of practical measurements of the heights of various structures were performed using one-way analysis of variance, and p < 0.05 was considered to indicate a statistically significant difference. Three different PDMS moulds were used, and 30 measurements were taken for each mould.
Young’s modulus measurement
The mechanical properties of a DNA film were characterised using nanoindentation (NanoIndenter XP; MTS, Eden Prairie, MN, USA) with a Berkovich diamond indenter. The Oliver–Pharr method was utilized to analyse the nanoindentation data to determine the representative modulus values with respect to the indentation depth55. The surface stiffness of the DNA film was measured based on the continuous stiffness measurement unit obtained using the NanoIndenter XP, and Poison’s ratio of DNA was assumed to be 0.3. The measured modulus was 5.39 GPa, which reflected the average value over a range of indentation depths from 100 to 200 nm. The tests were repeated in nine trials, using the same samples.
Simulation
The Structural Mechanics module of COMSOL Multiphysics software (www.comsol.com) was utilized for the simulations. The design of a single microneedle was a 3D cone-shaped structure of 750 μm in height, 200 μm in base diameter, and 10 μm in tip radius. The needle structure was considered a linear elastic material using the Young’s modulus, with an estimated Poisson’s ratio of 0.3. A fixed constraint was adopted such that the needle base only moved in the axial direction. The critical load factor of the microneedles was simulated while applying 5 N of axial loading. The microneedle tip was fixed in a position and the other end, i.e. the microneedle base, was allowed to move only in the axial direction.
UV–Vis spectroscopy
A spectrophotometer (Cary 5 G; Varian, Palo Alto, CA, USA) was used to measure the optical absorbance of the DOX-doped SDNA microneedles in the visible and UV regions (wavelengths between 800 and 190 nm). The spectrophotometer was equipped with two light sources: a deuterium arc lamp (near-infrared and visible) and a quartz W–halogen lamp (UV). The instrument also had two detectors: a cooled PbS detector (near-infrared) and a photomultiplier tube (visible and UV). The spectrophotometer measures the frequency-dependent light intensity passing either through a vacuum or through the sample.
PL measurements
PL and PL excitation spectra of DOX-doped SDNA microneedles were obtained at room temperature using a Xe-arc lamp-equipped fluorometer (FS-2; Scinco, Seoul, Korea) at 25 W. Excitation spectra were obtained at a fixed emission wavelength (597 nm), and emission spectra were measured by exciting samples at ~480 nm.
Microneedle injection imaging
SDNA microneedle patches were applied to whole pig cadaver skin (a female Micro-pig® weighing 25 kg) with a thickness of 2.0 ± 0.2 mm (Medi Kinetics Micropig®; Pyeongtaek, Korea). The test was approved by the Institutional Animal Care and Use Committee. All skin samples were received and stored at –80 °C until tests were conducted. Microneedle penetration was traced after injecting the SNDA microneedle array into the porcine skin in vitro. After applying the microneedle patch to the skin for 10 min, the needle-exposed side of the skin was examined by microscopy to identify the sites of microneedle insertion.
To view the cross-sectional images of microneedle-insertion sites, the microneedle-treated porcine skin samples were fixed for 20 h with formaldehyde (10%) and subsequently embedded in paraffin wax. The sample was cut into 20-μm sections using a microtome, and the slices were stained with haematoxylin and eosin. Then, a histological analysis was performed by microscopy to identify the insertion site of the SDNA microneedle.
Other microneedle-treated porcine skin samples were cryosectioned and bisected in the z-direction (30 μm). The sections were visualized by microscopy.
Electronic supplementary material
Acknowledgements
This work was supported by the Center for Advanced Meta-Materials (CAMM) funded by the Ministry of Science, ICT and Future Planning as Global Frontier Project (CAMM grant number 2014M3A6B3063707) and by the Industrial Strategic technology development program (grant number 10052641) funded by the Ministry of Trade, Industry & Energy (MI, Korea).
Author Contributions
Y.W.L. and J.H.J. initiated and designed the experiments. Y.W.L. developed the DNA microneedle, performed in vitro experiment, and wrote the first version of manuscript. S.R.D. prepared the SDNA solution and performed Fourier transform infrared spectroscopy and PL measurements. S.H.J. performed the COMSOL simulations. S.H.H. performed the nano patterning using DNA. J.H.K. performed the Young’s modulus measurement. All authors contributed to the data analysis. S.H.P. and J.H.J. initiated and supervised the research.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-09904-9
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sung Ha Park, Email: sunghapark@skku.edu.
Jun-Ho Jeong, Email: jhjeong@kimm.re.kr.
References
- 1.Niemeyer, C. M. & Mirkin, C. A. In Nanobiotechnology: concepts, applications and perspectives (John Wiley & Sons, 2004).
- 2.Blawas A, Reichert W. Protein patterning. Biomaterials. 1998;19:595–609. doi: 10.1016/S0142-9612(97)00218-4. [DOI] [PubMed] [Google Scholar]
- 3.Lemieux B, Aharoni A, Schena M. Overview of DNA chip technology. Mol. Breed. 1998;4:277–289. doi: 10.1023/A:1009654300686. [DOI] [Google Scholar]
- 4.Christman KL, Enriquez-Rios VD, Maynard HD. Nanopatterning proteins and peptides. Soft Matter. 2006;2:928–939. doi: 10.1039/b611000b. [DOI] [PubMed] [Google Scholar]
- 5.Kane RS, Takayama S, Ostuni E, Ingber DE, Whitesides GM. Patterning proteins and cells using soft lithography. Biomaterials. 1999;20:2363–2376. doi: 10.1016/S0142-9612(99)00165-9. [DOI] [PubMed] [Google Scholar]
- 6.Arora A, Prausnitz MR, Mitragotri S. Micro-scale devices for transdermal drug delivery. Int. J. Pharm. 2008;364:227–236. doi: 10.1016/j.ijpharm.2008.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen X, et al. Site‐Selectively Coated, Densely‐Packed Microprojection array patches for targeted delivery of vaccines to skin. Adv. Funct. Mater. 2011;21:464–473. doi: 10.1002/adfm.201000966. [DOI] [Google Scholar]
- 8.Daugherty AL, Mrsny RJ. Emerging technologies that overcome biological barriers for therapeutic protein delivery. Exp. Opin. Biol. Ther. 2003;3:1071–1081. doi: 10.1517/14712598.3.7.1071. [DOI] [PubMed] [Google Scholar]
- 9.Nir Y, Paz A, Sabo E, Potasman I. Fear of injections in young adults: prevalence and associations. Am. J. Trop. Med. Hyg. 2003;68:341–344. [PubMed] [Google Scholar]
- 10.Simonsen L, Kane A, Lloyd J, Zaffran M, Kane M. In focus-unsafe injections in the developing world and transmission of bloodborne pathogens: a review. Bull. World Health Organ. 1999;77:789–800. [PMC free article] [PubMed] [Google Scholar]
- 11.Bos JD, Meinardi MM. The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp. Dermatol. 2000;9:165–169. doi: 10.1034/j.1600-0625.2000.009003165.x. [DOI] [PubMed] [Google Scholar]
- 12.Al‐Qallaf B, Das DB. Optimizing microneedle arrays to increase skin permeability for transdermal drug delivery. Ann. N. Y. Acad. Sci. 2009;1161:83–94. doi: 10.1111/j.1749-6632.2009.04083.x. [DOI] [PubMed] [Google Scholar]
- 13.Badran M, Kuntsche J, Fahr A. Skin penetration enhancement by a microneedle device (Dermaroller®) in vitro: Dependency on needle size and applied formulation. Eur. J. Pharm. Sci. 2009;36:511–523. doi: 10.1016/j.ejps.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 14.Lin L, Pisano AP. Silicon-processed microneedles. J. Microelectromech. Syst. 1999;8:78–84. doi: 10.1109/84.749406. [DOI] [Google Scholar]
- 15.Omatsu T, et al. Metal microneedle fabrication using twisted light with spin. Opt. Express. 2010;18:17967–17973. doi: 10.1364/OE.18.017967. [DOI] [PubMed] [Google Scholar]
- 16.Cormier M, et al. Transdermal delivery of desmopressin using a coated microneedle array patch system. J. Controlled Release. 2004;97:503–511. doi: 10.1016/S0168-3659(04)00171-3. [DOI] [PubMed] [Google Scholar]
- 17.Davis SP, Martanto W, Allen MG, Prausnitz MR. Hollow metal microneedles for insulin delivery to diabetic rats. IEEE Trans. Biomed. Eng. 2005;52:909–915. doi: 10.1109/TBME.2005.845240. [DOI] [PubMed] [Google Scholar]
- 18.Wang PM, Cornwell M, Hill J, Prausnitz MR. Precise microinjection into skin using hollow microneedles. J. Invest. Dermatol. 2006;126:1080–1087. doi: 10.1038/sj.jid.5700150. [DOI] [PubMed] [Google Scholar]
- 19.Lee JW, Park J, Prausnitz MR. Dissolving microneedles for transdermal drug delivery. Biomaterials. 2008;29:2113–2124. doi: 10.1016/j.biomaterials.2007.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sullivan SP, et al. Dissolving polymer microneedle patches for influenza vaccination. Nat. Med. 2010;16:915–920. doi: 10.1038/nm.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Demir YK, Akan Z, Kerimoglu O. Characterization of polymeric microneedle arrays for transdermal drug delivery. PLoS One. 2013;8 doi: 10.1371/journal.pone.0077289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Demir YK, Kerimoglu O. Novel use of pectin as a microneedle base. Chem. Pharm. Bull. 2015;63:300–304. doi: 10.1248/cpb.c14-00759. [DOI] [PubMed] [Google Scholar]
- 23.Dangol, M. et al. Anti-obesity effect of a novel caffeine-loaded dissolving microneedle patch in high-fat diet-induced obese C57BL/6J mice. J. Controlled Release (2017). [DOI] [PubMed]
- 24.Kim S, et al. Enhanced transdermal delivery by combined application of dissolving microneedle patch on serum-treated skin. Mol. Pharm. 2017;14:2024–2031. doi: 10.1021/acs.molpharmaceut.7b00111. [DOI] [PubMed] [Google Scholar]
- 25.Davis SP, Landis BJ, Adams ZH, Allen MG, Prausnitz MR. Insertion of microneedles into skin: measurement and prediction of insertion force and needle fracture force. J. Biomech. 2004;37:1155–1163. doi: 10.1016/j.jbiomech.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 26.Aggarwal P, Johnston C. Geometrical effects in mechanical characterizing of microneedle for biomedical applications. Sensors Actuators B: Chem. 2004;102:226–234. doi: 10.1016/j.snb.2004.04.024. [DOI] [Google Scholar]
- 27.Park JH, Prausnitz MR. Analysis of Mechanical Failure of Polymer Microneedles by Axial Force. J. Korean Phys. Soc. 2010;56:1223–1227. doi: 10.3938/jkps.56.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Donnelly RF, et al. Optical coherence tomography is a valuable tool in the study of the effects of microneedle geometry on skin penetration characteristics and in-skin dissolution. J. Controlled Release. 2010;147:333–341. doi: 10.1016/j.jconrel.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 29.Wang L, Yoshida J, Ogata N, Sasaki S, Kajiyama T. Self-assembled supramolecular films derived from marine deoxyribonucleic acid (DNA)−cationic surfactant complexes: large-scale preparation and optical and thermal properties. Chem. Mater. 2001;13:1273–1281. doi: 10.1021/cm000869g. [DOI] [Google Scholar]
- 30.Zhang, G., Wang, L., Yoshida, J. & Ogata, N. Optical and optoelectronic materials derived from biopolymer deoxyribonucleic acid (DNA) (Asia-Pacific Optical and Wireless Communications Conference and Exhibit, International Society for Optics and Photonics, 2001).
- 31.Agudelo D, Bourassa P, Bérubé G, Tajmir-Riahi H. Intercalation of antitumor drug doxorubicin and its analogue by DNA duplex: structural features and biological implications. Int. J. Biol. Macromol. 2014;66:144–150. doi: 10.1016/j.ijbiomac.2014.02.028. [DOI] [PubMed] [Google Scholar]
- 32.Audrey S, Beatrice D, Loic JB. DNA biosensors and microarrays. Chem. Rev. 2008;108:109–139. doi: 10.1021/cr0684467. [DOI] [PubMed] [Google Scholar]
- 33.Kim K, et al. Drug delivery by a self-assembled DNA tetrahedron for overcoming drug resistance in breast cancer cells. Chemical Communications. 2013;49:2010–2012. doi: 10.1039/c3cc38693g. [DOI] [PubMed] [Google Scholar]
- 34.Yang X, Wang AH. Structural studies of atom-specific anticancer drugs acting on DNA. Pharmacol. Ther. 1999;83:181–215. doi: 10.1016/S0163-7258(99)00020-0. [DOI] [PubMed] [Google Scholar]
- 35.McGown LB, Joseph MJ, Pitner JB, Vonk GP, Vonk GP. The nucleic acid ligand. Anal. Chem. 1995;67:663A–668A. doi: 10.1021/ac00117a002. [DOI] [PubMed] [Google Scholar]
- 36.Dugasani SR, et al. Magnetic characteristics of copper ion-modified DNA thin films. Sci. Rep. 2013;3 doi: 10.1038/srep01819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dugasani SR, et al. Energy band gap and optical transition of metal ion modified double crossover DNA lattices. ACS Appl. Mater. Interfaces. 2014;6:17599–17605. doi: 10.1021/am503614x. [DOI] [PubMed] [Google Scholar]
- 38.Mahadevan S, Palaniandavar M. Spectroscopic and voltammetric studies of copper (II) complexes of bis (pyrid-2-yl)-di/trithia ligands bound to calf thymus DNA. Inorg. Chim. Acta. 1997;254:291–302. doi: 10.1016/S0020-1693(96)05175-4. [DOI] [Google Scholar]
- 39.Kwak H, et al. Development of a sustained-release recombinant human growth hormone formulation. J. Controlled Release. 2009;137:160–165. doi: 10.1016/j.jconrel.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 40.Lee JW, Choi S, Felner EI, Prausnitz MR. Dissolving microneedle patch for transdermal delivery of human growth hormone. Small. 2011;7:531–539. doi: 10.1002/smll.201001091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Edens C, Collins ML, Ayers J, Rota PA, Prausnitz MR. Measles vaccination using a microneedle patch. Vaccine. 2013;31:3403–3409. doi: 10.1016/j.vaccine.2012.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jacoby E, Jarrahian C, Hull HF, Zehrung D. Opportunities and challenges in delivering influenza vaccine by microneedle patch. Vaccine. 2015;33:4699–4704. doi: 10.1016/j.vaccine.2015.03.062. [DOI] [PubMed] [Google Scholar]
- 43.Wolfe, D. B., Qin, D. & Whitesides, G. M. Rapid prototyping of microstructures by soft lithography for biotechnology. Microeng. Biotechnol. 81–107 (2010). [DOI] [PubMed]
- 44.Bhattacharya S, Datta A, Berg JM, Gangopadhyay S. Studies on surface wettability of poly (dimethyl) siloxane (PDMS) and glass under oxygen-plasma treatment and correlation with bond strength. J. Microelectromech. Syst. 2005;14:590–597. doi: 10.1109/JMEMS.2005.844746. [DOI] [Google Scholar]
- 45.Liu X, Zhang C, Xu W, Ouyang C. Controlled release of heparin from blended polyurethane and silk fibroin film. Mater. Lett. 2009;63:263–265. doi: 10.1016/j.matlet.2008.10.006. [DOI] [Google Scholar]
- 46.Fritz L, Hofmann D. Molecular dynamics simulations of the transport of water-ethanol mixtures through polydimethylsiloxane membranes. Polymer. 1997;38:1035–1045. doi: 10.1016/S0032-3861(96)00600-3. [DOI] [Google Scholar]
- 47.Gnapareddy B, et al. Chemical and physical characteristics of doxorubicin hydrochloride drug-doped Salmon DNA thin films. Sci. Rep. 2015;5 doi: 10.1038/srep12722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kalichevsky M, Jaroszkiewicz E, Ablett S, Blanshard J, Lillford P. The glass transition of amylopectin measured by DSC, DMTA and NMR. Carbohydr. Polym. 1992;18:77–88. doi: 10.1016/0144-8617(92)90129-E. [DOI] [Google Scholar]
- 49.Park J, Allen MG, Prausnitz MR. Biodegradable polymer microneedles: fabrication, mechanics and transdermal drug delivery. J. Controlled Release. 2005;104:51–66. doi: 10.1016/j.jconrel.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 50.Loizidou EZ, et al. Structural characterisation and transdermal delivery studies on sugar microneedles: Experimental and finite element modelling analyses. Eur. J. Pharm. Biopharm. 2015;89:224–231. doi: 10.1016/j.ejpb.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 51.Hynek D, et al. Electrochemical study of doxorubicin interaction with different sequences of single stranded oligonucleotides, Part I. Int. J. Electrochem Sci. 2012;7:13–33. [Google Scholar]
- 52.Fu PK, Turro C. Energy transfer from nucleic acids to Tb (III): Selective emission enhancement by single DNA mismatches. J. Am. Chem. Soc. 1999;121:1–7. doi: 10.1021/ja9826082. [DOI] [Google Scholar]
- 53.Kelley SO, Barton JK, Jackson NM, Hill MG. Electrochemistry of methylene blue bound to a DNA-modified electrode. Bioconjug. Chem. 1997;8:31–37. doi: 10.1021/bc960070o. [DOI] [PubMed] [Google Scholar]
- 54.Hajian R, Shams N, Mohagheghian M. Study on the interaction between doxorubicin and deoxyribonucleic acid with the use of methylene blue as a probe. J. Braz. Chem. Soc. 2009;20:1399–1405. doi: 10.1590/S0103-50532009000800003. [DOI] [Google Scholar]
- 55.Oliver WC, Pharr GM. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992;7:1564–1583. doi: 10.1557/JMR.1992.1564. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.