Abstract
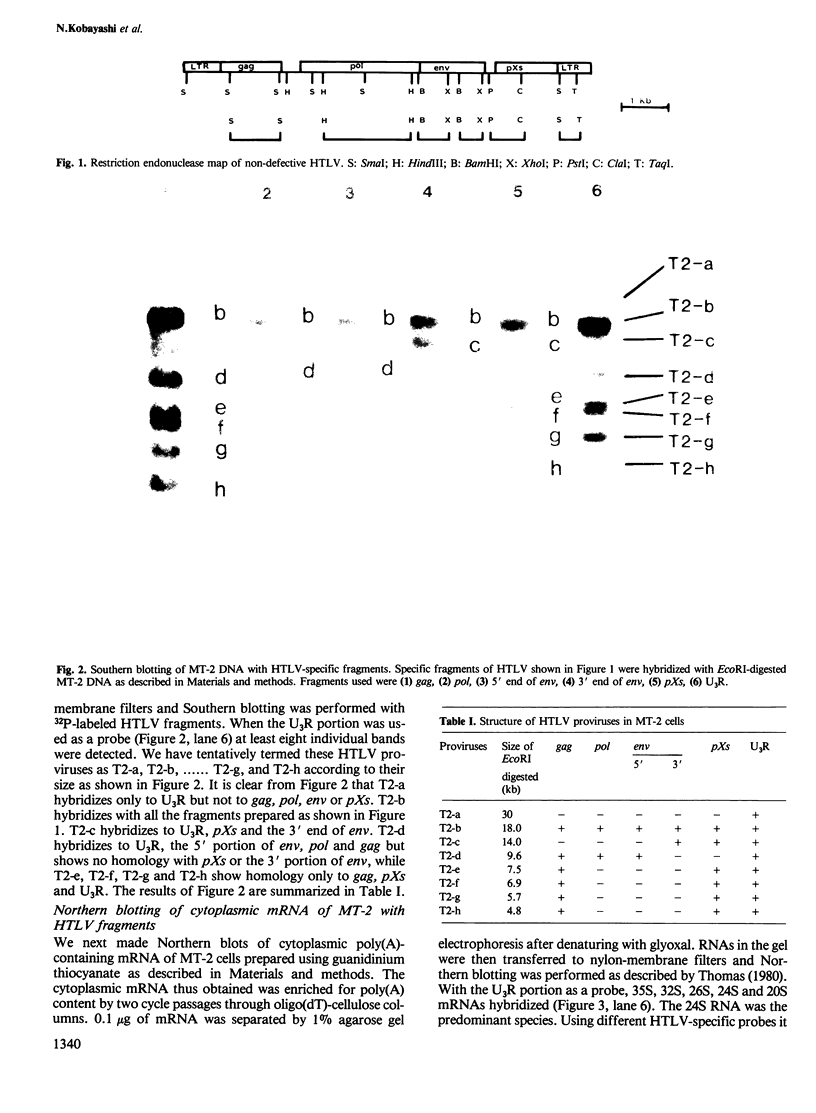
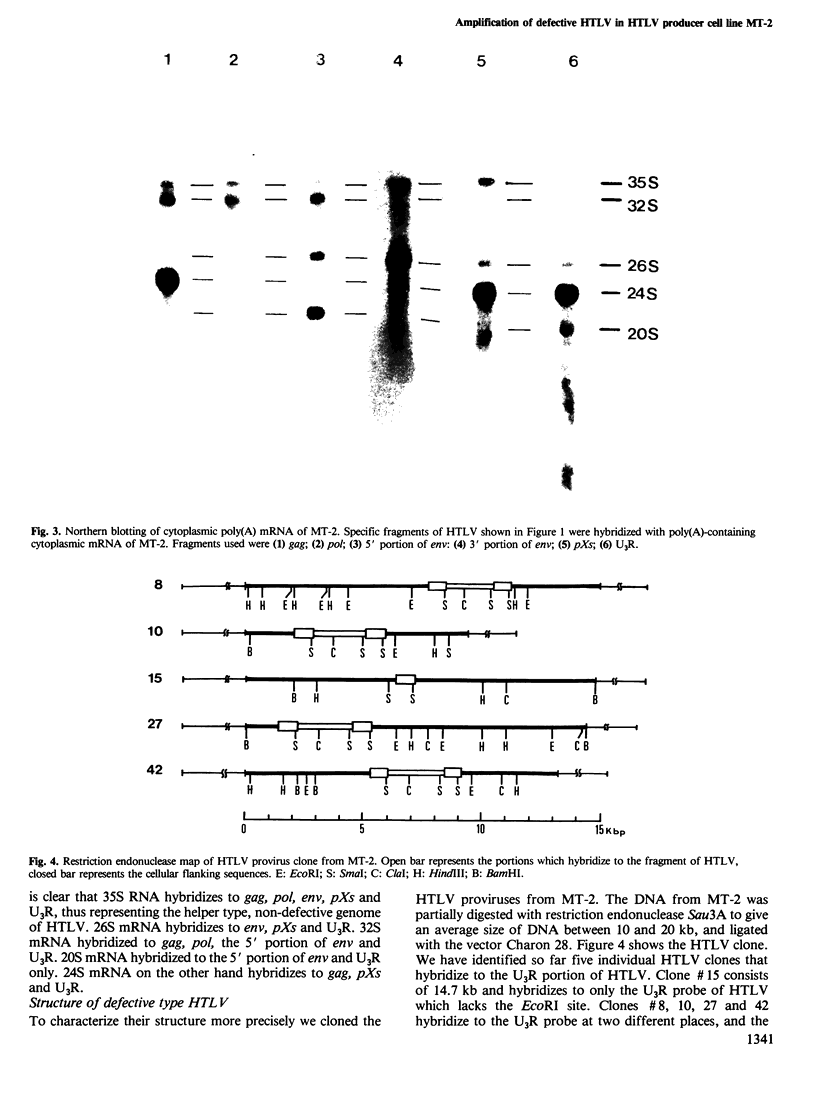
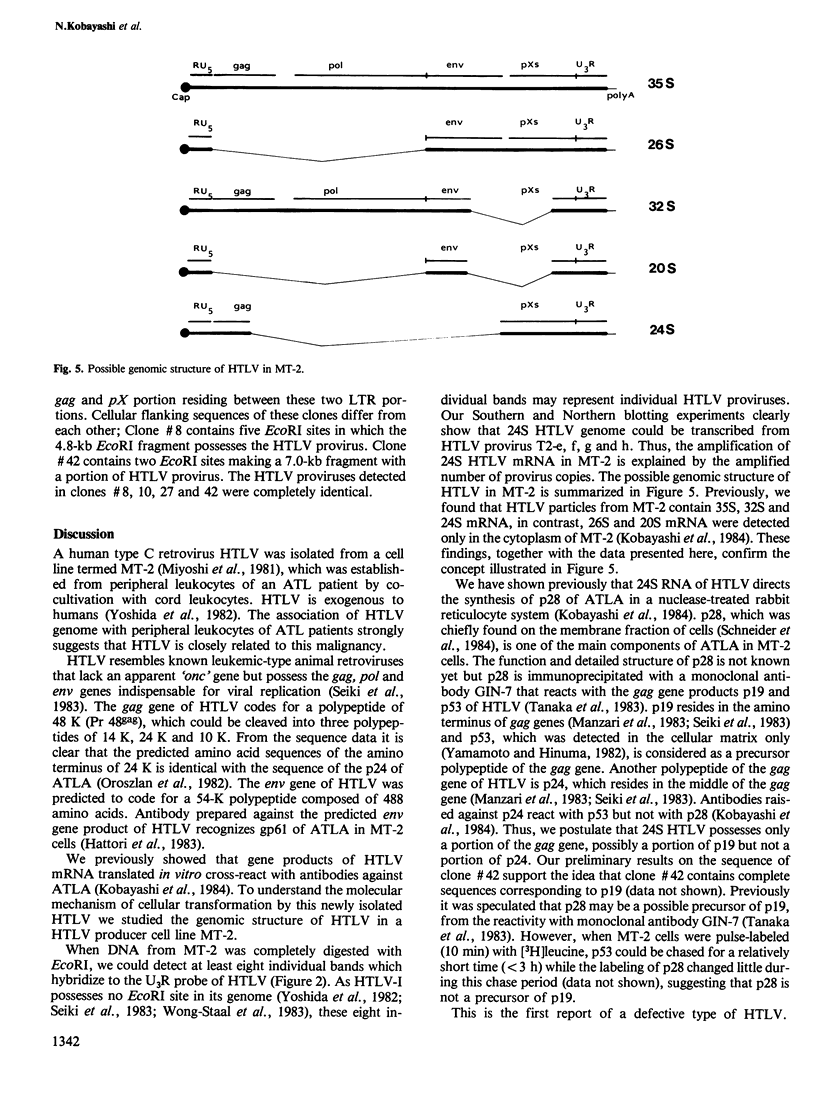
We studied the genomic structure of human T-cell leukemia virus (HTLV) in the HTLV producer cell line MT-2. Southern blotting revealed that at least eight HTLV proviruses were integrated in the chromosomes of MT-2 cells. The genomic structure of these proviruses was analyzed using fragments of cloned HTLV that were specific to gag, pol, env, pXs and U3R genes as probes. We have identified a complete genome of HTLV in MT-2 (non-defective type). However, seven of the eight proviruses had defective genomes. Provirus T2-a contains only the U3R (LTR) of HTLV and T2-b corresponds to the non-defective genome. T2-c possesses only a portion of env, and pXs and U3R. T2-d consists of gag, pol, part of env and U3R. On the other hand, T2-e, f, g and h consist of gag, pXs and U3R. Northern blotting experiments with mRNA from MT-2 cells supported the evidence of amplification of the gag-pXs gene of HTLV. 26S mRNA is considered to be a subgenomic species of 35S RNA. 32S mRNA may represent the T2-d provirus which lacks a portion of env and pXs, while 20S mRNA was a subgenomic species. The gag-pXs gene may correspond to 24S mRNA, the amount which was amplified in MT-2 cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Stephenson J. R. Endogenous type-C RNA viruses of mammalian cells. Biochim Biophys Acta. 1976 Dec 23;458(4):323–354. doi: 10.1016/0304-419x(76)90006-8. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catovsky D., Greaves M. F., Rose M., Galton D. A., Goolden A. W., McCluskey D. R., White J. M., Lampert I., Bourikas G., Ireland R. Adult T-cell lymphoma-leukaemia in Blacks from the West Indies. Lancet. 1982 Mar 20;1(8273):639–643. doi: 10.1016/s0140-6736(82)92200-0. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Hattori S., Imagawa K., Shimizu F., Hashimura E., Seiki M., Yoshida M. Identification of envelope glycoprotein encoded by env gene of human T-cell leukemia virus. Gan. 1983 Dec;74(6):790–793. [PubMed] [Google Scholar]
- Hinuma Y., Komoda H., Chosa T., Kondo T., Kohakura M., Takenaka T., Kikuchi M., Ichimaru M., Yunoki K., Sato I. Antibodies to adult T-cell leukemia-virus-associated antigen (ATLA) in sera from patients with ATL and controls in Japan: a nation-wide sero-epidemiologic study. Int J Cancer. 1982 Jun 15;29(6):631–635. doi: 10.1002/ijc.2910290606. [DOI] [PubMed] [Google Scholar]
- Hinuma Y., Nagata K., Hanaoka M., Nakai M., Matsumoto T., Kinoshita K. I., Shirakawa S., Miyoshi I. Adult T-cell leukemia: antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6476–6480. doi: 10.1073/pnas.78.10.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyanaraman V. S., Sarngadharan M. G., Bunn P. A., Minna J. D., Gallo R. C. Antibodies in human sera reactive against an internal structural protein of human T-cell lymphoma virus. Nature. 1981 Nov 19;294(5838):271–273. doi: 10.1038/294271a0. [DOI] [PubMed] [Google Scholar]
- Kalyanaraman V. S., Sarngadharan M. G., Poiesz B., Ruscetti F. W., Gallo R. C. Immunological properties of a type C retrovirus isolated from cultured human T-lymphoma cells and comparison to other mammalian retroviruses. J Virol. 1981 Jun;38(3):906–915. doi: 10.1128/jvi.38.3.906-915.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi N., Yamamoto N., Koyanagi Y., Schneider J., Hunsmann G., Hatanaka M. Translation of HTLV (human T-cell leukemia virus) RNA in a nuclease-treated rabbit reticulocyte system. EMBO J. 1984 Feb;3(2):321–325. doi: 10.1002/j.1460-2075.1984.tb01804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzari V., Wong-Staal F., Franchini G., Colombini S., Gelmann E. P., Oroszlan S., Staal S., Gallo R. C. Human T-cell leukemia-lymphoma virus (HTLV): cloning of an integrated defective provirus and flanking cellular sequences. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1574–1578. doi: 10.1073/pnas.80.6.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi I., Kubonishi I., Yoshimoto S., Akagi T., Ohtsuki Y., Shiraishi Y., Nagata K., Hinuma Y. Type C virus particles in a cord T-cell line derived by co-cultivating normal human cord leukocytes and human leukaemic T cells. Nature. 1981 Dec 24;294(5843):770–771. doi: 10.1038/294770a0. [DOI] [PubMed] [Google Scholar]
- Oroszlan S., Sarngadharan M. G., Copeland T. D., Kalyanaraman V. S., Gilden R. V., Gallo R. C. Primary structure analysis of the major internal protein p24 of human type C T-cell leukemia virus. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1291–1294. doi: 10.1073/pnas.79.4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poiesz B. J., Ruscetti F. W., Gazdar A. F., Bunn P. A., Minna J. D., Gallo R. C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poiesz B. J., Ruscetti F. W., Reitz M. S., Kalyanaraman V. S., Gallo R. C. Isolation of a new type C retrovirus (HTLV) in primary uncultured cells of a patient with Sézary T-cell leukaemia. Nature. 1981 Nov 19;294(5838):268–271. doi: 10.1038/294268a0. [DOI] [PubMed] [Google Scholar]
- Popovic M., Sarin P. S., Robert-Gurroff M., Kalyanaraman V. S., Mann D., Minowada J., Gallo R. C. Isolation and transmission of human retrovirus (human t-cell leukemia virus). Science. 1983 Feb 18;219(4586):856–859. doi: 10.1126/science.6600519. [DOI] [PubMed] [Google Scholar]
- Posner L. E., Robert-Guroff M., Kalyanaraman V. S., Poiesz B. J., Ruscetti F. W., Fossieck B., Bunn P. A., Jr, Minna J. D., Gallo R. C. Natural antibodies to the human T cell lymphoma virus in patients with cutaneous T cell lymphomas. J Exp Med. 1981 Aug 1;154(2):333–346. doi: 10.1084/jem.154.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitz M. S., Jr, Poiesz B. J., Ruscetti F. W., Gallo R. C. Characterization and distribution of nucleic acid sequences of a novel type C retrovirus isolated from neoplastic human T lymphocytes. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1887–1891. doi: 10.1073/pnas.78.3.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert-Guroff M., Ruscetti F. W., Posner L. E., Poiesz B. J., Gallo R. C. Detection of the human T cell lymphoma virus p19 in cells of some patients with cutaneous T cell lymphoma and leukemia using a monoclonal antibody. J Exp Med. 1981 Dec 1;154(6):1957–1964. doi: 10.1084/jem.154.6.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider J., Yamamoto N., Hinuma Y., Hunsmann G. Sera from adult T-cell leukemia patients react with envelope and core polypeptides of adult T-cell leukemia virus. Virology. 1984 Jan 15;132(1):1–11. doi: 10.1016/0042-6822(84)90086-2. [DOI] [PubMed] [Google Scholar]
- Seiki M., Hattori S., Hirayama Y., Yoshida M. Human adult T-cell leukemia virus: complete nucleotide sequence of the provirus genome integrated in leukemia cell DNA. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3618–3622. doi: 10.1073/pnas.80.12.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y., Koyanagi Y., Chosa T., Yamamoto N., Hinuma Y. Monoclonal antibody reactive with both p28 and p19 of adult T-cell leukemia virus-specific polypeptides. Gan. 1983 Jun;74(3):327–330. [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama T., Yodoi J., Sagawa K., Takatsuki K., Uchino H. Adult T-cell leukemia: clinical and hematologic features of 16 cases. Blood. 1977 Sep;50(3):481–492. [PubMed] [Google Scholar]
- Wong-Staal F., Hahn B., Manzari V., Colombini S., Franchini G., Gelmann E. P., Gallo R. C. A survey of human leukaemias for sequences of a human retrovirus. Nature. 1983 Apr 14;302(5909):626–628. doi: 10.1038/302626a0. [DOI] [PubMed] [Google Scholar]
- Yamamoto N., Hinuma Y. Antigens in an adult T-cell leukemia virus-producer cell line: reactivity with human serum antibodies. Int J Cancer. 1982 Sep 15;30(3):289–293. doi: 10.1002/ijc.2910300306. [DOI] [PubMed] [Google Scholar]
- Yamamoto N., Schneider J., Hinuma Y., Hunsmann G. Adult T-cell leukemia-associated antigen (ATLA): detection of a glycoprotein in cell- and virus-free supernatant. Z Naturforsch C. 1982 Jul-Aug;37(7-8):731–732. doi: 10.1515/znc-1982-7-828. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Miyoshi I., Hinuma Y. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2031–2035. doi: 10.1073/pnas.79.6.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]