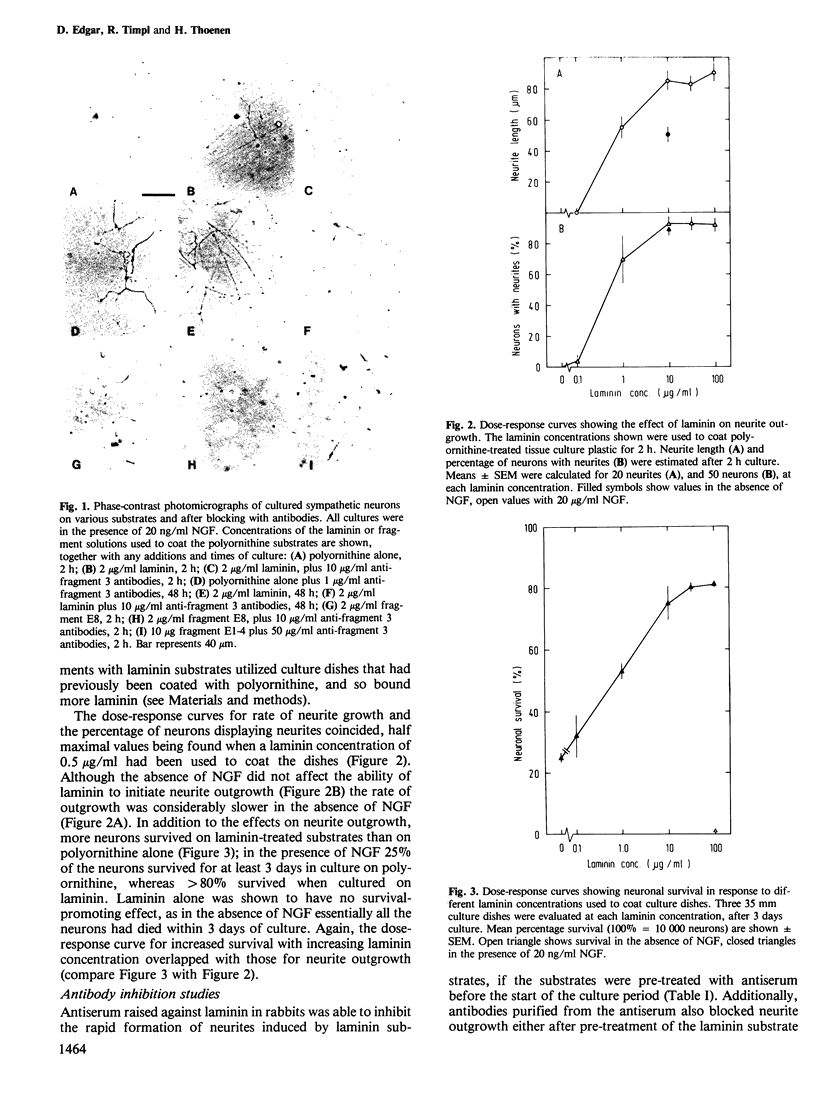
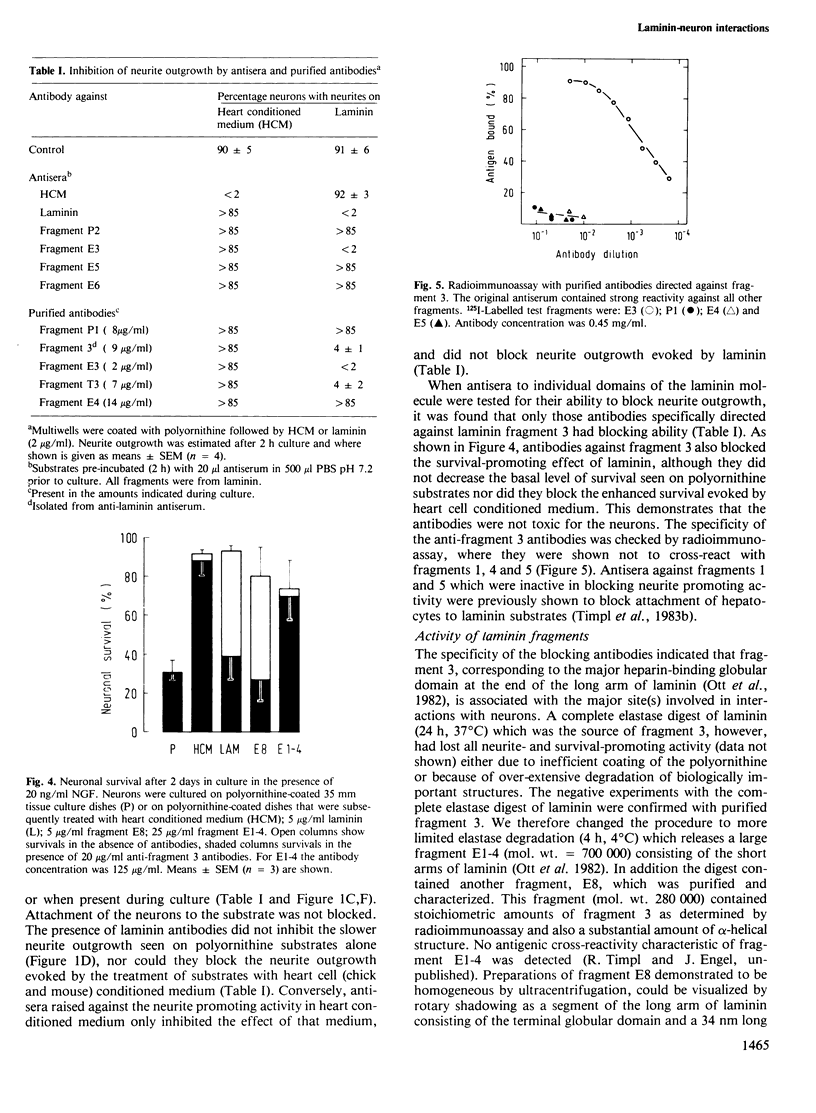
Abstract
The survival of cultured chick sympathetic neurons and the outgrowth of neurites were stimulated by the basement membrane protein laminin coated onto polyornithine culture substrates. The survival-potentiating activity was dependent on the presence of nerve growth factor. Both effects of laminin could be completely inhibited by affinity-purified antibodies against laminin fragment 3, the product of a limited proteolysis that corresponds to the heparin-binding globular domain at the end of the long arm of the laminin molecule. Antibodies against other laminin fragments were inactive, including those against previously determined cell-binding domains. A large laminin fragment, E8, was produced by brief elastase digestion and shown to consist of fragment 3 and an adjacent rod-like structure. Although lacking the cell binding domains, fragment E8 potentiated both neuronal survival and neurite outgrowth, and these effects could be blocked by antibodies against fragment 3. Weak survival and neurite potentiating activity was also detected in another fragment corresponding to the short arms of laminin, but as these effects were not inhibited by any of the antibodies tested they probably arose de novo during proteolysis. The heparin-binding domain of laminin is therefore responsible for its effects on neurons.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barde Y. A., Edgar D., Thoenen H. New neurotrophic factors. Annu Rev Physiol. 1983;45:601–612. doi: 10.1146/annurev.ph.45.030183.003125. [DOI] [PubMed] [Google Scholar]
- Barde Y. A., Edgar D., Thoenen H. Sensory neurons in culture: changing requirements for survival factors during embryonic development. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1199–1203. doi: 10.1073/pnas.77.2.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Van Evercooren A., Kleinman H. K., Ohno S., Marangos P., Schwartz J. P., Dubois-Dalcq M. E. Nerve growth factor, laminin, and fibronectin promote neurite growth in human fetal sensory ganglia cultures. J Neurosci Res. 1982;8(2-3):179–193. doi: 10.1002/jnr.490080208. [DOI] [PubMed] [Google Scholar]
- Carbonetto S., Gruver M. M., Turner D. C. Nerve fiber growth in culture on fibronectin, collagen, and glycosaminoglycan substrates. J Neurosci. 1983 Nov;3(11):2324–2335. doi: 10.1523/JNEUROSCI.03-11-02324.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. Induction of neurite outgrowth by a conditioned-medium factor bound to the culture substratum. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5210–5213. doi: 10.1073/pnas.75.10.5210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornbrooks C. J., Carey D. J., McDonald J. A., Timpl R., Bunge R. P. In vivo and in vitro observations on laminin production by Schwann cells. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3850–3854. doi: 10.1073/pnas.80.12.3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar D., Barde Y. A., Thoenen H. Subpopulations of cultured chick sympathetic neurones differ in their requirements for survival factors. Nature. 1981 Jan 22;289(5795):294–295. doi: 10.1038/289294a0. [DOI] [PubMed] [Google Scholar]
- Edgar D., Thoenen H. Modulation of NGF-induced survival of chick sympathetic neurons by contact with a conditioned medium factor bound to the culture substrate. Brain Res. 1982 Sep;281(1):89–92. doi: 10.1016/0165-3806(82)90115-8. [DOI] [PubMed] [Google Scholar]
- Engel J., Odermatt E., Engel A., Madri J. A., Furthmayr H., Rohde H., Timpl R. Shapes, domain organizations and flexibility of laminin and fibronectin, two multifunctional proteins of the extracellular matrix. J Mol Biol. 1981 Jul 25;150(1):97–120. doi: 10.1016/0022-2836(81)90326-0. [DOI] [PubMed] [Google Scholar]
- Greif K. F., Reichardt L. F. Appearance and distribution of neuronal cell surface and synaptic vesicle antigens in the developing rat superior cervical ganglion. J Neurosci. 1982 Jul;2(7):843–852. doi: 10.1523/JNEUROSCI.02-07-00843.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawrot E. Cultured sympathetic neurons: effects of cell-derived and synthetic substrata on survival and development. Dev Biol. 1980 Jan;74(1):136–151. doi: 10.1016/0012-1606(80)90057-3. [DOI] [PubMed] [Google Scholar]
- Helfand S. L., Smith G. A., Wessells N. K. Survival and development in culture of dissociated parasympathetic neurons from ciliary ganglia. Dev Biol. 1976 Jun;50(2):541–547. doi: 10.1016/0012-1606(76)90174-3. [DOI] [PubMed] [Google Scholar]
- Kjellén L., Oldberg A., Hök M. Cell-surface heparan sulfate. Mechanisms of proteoglycan-cell association. J Biol Chem. 1980 Nov 10;255(21):10407–10413. [PubMed] [Google Scholar]
- Kligman D. Isolation of a protein from bovine brain which promotes neurite extension from chick embryo cerebral cortex neurons in defined medium. Brain Res. 1982 Oct 28;250(1):93–100. doi: 10.1016/0006-8993(82)90955-6. [DOI] [PubMed] [Google Scholar]
- Lander A. D., Fujii D. K., Gospodarowicz D., Reichardt L. F. Characterization of a factor that promotes neurite outgrowth: evidence linking activity to a heparan sulfate proteoglycan. J Cell Biol. 1982 Sep;94(3):574–585. doi: 10.1083/jcb.94.3.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laterra J., Silbert J. E., Culp L. A. Cell surface heparan sulfate mediates some adhesive responses to glycosaminoglycan-binding matrices, including fibronectin. J Cell Biol. 1983 Jan;96(1):112–123. doi: 10.1083/jcb.96.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesot H., Kühl U., Mark K. Isolation of a laminin-binding protein from muscle cell membranes. EMBO J. 1983;2(6):861–865. doi: 10.1002/j.1460-2075.1983.tb01514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinoff H. L., Wicha M. S. Isolation of a cell surface receptor protein for laminin from murine fibrosarcoma cells. J Cell Biol. 1983 May;96(5):1475–1479. doi: 10.1083/jcb.96.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manthorpe M., Engvall E., Ruoslahti E., Longo F. M., Davis G. E., Varon S. Laminin promotes neuritic regeneration from cultured peripheral and central neurons. J Cell Biol. 1983 Dec;97(6):1882–1890. doi: 10.1083/jcb.97.6.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manthorpe M., Varon S., Adler R. Neurite-promoting factor in conditioned medium from RN22 Schwannoma cultures: bioassay, fractionation, and properties. J Neurochem. 1981 Sep;37(3):759–767. doi: 10.1111/j.1471-4159.1982.tb12552.x. [DOI] [PubMed] [Google Scholar]
- Morris J. E., Ting Y. P. Comparison of proteoglycans extracted by saline and guanidinium chloride from cultured chick retinas. J Neurochem. 1981 Dec;37(6):1594–1602. doi: 10.1111/j.1471-4159.1981.tb06332.x. [DOI] [PubMed] [Google Scholar]
- Ott U., Odermatt E., Engel J., Furthmayr H., Timpl R. Protease resistance and conformation of laminin. Eur J Biochem. 1982 Mar;123(1):63–72. doi: 10.1111/j.1432-1033.1982.tb06499.x. [DOI] [PubMed] [Google Scholar]
- Palm S. L., Furcht L. T. Production of laminin and fibronectin by Schwannoma cells: cell-protein interactions in vitro and protein localization in peripheral nerve in vivo. J Cell Biol. 1983 May;96(5):1218–1226. doi: 10.1083/jcb.96.5.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins M. E., Ji T. H., Hynes R. O. Cross-linking of fibronectin to sulfated proteoglycans at the cell surface. Cell. 1979 Apr;16(4):941–952. doi: 10.1016/0092-8674(79)90109-0. [DOI] [PubMed] [Google Scholar]
- Rao C. N., Margulies I. M., Tralka T. S., Terranova V. P., Madri J. A., Liotta L. A. Isolation of a subunit of laminin and its role in molecular structure and tumor cell attachment. J Biol Chem. 1982 Aug 25;257(16):9740–9744. [PubMed] [Google Scholar]
- Rao N. C., Barsky S. H., Terranova V. P., Liotta L. A. Isolation of a tumor cell laminin receptor. Biochem Biophys Res Commun. 1983 Mar 29;111(3):804–808. doi: 10.1016/0006-291x(83)91370-0. [DOI] [PubMed] [Google Scholar]
- Rogers S. L., Letourneau P. C., Palm S. L., McCarthy J., Furcht L. T. Neurite extension by peripheral and central nervous system neurons in response to substratum-bound fibronectin and laminin. Dev Biol. 1983 Jul;98(1):212–220. doi: 10.1016/0012-1606(83)90350-0. [DOI] [PubMed] [Google Scholar]
- Rohde H., Wick G., Timpl R. Immunochemical characterization of the basement membrane glycoprotein laminin. Eur J Biochem. 1979 Dec;102(1):195–201. doi: 10.1111/j.1432-1033.1979.tb06280.x. [DOI] [PubMed] [Google Scholar]
- Schubert D., LaCorbiere M. A role of secreted glycosaminoglycans in cell-substratum adhesion. J Biol Chem. 1980 Dec 10;255(23):11564–11569. [PubMed] [Google Scholar]
- Suda K., Barde Y. A., Thoenen H. Nerve growth factor in mouse and rat serum: correlation between bioassay and radioimmunoassay determinations. Proc Natl Acad Sci U S A. 1978 Aug;75(8):4042–4046. doi: 10.1073/pnas.75.8.4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terranova V. P., Rao C. N., Kalebic T., Margulies I. M., Liotta L. A. Laminin receptor on human breast carcinoma cells. Proc Natl Acad Sci U S A. 1983 Jan;80(2):444–448. doi: 10.1073/pnas.80.2.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terranova V. P., Rohrbach D. H., Martin G. R. Role of laminin in the attachment of PAM 212 (epithelial) cells to basement membrane collagen. Cell. 1980 Dec;22(3):719–726. doi: 10.1016/0092-8674(80)90548-6. [DOI] [PubMed] [Google Scholar]
- Timpl R. Antibodies to collagens and procollagens. Methods Enzymol. 1982;82(Pt A):472–498. doi: 10.1016/0076-6879(82)82079-x. [DOI] [PubMed] [Google Scholar]
- Timpl R., Johansson S., van Delden V., Oberbäumer I., Hök M. Characterization of protease-resistant fragments of laminin mediating attachment and spreading of rat hepatocytes. J Biol Chem. 1983 Jul 25;258(14):8922–8927. [PubMed] [Google Scholar]
- Timpl R., Rohde H., Robey P. G., Rennard S. I., Foidart J. M., Martin G. R. Laminin--a glycoprotein from basement membranes. J Biol Chem. 1979 Oct 10;254(19):9933–9937. [PubMed] [Google Scholar]
- Yamada K. M. Cell surface interactions with extracellular materials. Annu Rev Biochem. 1983;52:761–799. doi: 10.1146/annurev.bi.52.070183.003553. [DOI] [PubMed] [Google Scholar]