Abstract
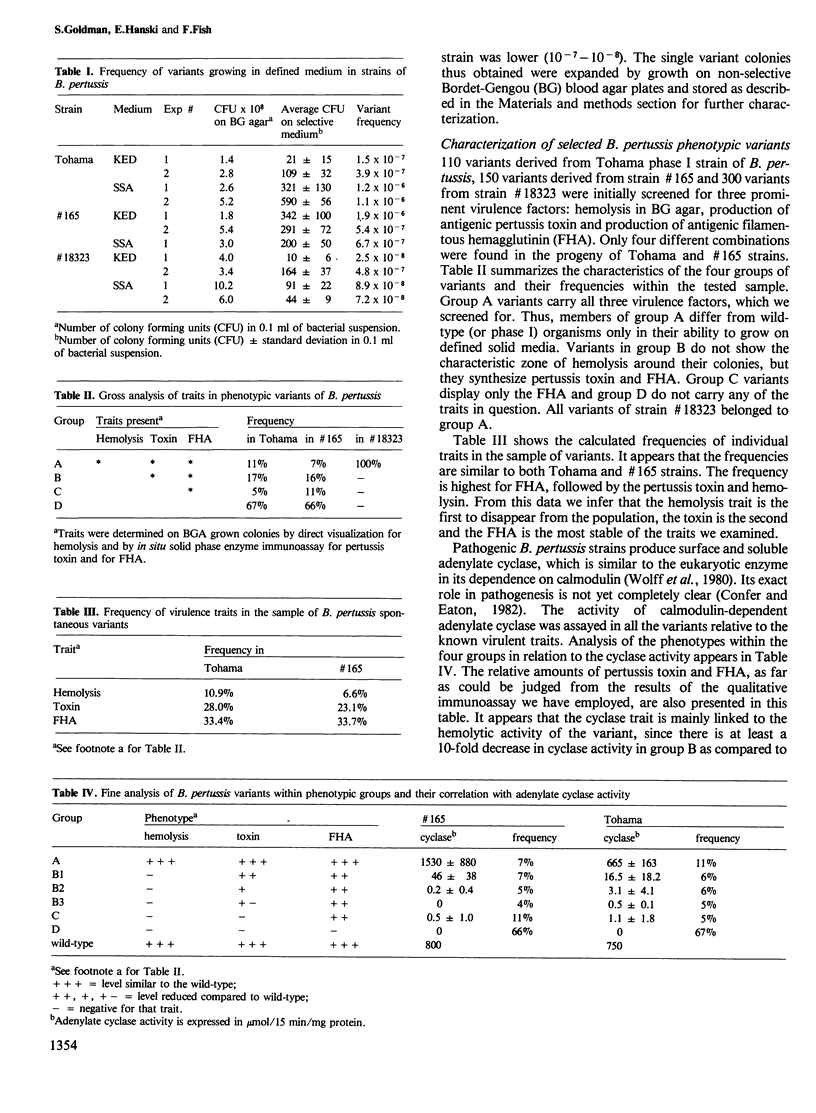
Pathogenic strains of Bordetella pertussis undergo spontaneous phase variation and become non-pathogenic upon culturing in vitro. Spontaneous variants of the Tohama and #165 pathogenic strains of B. pertussis were selected by their ability to grow on synthetic and semi-synthetic solid media. The frequency of these variants was between 10(-6) and 10(-7). About 250 variant strains were screened for the presence of virulence-associated traits, such as production of hemolysin, pertussis toxin and filamentous hemagglutinin (FHA). Only four different combinations of the traits were found: 7-11% of the variants displayed all traits, 17% of the variants carried the toxin and FHA, 5-11% carried FHA only and 66% were devoid of all virulence traits. The strains which had at least one virulence trait also demonstrated some adenylate cyclase activity. The disappearance of hemolysin quantitatively affected the other traits. These results suggest that phase variation in B. pertussis is a non-random process, involving multistep disappearance of virulence factors in the following order: hemolysin, pertussis toxin and FHA. In contrast, all 300 variants of strain #18323 of B. pertussis, which were able to grow on the selective solid media, carried all the virulence traits. This is in accordance with the strain's unique intracerebral growth capability.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buckel P., Zehelein E. Expression of Pseudomonas fluorescens D-galactose dehydrogenase in E. coli. Gene. 1981 Dec;16(1-3):149–159. doi: 10.1016/0378-1119(81)90071-8. [DOI] [PubMed] [Google Scholar]
- Confer D. L., Eaton J. W. Phagocyte impotence caused by an invasive bacterial adenylate cyclase. Science. 1982 Sep 3;217(4563):948–950. doi: 10.1126/science.6287574. [DOI] [PubMed] [Google Scholar]
- Elwell L. P., Shipley P. L. Plasmid-mediated factors associated with virulence of bacteria to animals. Annu Rev Microbiol. 1980;34:465–496. doi: 10.1146/annurev.mi.34.100180.002341. [DOI] [PubMed] [Google Scholar]
- Guinée P. A., Jansen W. H., Agterberg C. M. Detection of the K99 antigen by means of agglutination and immunoelectrophoresis in Escherichia coli isolates from calves and its correlation with entertoxigenicity. Infect Immun. 1976 May;13(5):1369–1377. doi: 10.1128/iai.13.5.1369-1377.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KASUGA T., NAKASE Y., UKISHIMA K., TAKATSU K. Studies on Haemophilis pertussis. III. Some properties of each phase of H. pertussis. Kitasato Arch Exp Med. 1954 Sep;27(3):37–47. [PubMed] [Google Scholar]
- Kleckner N. Translocatable elements in procaryotes. Cell. 1977 May;11(1):11–23. doi: 10.1016/0092-8674(77)90313-0. [DOI] [PubMed] [Google Scholar]
- LACEY B. W. Antigenic modulation of Bordetella pertussis. J Hyg (Lond) 1960 Mar;58:57–93. doi: 10.1017/s0022172400038134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McPheat W. L., Wardlaw A. C., Novotny P. Modulation of Bordetella pertussis by nicotinic acid. Infect Immun. 1983 Aug;41(2):516–522. doi: 10.1128/iai.41.2.516-522.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakane P. K., Kawaoi A. Peroxidase-labeled antibody. A new method of conjugation. J Histochem Cytochem. 1974 Dec;22(12):1084–1091. doi: 10.1177/22.12.1084. [DOI] [PubMed] [Google Scholar]
- Peppler M. S. Isolation and characterization of isogenic pairs of domed hemolytic and flat nonhemolytic colony types of Bordetella pertussis. Infect Immun. 1982 Mar;35(3):840–851. doi: 10.1128/iai.35.3.840-851.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STANDFAST A. F. B. The phase I of Haemophilus pertussis. J Gen Microbiol. 1951 Aug;5(3):531–545. doi: 10.1099/00221287-5-3-531. [DOI] [PubMed] [Google Scholar]
- STANDFAST A. F. Some factors influencing the virulence for mice of Bordetella pertussis by the intracerebral route. Immunology. 1958 Apr;1(2):123–134. [PMC free article] [PubMed] [Google Scholar]
- Salomon Y. Adenylate cyclase assay. Adv Cyclic Nucleotide Res. 1979;10:35–55. [PubMed] [Google Scholar]
- Salonen E. M., Vaheri A. Rapid solid-phase enzyme immunoassay for antibodies to viruses and other microbes: effects of polyethylene glycol. J Immunol Methods. 1981;41(1):95–103. doi: 10.1016/0022-1759(81)90277-5. [DOI] [PubMed] [Google Scholar]
- Sato Y., Izumiya K., Sato H., Cowell J. L., Manclark C. R. Aerosol infection of mice with Bordetella pertussis. Infect Immun. 1980 Jul;29(1):261–266. doi: 10.1128/iai.29.1.261-266.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M., Zieg J., Silverman M., Mandel G., Doolittle R. Phase variation: evolution of a controlling element. Science. 1980 Sep 19;209(4463):1370–1374. doi: 10.1126/science.6251543. [DOI] [PubMed] [Google Scholar]
- Stainer D. W., Scholte M. J. A simple chemically defined medium for the production of phase I Bordetella pertussis. J Gen Microbiol. 1970 Oct;63(2):211–220. doi: 10.1099/00221287-63-2-211. [DOI] [PubMed] [Google Scholar]
- Weiss A. A., Falkow S. Plasmid transfer to Bordetella pertussis: conjugation and transformation. J Bacteriol. 1982 Oct;152(1):549–552. doi: 10.1128/jb.152.1.549-552.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A. A., Falkow S. Transposon insertion and subsequent donor formation promoted by Tn501 in Bordetella pertussis. J Bacteriol. 1983 Jan;153(1):304–309. doi: 10.1128/jb.153.1.304-309.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A. A., Hewlett E. L., Myers G. A., Falkow S. Tn5-induced mutations affecting virulence factors of Bordetella pertussis. Infect Immun. 1983 Oct;42(1):33–41. doi: 10.1128/iai.42.1.33-41.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff J., Cook G. H., Goldhammer A. R., Berkowitz S. A. Calmodulin activates prokaryotic adenylate cyclase. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3841–3844. doi: 10.1073/pnas.77.7.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]


