Abstract
Essentials.
Tissue factor (TF) represents a central link between hemostasis and inflammation.
We studied the roles of myeloid and airway epithelial TF in acid‐caused acute lung injury (ALI).
TF on myeloid cells displays a non‐coagulatory role regulating the inflammatory response in ALI.
Airway epithelial TF contributes to hemostatic functions, but is dispensable in ALI pathogenesis.
Summary
Introduction
Acute lung injury (ALI) is a life‐threatening condition characterized by damaged alveolar–capillary structures and activation of inflammatory and hemostatic processes. Tissue factor (TF) represents a crucial link between inflammation and coagulation, as inflammatory mediators induce myeloid TF expression, and TF initiates extrinsic coagulation.
Objective
As pulmonary inflammation stimulates TF expression and TF modulates immune responses, we aimed to elucidate its impact on ALI. In particular, we wanted to distinguish the contributions of TF expressed on airway epithelial cells and TF expressed on myeloid cells.
Methods
Mice with different cell type‐specific TF deficiency and wild‐type littermates were intratracheally treated with hydrochloric acid, and leukocyte recruitment, cytokine levels, thrombin–antithrombin (TAT) complexes and pulmonary protein‐rich infiltrates were analyzed.
Results
Our data demonstrate that a lack of epithelial TF did not influence acute responses, as bronchoalveolar neutrophil accumulation 8 h after ALI induction was unaltered. However, it led to mild, prolonged inflammation, as pulmonary leukocyte and erythrocyte numbers were still increased after 24 h, whereas those in wild‐type mice had returned to basal levels. In contrast, myeloid TF was primarily involved in regulating the acute phase of ALI without affecting local coagulation, as indicated by increased bronchoalveolar neutrophil infiltration, pulmonary interleukin‐6 levels, and edema formation, but equal TAT complex formation, 8 h after ALI induction. This augmented inflammatory response associated with myeloid TF deficiency was confirmed in vitro, as lipopolysaccharide‐stimulated TF‐deficient alveolar macrophages released increased levels of chemokine (C‐X‐C motif) ligand 1 and tumor necrosis factor‐α as compared with wild‐type macrophages.
Conclusion
We conclude that myeloid TF dampens inflammation in acid‐induced ALI.
Keywords: acute lung injury, blood coagulation, inflammation, macrophages, tissue factor
Introduction
Tissue factor (TF), also referred to as factor III, thromboplastin, or CD142, is a glycosylated integral transmembrane protein that initiates the extrinsic coagulation cascade. TF is constitutively expressed on the surface by various extravascular tissues, such as pulmonary epithelium. Upon injury, e.g. to the alveolar–endothelial barrier, extravascular TF is exposed to plasmatic coagulation factors, e.g. FVII, and initiates clot formation. The generated thrombin triggers fibrin formation, which stabilizes the primary platelet plug. Hence, TF is important for protecting vascular integrity, and thus preventing uncontrolled and life‐threatening blood loss 1, 2, 3. Upon infection or inflammation, TF expression is readily induced on leukocytes that are usually devoid of surface TF. These TF‐expressing leukocytes comprise members of the first line of defense, such as macrophages, but also circulating monocytes or neutrophils. Furthermore, blood vessel‐lining endothelial cells are able to synthesize TF 4, 5, 6. Stimuli triggering bloodborne TF expression during infection include pathogen‐associated molecular patterns, e.g. lipopolysaccharide (LPS) or lipoteichoic acid, and endogenous inflammatory cytokines, e.g. tumor necrosis factor (TNF)‐α and interleukin (IL)‐1β 7, 8. This process involves several transcription factors that are essential for immune functions, such as nuclear factor‐κB (NF‐κB), AP‐1, nuclear factor of activated T cells, and early growth response‐1 9, 10, 11, 12. Hence, the properties of TF are not strictly confined to hemostatic functions, but also promote host defense and inflammation 13. Therefore, reduced TF activity in mice leads to more severe bacteremia and increased mortality 14. However, uncontrolled coagulation cascade activation markedly contributes to disseminated intravascular coagulation. Correspondingly, reduced TF expression protects mice from systemic endotoxemia 15, indicating that finely balanced TF expression is essential for adequate immune responses during sepsis. Furthermore, TF inhibition prevents secondary lung injury in a baboon sepsis model 16. However, it remains incompletely understood how TF contributes to local infection and inflammation in organs such as the lung.
Acute lung injury (ALI) represents a multifactorial disease that can be induced by aspiration of non‐infectious acidic gastric contents as well as by pathogenic lung infections. The role of TFs in different pathologies of lung injury are still undefined. A recent study investigating low‐TF mice subjected to LPS‐induced ALI suggested that reduced TF expression exacerbates disease pathogenesis 17. Cell type‐specific TF deficiency in type I and type II lung epithelial cells worsens LPS‐induced ALI by increasing alveolar permeability and hemorrhage. In contrast, mice devoid of myeloid TF show no overt differences in the severity of lung damage 18. In the present study, we focused on cell type‐specific roles of TF in acid‐induced ALI. This clinically relevant mouse model mimics aspiration of gastric contents leading to lung complications (particularly in patients admitted to intensive care units), which accounts for 11% of all ALI cases in clinics 19, and is characterized by the release of endogenous alarm signals, damage‐associated molecular patterns (DAMPs), and simultaneous Toll‐like receptor activation 20, 21. The resulting inflammation is associated with endothelial cell activation, leukocyte and platelet influx, activation of coagulation, fibrin deposition, and, eventually, damage to the alveolar–capillary barrier 22. Notably, activation of the coagulation cascade plays a relevant role in disease progression 23.
As coagulatory and inflammatory processes affect the pathogenesis of ALI, we wanted to elucidate the impact of TF on these events during ALI. Thus, we endeavored to elucidate whether the presence of TF is required to protect the integrity of alveolar–capillary structures. Moreover, we aimed to define cell type‐specific functions of TF, and therefore performed acid‐induced ALI in mice with TF deficiency in either myeloid cells (monocytes, macrophages, and granulocytes) or alveolar type I and type II epithelial cells. Our data indicate that TF acts in a cell type‐specific manner, and that myeloid TF, in contrast to airway epithelial TF, dampens inflammatory responses during acid‐induced ALI.
Materials and methods
Mice
All experiments and animal studies were conducted according to institutional guidelines and were approved by the Animal Care and Use Committee of the Medical University of Vienna (BMWF‐66.009/0320‐II/3b/2013; BMWF‐66.009/0254‐ll/3b/2013).
All animal experimentation was littermate‐controlled. We used previously described LysM cre+/−TFflox/flox (referred to as TF∆mye) 24, 25 or SPC cre+/−TFflox/flox (referred to as TF∆epi) 18 mice on a C57BL/6J genetic background (backcrossed for six generations), and 8–12‐week‐old, sex‐matched littermates (control LysM cre−/−TFflox/flox and SpcCre−/−TFflox/flox, referred as TF+/+ or wild‐type).
Direct PCR of lysed tissue was performed for genotyping. (Primers: TF forward, 5′‐ATGAGGAGCTGTGTTAAAGGGTCGCAGA‐3′; TF reverse, 5′‐TGCAGTAAATGCACGTGTCTGCCAT‐3′; Cre forward, 5′‐TCGCGATTATCTTCTATATCTTCAG‐3′; Cre reverse, 5′‐GCTCGACCAGTTTAGTTACCC‐3′.)
Anesthesia
Mice were anesthetized with Ketaminol (50 mg kg−1) (Intervet International, Unterschleißheim, Germany) and Xylasol (5 mg kg−1) (Dr E. Gräub, Bern, Switzerland) or Forane (isoflurane; Abbott Laboratories, Queenborough, UK).
ALI and administration of anti‐TF antibody
Sedated mice were intratracheally treated with 50 μL of 0.1 m hydrochloric acid (HCl) to induce ALI.
Wild‐type mice (C57BL/6J genetic background) were intraperitoneally injected once with monoclonal inhibitory rat anti‐mouse TF (1H1) (20 μg g−1) prior to ALI induction.
Bronchoalveolar lavage fluid (BALF) and alveolar macrophage isolation and stimulation
For quantification of cytokines and leukocytes in the bronchoalveolar space, the trachea was exposed and punctured with a vein catheter, and the bronchoalveolar space was lavaged with 1 mL of phosphate‐buffered saline (PBS). Cells were pelleted for flow cytometry (600 × g, 10 min), and supernatants were used for ELISA.
Alveolar macrophages were isolated by repeating lavaging (10 times), and seeded into non‐tissue culture‐treated plates (2 h, 37 °C, and 5% CO2; RPMI medium (Sigma‐Aldrich, Vienna, Austria) dosed with 10% fetal bovine serum, 1% penicillin, streptomycin, and fungizone, and 1% l‐glutamine) before non‐adherent lymphocytes were discarded.
Alveolar macrophages were stimulated with 10 ng mL−1 or 100 ng mL−1 LPS (LPS‐EB ultrapure from Escherichia coli O111:B4; Invivogen, Toulouse, France) for the indicated times, and supernatant or cells with peqGOLD TriFast (VWR, Darmstadt, Germany) were harvested.
Bone marrow‐derived macrophage (BMDM) isolation
Murine bone marrow was isolated and incubated with granulocyte–macrophage colony‐stimulating factor (GM‐CSF) (10 ng mL−1) for 1 week to generate BMDMs, which were then stimulated with LPS (10 ng mL−1) for the indicated times.
Lung homogenization for ELISA and western blotting
Lungs were homogenized in 4 μL of PBS mg–1 with 5‐mm beads in a Precellys24 homogenizer (Bertin Technologies, Aix‐en‐Provence, France). Supernatants were mixed with Greenberger Lysis buffer and protease inhibitor (Roche Life Sciences, Basel, Switzerland), incubated (20 min on ice), and centrifuged (135 × g, 15 min, 4 °C), and the supernatant was used for ELISA.
For immunoblotting, lungs were homogenized in 10 μL of RIPA buffer mg–1, and supernatants were mixed with Laemmli buffer.
RNA isolation and quantitative PCR (qPCR)
RNA was isolated from TriFast‐homogenized lungs or macrophages, transcribed into cDNA (High‐Capacity cDNA Reverse transcription Kit; Applied Biosystems, Carlsbad, CA, USA), and analyzed by qPCR with Fast SYBR Green Master Mix (Applied Biosystems, Carlsbad, CA, USA) on a StepOne Real‐Time PCR System (Applied Biosystems, Foster City, CA, USA). The results were evaluated according to Pfaffl 26 and expressed as fold control values. Primer sequences are supplied in Data S1.
Western blotting
Proteins were separated by SDS‐PAGE (10% acrylamide gels), and semi‐dry‐blotted onto a poly(vinylidene difluoride) membrane. The fibrin β‐monomer 59D8 and anti‐β‐actin (Sigma‐Aldrich) and horseradish peroxidase‐linked secondary antibodies were used. Immunoblots were developed with SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Fisher Scientific, Waltham, MA, USA), imaged with FluorChem HD2 Chemiluminesence Imager (Alpha Innotech, San Leandro, CA, USA), and densitometrically analyzed with imagej 1.47v. Bands were normalized to β‐actin.
Flow cytometry
BALF cells were incubated (20 min) with anti‐CD45–PerCP, anti‐F4/80–fluorescein isothiocyanate, anti‐Ly6G/Ly6C–phycoerythrin, and anti‐Ter119–allophycocyanin (1 : 80; BioLegend, London, UK), fixed with 1% formaldehyde, and measured by flow cytometry (BD Accuri flow cytometer with BD accuric6, and flowjo 9 [Treestar, Ashland, OR, USA]). The following gating strategy was applied. CD45+ cells (leukocytes) were analyzed for F4/80 positivity (macrophages). Ly6C+ macrophages were termed inflammatory macrophages. CD45+ F4/80− cells were analyzed for Ly6G expression (neutrophils).
Total cell counts of BALF were calculated by multiplying the events by
Histologic analysis
The left lung was extracted, fixed in 4% formaldehyde, and embedded in paraffin. Three‐micrometer lung sections were stained with anti‐mouse GR‐1 or TF antibody, and counterstained with hematoxylin or with hematoxylin and eosin (Carl Roth, Karlsruhe, Germany). Images were obtained with an Axio Imager.Z1 microscope (Carl Zeiss, Vienna, Austria).
TF activity assay
TF activity was determined as previously described 27. Briefly, BALF was diluted 1 : 6 with Hanks’ balanced salt solution with 0.1% bovine serum albumin (HBSA), and microvesicles were pelleted (18 000 × g, 20 min, 4 °C), washed, and incubated with anti‐mouse TF (1H1) (100 μg mL−1) or control IgG (15 min) before addition of FVII (4.88 nm), FX (150 nm), and CaCl2 (10 mm). The reaction was stopped by addition of EDTA HBSA buffer before addition of chromogenic substrate and absorbance measurement. TF‐dependent activated FX (FXa) generation was determined by subtracting FXa levels generated in the presence of TF antibody from the levels in IgG control samples.
ELISA
ELISAs were performed according to manufacturer's protocols. Myeloperoxidase (MPO), IL‐33 and IL‐6, and chemokine (C‐X‐C‐motif) ligand‐1 (KC), TNF‐α and thrombin–antithrombin (TAT) ELISAs were purchased from eBioscience (San Diego, USA), R&D Systems (Minneapolis, MN, USA) and Siemens Healthcare Diagnostics (Vienna, Austria), respectively. Absorbance was measured at 450 nm with an EL808 Ultra Microplate Reader (Bio‐Tek Instruments, Winooski, VT, USA).
Bicinchoninic acid (BCA) assay
The protein concentration in BALF was determined with a Pierce BCA Protein Assay Kit (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer's guidelines.
Edema formation
Murine lungs were extracted, weighed, dried at 55 °C for 48 h, and weighed again. The ratio between wet weight and dry weight was calculated.
Statistical analysis
Data were depicted as bar diagrams showing the mean ± standard error of the mean, as box plots indicating the median, the first and third quartiles, and the minimum and maximum, or as scatter plots with indicated means. The results were evaluated with graphpad prism 5 (GraphPad, San Diego, CA, USA) by one‐way‐anova with Dunn's multiple comparisons test, two‐way anova with Sidak's multiple comparisons test, unpaired, two‐tailed t‐tests, or the Mann–Whitney test, if the samples did not pass the Kolmogorov–Smirnov test for normal distribution. A P‐value of < 0.05 was considered to be significant.
Results
TF expression during acid‐induced ALI
Activation of coagulation is an integral step in the pathology of ALI. We established a mouse model of acid‐induced ALI by intratracheal administration of HCl. Lung TF mRNA expression was increased within the first 8 h of ALI, which represents the most critical time in the pathology (Fig. 1A), whereas we could not detect simultaneous accumulation of fibrin β‐monomers in lungs (Fig. 1B–D). This indicates rather modest activation of coagulation during acid‐induced ALI. To investigate the role of TF in acid‐induced ALI, we used mice with a cell type‐specific TF deficiency (Fig. S1) either in myeloid cells (TFΔmye) or in lung epithelial cells (TFΔepi) in littermate‐controlled experiments. We found a significant increase in TF mRNA expression in TFΔmye mice during ALI, which was significantly more pronounced than in wild‐type mice (Fig. 1E). This indicates that non‐myeloid cells upregulate TF during ALI. As fibrin generation was only moderately affected during ALI, we used thrombin, the protease‐cleaving fibrinogen, as a functional readout for coagulation activation. Formed thrombin becomes rapidly inactivated via binding of antithrombin III, leading to the formation of TAT complexes. However, we found only modest TAT complex upregulation in BALF 8 h after ALI, perhaps because of low FVII and FX BALF levels. TAT complex formation only tends to be increased in TFΔmye mice (Fig. 1F). In vitro, we confirmed TF deletion at the mRNA level in alveolar macrophages with and without LPS stimulation (Fig. 1G).
Figure 1.
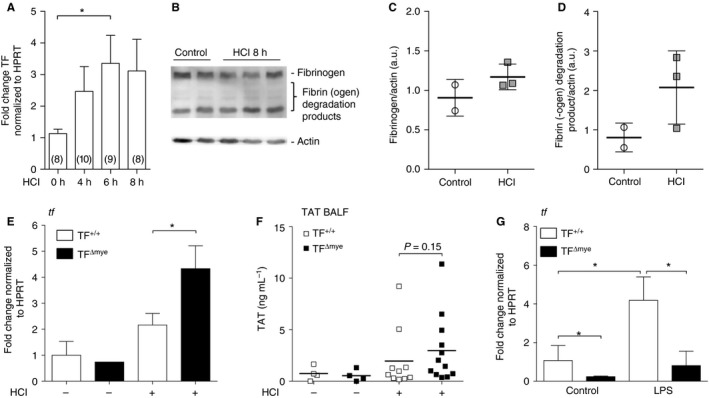
Tissue factor (TF) expression and activation of coagulation during acute lung injury (ALI). (A) TF mRNA expression in lungs of wild‐type mice after HCl‐induced ALI was determined by qPCR at the indicated time points. Data show fold control (0 h) values normalized to hypoxanthine guanine phosphoribosyl transferase (HPRT); numbers are shown in parentheses. (B) Western blot analysis for fibrin‐β of homogenized lung samples 8 h after HCl treatment (n = 3) and of controls (n = 2); actin was used as a loading control. (C, D) Western blot densitometric analysis of (C) fibrinogen and (D) the major fibrin(ogen) degradation product. (E) TF mRNA expression in total lung tissue of control (n TF +/+ = 2; n TF Δmye = 1) and HCl‐treated (n TF +/+ = 9; n TF Δmye = 4) myeloid TF‐deficient (TF Δmye) or wild‐type (TF +/+) mice was analyzed by q‐PCR 8 h after treatment. (F) Thrombin–antithrombin (TAT) complexes in the bronchoalveolar lavage fluid (BALF) of control (n = 4) and HCl‐treated (n TF +/+ = 10; n TF Δmye = 12) wild‐type or myeloid TF‐deficient mice 8 h after treatment. (G) TF mRNA expression of alveolar macrophages, isolated from lungs of myeloid TF knockout mice and wild‐type littermates and stimulated with lipopolysaccharide (LPS) (100 ng mL−1) in vitro for 3 h; n = 5. For statistical analysis, one‐way‐anova with Dunn's multiple comparisons test (A), unpaired Student's t‐tests (E, F) or the Mann–Whitney test (G) were performed; *P < 0.05. Littermate‐controlled experiments were performed. a.u., arbitrary units.
TF on myeloid cells negatively regulates leukocyte recruitment during acid‐induced ALI
One of the most prominent features of ALI is leukocyte influx, in particular of neutrophils, into the bronchoalveolar compartment. As we observed only a minor impact of TF induction on coagulation, we investigated whether upregulated TF could influence inflammation during ALI. Acid‐induced ALI led to significantly enhanced leukocyte extravasation in TFΔmye mice as compared with wild‐type littermates after 8 h, as evidenced by increased CD45+ leukocyte numbers (Fig. 2A). We further identified CD45+ F4/80− Ly6G+ cells, representing neutrophils, as strongly contributing to increased cellular influx. Significantly more neutrophils migrated into the bronchoalveolar compartment in TFΔmye mice 8 h after acid‐induced ALI (Fig. 2B). To verify that the majority of neutrophils transmigrated through the interstitial barrier, we stained lung sections for the neutrophil marker GR‐1 (brown staining, arrows), which recognizes both neutrophils and, to a lesser extent, activated macrophages. We observed that most of the neutrophils were present within the alveoli, and that only a subfraction were present in interstitial layers (Fig. 2C). Furthermore, leukocyte accumulation and lung damage in TFΔmye mice were slightly increased as compared with wild‐type mice (Fig 2D). In contrast, flow cytometric analysis of macrophage subsets characterized by either F4/80+ (Fig. 2E; Fig. S2A) or F4/80+ CD11c+ (Fig. S2B) revealed no difference between TFΔmye mice and wild‐type littermates. Only inflammatory macrophages (Fig. 2F), characterized by F4/80+ and Ly6C+ 28, 29, were marginally, but not significantly (P = 0.09), increased in number. To analyze the specificity of this phenomenon, we applied a leukocyte recruitment model to TFΔmye and wild‐type mice. To this end, we induced thioglycollate‐elicited sterile inflammation, and analyzed leukocyte influx in an early phase (4 h) and a late phase (72 h). However, no differences in infiltrating leukocytes, macrophages or neutrophils were observed, perhaps because of the strong inflammatory stimulus (Fig. S3A–D). In vitro analysis of alveolar macrophages revealed that, in response to LPS, TF deficiency increased the release of the chemokine KC, which primarily attracts neutrophils (Fig. 2G). These data may indicate that myeloid TF plays a lung‐specific role in regulating leukocyte alveolar trafficking.
Figure 2.
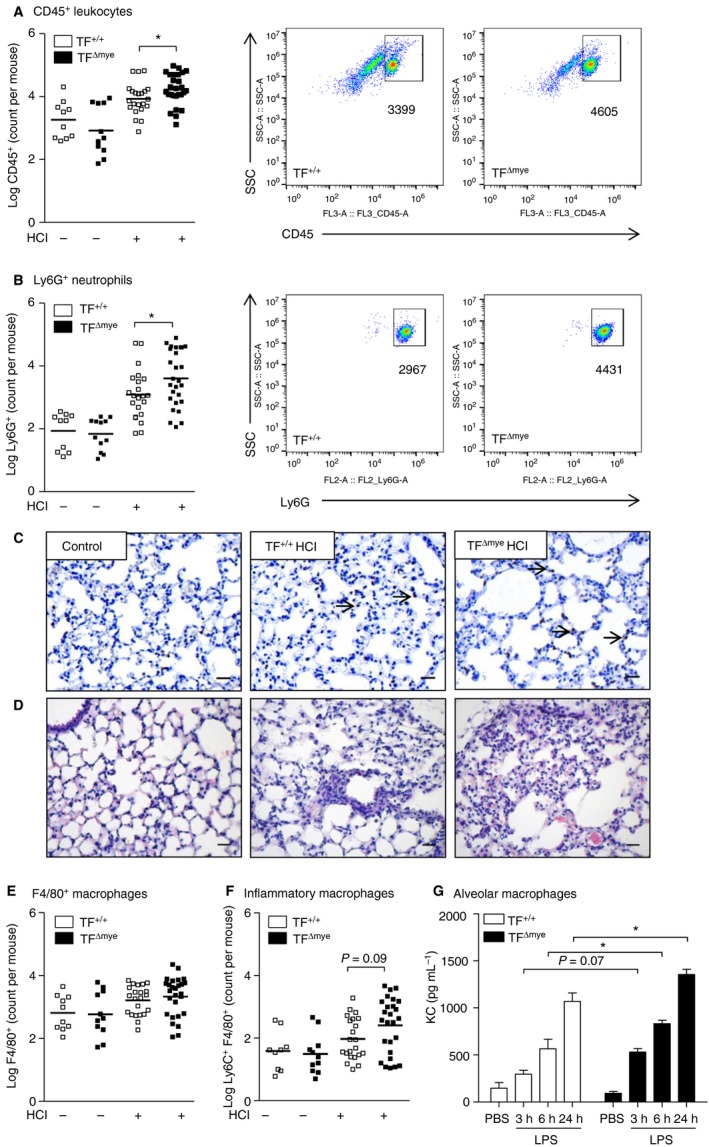
The effect of myeloid tissue factor (TF) on pulmonary leukocyte recruitment during acute lung injury (ALI). (A, B, E, F) Flow cytometric analysis of leukocytes in the bronchoalveolar lavage fluid of control mice (n TF +/+ = 10; n TF Δmye = 11) and HCl‐treated (n TF +/+ = 22; n TF Δmye = 27) myeloid TF‐deficient mice or wild‐type mice 8 h after ALI. (A) CD45+ leukocytes. (B) CD45+ Ly6G+ F4/80− neutrophils. (E) CD45+ F4/80+ macrophages. (F) CD45+ Ly6C+ F4/80+ inflammatory macrophages (n control TF +/+ = 9; n control TF Δmye = 11; n HC l TF +/+ = 21; n HC l TF Δmye = 26). (A, B) Representative blots of HCl‐treated mice. (C,D) Lung sections of myeloid TF‐deficient mice or wild‐type mice 8 h after ALI induction stained for GR‐1 and hematoxylin (C) or hematoxylin and eosin (D). Scale bar: 25 μm. Magnification: × 200. Arrows indicate neutrophils. (G) Alveolar macrophages were stimulated with lipopolysaccharide (LPS) for the indicated time points in vitro, and released chemokine (C‐X‐C‐motif) ligand‐1 (KC) was measured by ELISA; n = 3. For statistical analysis (A–F), unpaired Student's t‐tests and (G) two‐way anova with Sidak's multiple comparison were performed; *P < 0.05. Littermate‐controlled experiments were performed. PBS, phosphate‐buffered saline.
TF dampens the inflammatory response in acid‐induced ALI
Another hallmark of acid‐induced ALI is the induction of an inflammatory response, which is usually triggered by endogenous alarmins or DAMPs, owing to cellular damage. DAMPs include nuclear components such as histones or high‐mobility group box 1, but also IL‐33 30. We observed a strong increase in the release of IL‐33 into the bronchoalveolar compartment in response to ALI, but no differences between TFΔmye and wild‐type littermate mice (Fig. 3A). Although granulocyte numbers in TFΔmye mice (Fig. 2B) were markedly increased, we found only a slight trend for increased MPO levels in BALF and lung homogenates in TFΔmye mice (Fig. 3B). However, analysis of total protein in BALF revealed an increased protein content in TFΔmye mice subjected to acid‐induced ALI as compared with wild‐type mice (Fig. 3C). This finding points towards increased vascular leakage and loss of alveolar–capillary barrier function in the lungs of TFΔmye mice. Next, we measured the level of pulmonary IL‐6 of acid‐treated mice, which we considered to be the most robust marker for inflammation in this rather mild inflammatory model. We observed significantly increased IL‐6 production and release in BALF and total lung after acid‐induced ALI in TFΔmye mice as compared with wild‐type mice (Fig. 3D,E). However, pulmonary IL‐1β and IL‐12 p40 levels were unaltered (Fig. S4A,B).
Figure 3.
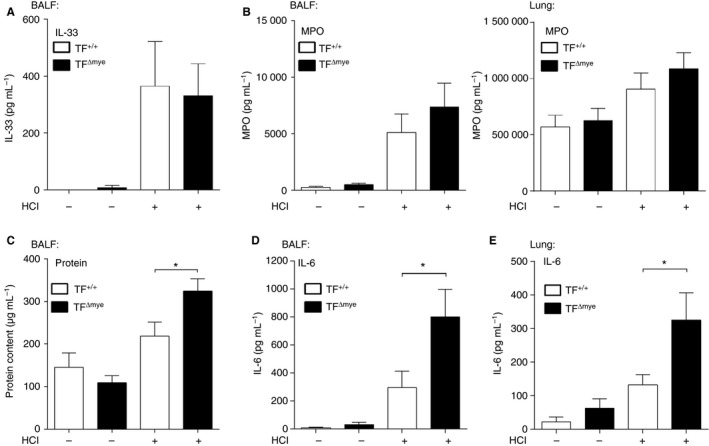
Increased inflammation in myeloid tissue factor (TF)‐deficient mice in response to acid‐induced acute lung injury (ALI). (A–D) Interleukin (IL)‐33 (A) (n control TF +/+ = 6, n control TF Δmye = 5, n HC l TF +/+ = 7, n HC l TF Δmye = 8), myeloperoxidase (MPO) (B) (bronchoalveolar lavage fluid [BALF] – n control TF +/+ = 6, n control TF Δmye = 4, n HC l TF +/+ = 11, n HC l TF Δmye = 12; lung – n control TF +/+ = 5, n control TF Δmye = 5, n HC l TF +/+ = 14, n HC l TF Δmye = 14) and IL‐6 (D) (n control TF +/+ = 6, n control TF Δmye = 5, n HC l TF +/+ = 10, n HC l TF Δmye = 12) were measured in BALF by ELISA. (C) The protein content in BALF was measured with a bicinchoninic acid protein assay (n control TF +/+ = 3, n control TF Δmye = 3, n HC l TF +/+ = 7, n HC l TF Δmye = 10). (E) IL‐6 levels in total lung tissue were determined by ELISA (n control TF +/+ = 7, n control TF Δmye = 8; n HC l TF +/+ = 11, n HC l TF Δmye = 13). Parameters were analyzed 8 h after HCl treatment. For statistical analysis, unpaired Student's t‐tests (A–E) were performed; *P < 0.05. Littermate‐controlled experiments were performed.
TF dampens the inflammatory response of macrophages in vitro
To determine the possible source of increased IL‐6 levels in HCl‐treated TFΔmye mice and the impact of myeloid TF on inflammatory responses of macrophages, we isolated alveolar macrophages from naive TFΔmye mice and wild‐type littermates. Analysis of IL‐6 at the mRNA and protein levels revealed a significant increase in IL‐6 mRNA 3 h after LPS stimulation (Fig. 4A,B). Moreover, TNF‐α mRNA and protein levels were significantly increased 3 h after LPS treatment (Fig. 4C,D). As alveolar macrophages are very limited in number, and their development depends on GM‐CSF 31, we also used GM‐CSF‐induced BMDMs. LPS‐stimulated TF‐deficient BMDMs expressed similar levels of IL‐6 mRNA and protein (Fig. 4E,F); however, TNF‐α secretion was significantly increased (Fig. 4G). To identify potential signaling pathways affected by TF, we analyzed the expression of EGR1, IκBα, and STAT1. We found that TF significantly reduced the expression of EGR1 1 h after LPS stimulation, whereas IκBα and STAT1 were similarly expressed (Fig. 4H–J), indicating that the mitogen‐activated kinase pathway was primarily affected, rather than the interferon and NF‐κB pathways.
Figure 4.
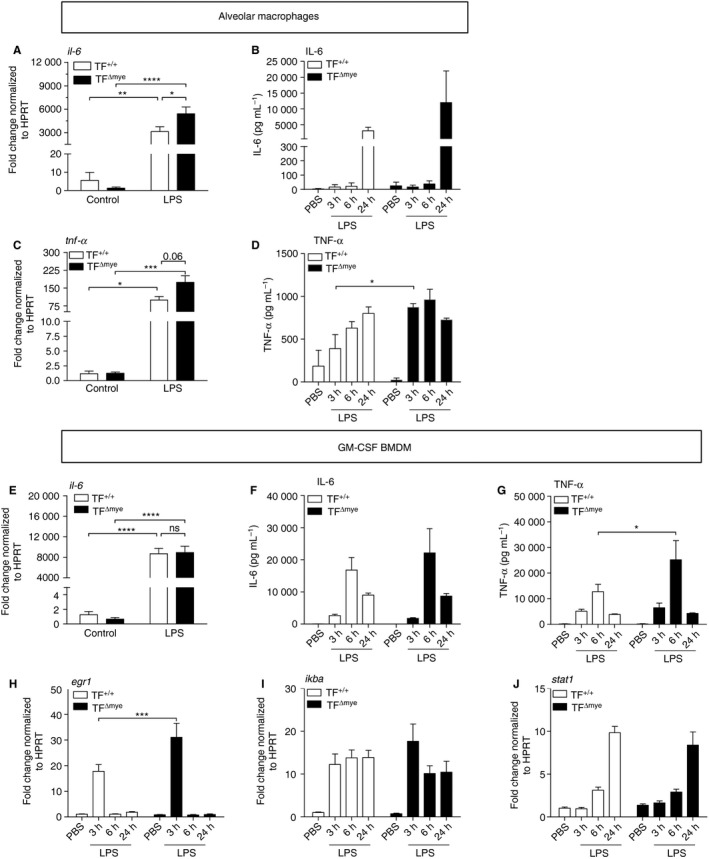
Myeloid tissue factor (TF) deficiency promotes a proinflammatory phenotype of alveolar and bone marrow‐derived macrophages (BMDMs). (A–D) Alveolar macrophages of TF Δmye and TF +/+ mice were isolated and stimulated with lipopolysaccharide (LPS) (10 ng mL−1) or vehicle for the indicated time points. Interleukin (IL)‐6 (A) (n = 5) and tumor necrosis factor (TNF)‐α (C) (n = 3) mRNA expression in isolated alveolar macrophages, stimulated with LPS or vehicle for 3 h were determined by qPCR. Data are depicted as fold TF +/+ control. (B, D) IL‐6 (B) and TNF‐α (D) protein levels in supernatants of alveolar macrophages were measured by ELISA at the indicated time points after LPS stimulation; n = 3. (E–J) Bone marrow cells of TFΔmye and TF+/+ mice were differentiated towards macrophages by granulocyte–macrophage colony‐stimulating factor (GM‐CSF) (10 ng mL−1) and stimulated with LPS (10 ng mL−1) or vehicle at the indicated time points. (E) IL‐6 mRNA (n control TF+/+ = 5; all others n = 6) expression 3 h after stimulation was quantified by qPCR, and the data are depicted as fold TF+/+ control. (F) IL‐6 (n 6 h = 4; all others n = 6). (G) TNF‐α (n = 6) levels in supernatants were determined by ELISA. (H–J) EGR1 (H) (n = 5–6), IKBα (I) (n = 5–6) and STAT1 (J) (n = 5–6) were measured by qPCR, and are depicted as fold TF+/+ control. For statistical analysis, two‐way anova with Sidak's multiple comparison was performed; *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Littermate‐controlled experiments were performed. HPRT, hypoxanthine guanine phosphoribosyl transferase; NS, not significant; PBS, phosphate‐buffered saline.
As TF activity leads to activated FVII (FVIIa) and thrombin generation, we examined the effects of 3 h of human FVIIa and thrombin stimulation on wild‐type BMDMs. We found that concomitant stimulation with LPS and thrombin significantly reduced the expression of IL‐6 and tended to decrease the expression of TNF‐α and IκBα (Fig. S5A–C). Similarly, FVIIa showed a tendency to reduce IL‐6 levels and, to a lesser extent, TNF‐α and IκBα mRNA levels (Fig. S5D–F). However, in vivo, other mechanisms, such as the intrinsic coagulation cascade, can also contribute to thrombin generation. Moreover, TF can directly signal and thereby modulate macrophage functions.
These results indicate that the differential expression of proinflammatory cytokines during ALI is probably dependent on TF surface expression on innate immune cells.
TF on epithelial cells does not contribute to tissue inflammation 8 h after acid‐induced ALI
To determine the contribution of lung epithelial TF to the pathophysiology of acid‐induced ALI, we used TFΔepi mice. We used immunostaining to confirm that there was appropriate TF deficiency in the lungs of TFΔepi mice 18 (Fig. S1B). Analysis of TF mRNA expression in whole lung tissue during ALI revealed a trend for increased TF expression 8 h after acid instillation in wild‐type mice. In contrast, TF mRNA levels were strongly reduced in TFΔepi mice (Fig. 5A), indicating that epithelial cells express the majority of lung TF mRNA during ALI. The SPC cre efficiently targets TF expression in most of the lung epithelial cells 32. Moreover, upon LPS‐induced ALI, lung epithelial cells represent the major TF‐expressing cell type responsible for protection of alveolar–capillary integrity 18. Hence, we wanted to determine whether this is also the case during acid‐induced ALI. Measurement of TF activity revealed slightly increased FXa generation and TAT complex formation 8 h after disease induction in wild‐type littermates. In comparison, TFΔepi mice showed a trend for reduced FXa generation but no differences in TAT complex formation (Fig. 5B,C). Next, we tested endothelial and epithelial barrier function by measuring BALF protein levels. Surprisingly, we did not observe significant differences in BALF protein levels and edema formation between acid‐treated TFΔepi mice and wild‐type mice (Fig. 5D,E). Similarly, we did not observe alterations in erythrocyte levels in BALF (Fig. 5J). To test the inflammatory response, we measured pulmonary IL‐6 mRNA expression and IL‐6 protein levels in BALF and lung tissue, and pulmonary neutrophil transmigration, but did not detect any differences between acid‐treated TFΔepi mice and wild‐type littermates (Fig. 5F–I).
Figure 5.
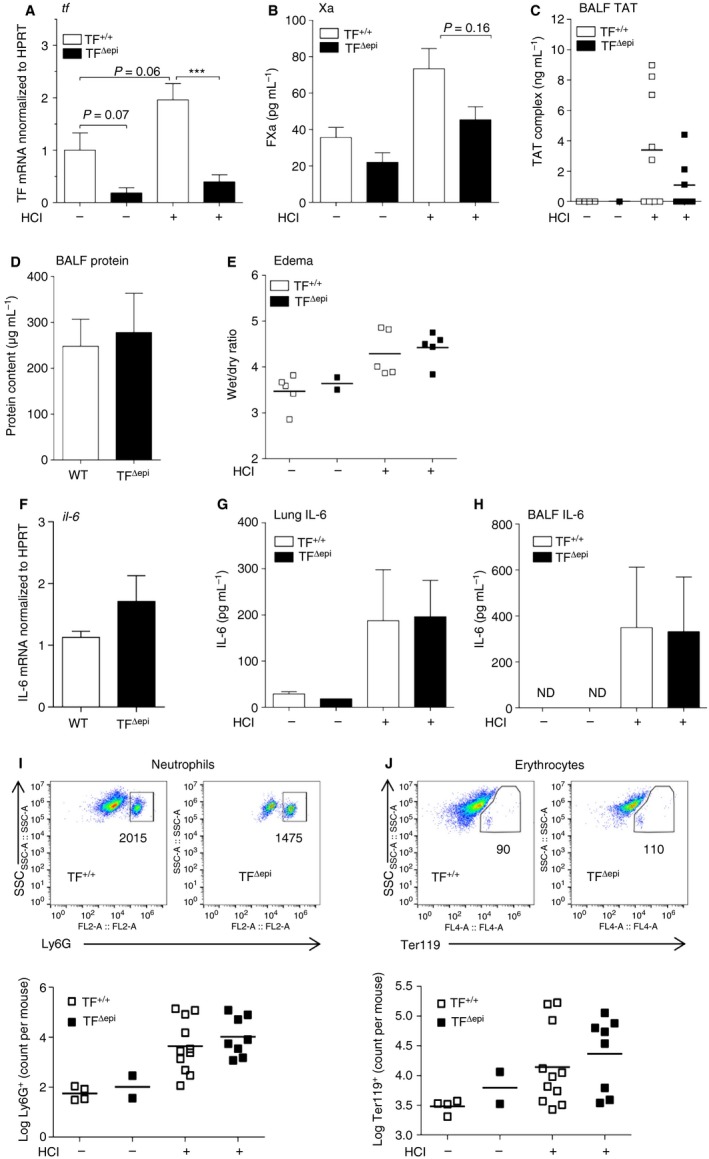
Epithelial tissue factor (TF) plays a minor role in acid‐induced acute lung injury (ALI). Epithelial TF‐deficient and wild‐type (WT) mice were treated with HCl for 8 h. (A) TF mRNA expression of lung tissue was determined by qPCR, and is depicted as fold wild‐type control (n control TF +/+ = 5; n control TF Δmye = 3; n HC l TF +/+ = 9; n HC l TF Δmye = 10). (B, C) Activated factor X (FXa) generation (B) (n control TF +/+ = 4; n control TF Δmye = 2; n HC l TF +/+ = 6; n HC l TF Δmye = 4) and thrombin–antithrombin (TAT) complex (C) were measured in bronchoalveolar lavage fluid (BALF) (n control TF +/+ = 4; n control TF Δmye = 1; n HC l TF +/+ = 9; n HC l TF Δmye = 7). (D) Protein content in BALF samples (n control TF +/+ = 4; n control TF Δmye = 2; n HC l TF +/+ = 10; n HC l TF Δmye = 6). (E) Lung edema was determined by measuring the weight ratio of wet and dried whole lung tissue (n control TF Δmye = 2; all others n = 5) (F–H) IL‐6 mRNA expression (F) (n control = 4; n HC l TF +/+ = 5; n HC l TF Δmye = 8) and IL‐6 protein levels in (G) total lung tissue (n control TF Δmye = 1; all others n = 4) and (H) BALF (n control TF +/+ = 4; n control TF Δmye = 1; n HC l TF +/+ = 6; n HC l TF Δmye = 3). (I, J) Flow cytometric analysis of (I) neutrophils (CD45+ Ly6G+ F4/80−) and (J) erythrocytes (Ter119+) in the BALF of epithelial TF‐deficient and WT mice (n control TF +/+ = 4; n control TF Δmye = 2; n HC l TF +/+ = 11; n HC l TF Δmye = 8). For statistical analysis, the Mann–Whitney test was performed (A, B); ***P < 0.001. Littermate‐controlled experiments were performed. HPRT, hypoxanthine guanine phosphoribosyl transferase; ND, not detectable.
Restoration of tissue integrity is disturbed at late stages of acid‐induced ALI in TFΔepi mice but not in TFΔmye mice
The current literature on TF in ALI pathogenesis is focused on early responses in disease progression. Therefore, we aimed to elucidate the effects of TF deletion at late stages of ALI. Twenty‐four hours after acid treatment, the initial injury and inflammation in wild‐type mice had resolved. Extravasation of leukocytes, including neutrophils and macrophages, as well as erythrocytes and protein infiltrates, returned to baseline levels (Fig. 6A–H). Surprisingly, we observed increased leukocyte counts in the bronchoalveolar compartment in TFΔepi mice as compared with wild‐type littermates at this time point, suggesting that TFΔepi mice were unable to completely resolve the mild acid‐induced injury (Fig. 6A). Notably, most of this increase could be attributed to macrophages, and not to neutrophils (Fig. 6B,C). In contrast, TFΔmye mice did not show any overt pulmonary inflammation 24 h after ALI induction, as indicated by baseline levels of leukocytes, macrophages and neutrophils in BALF (Fig. 6D–F). Similarly, we found significantly more erythrocytes in HCl‐treated TFΔepi mice and a trend for more protein in the BALF (Fig. 6G), whereas TFΔmye mice and wild‐type mice did not show increased levels in comparison with untreated mice (Fig. 6H). Edema formation was still slightly increased in all four HCl‐treated groups, but no differences between genotypes were observed (Fig. 6I,J).
Figure 6.
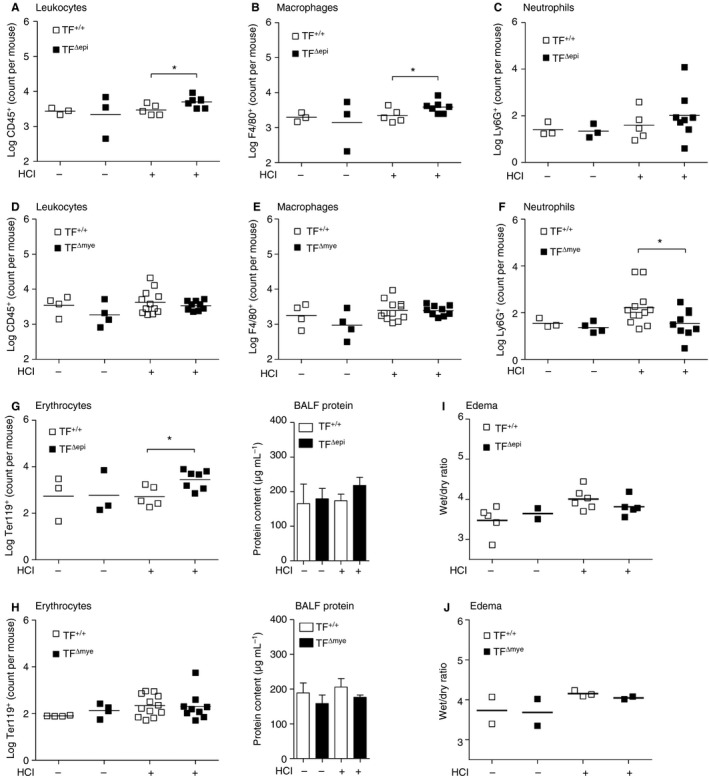
Leukocyte extravasation into the lung 24 h after HCl‐induced acute lung injury (ALI) in epithelial and myeloid tissue factor (TF)‐deficient mice. The presence of (A, D) leukocytes (CD45+), (B, E) macrophages (CD45+ F4/80+), (C, F) neutrophils (CD45+ Ly6G+ F4/80−) and (G, H) erythrocytes (Ter119+) in the bronchoalveolar lavage fluid (BALF) was determined by flow cytometry. (I, J) BALF protein was measured with a bicinchoninic acid assay. (A–C, G) n control = 2–3; n HCl TF+/+ = 5; n HCl TFΔepi = 7–8. (D–F, H) n control = 3–4; n HCl TF+/+ = 12; n HCl TFΔmye = 9. (I) n control TF+/+ = 5; n control TF+/+ = 2; n HCl TF+/+ = 6; n HCl TFΔepi = 5. (J) n = 2–3. For statistical analysis, unpaired Student's t‐tests were performed; *P < 0.05. Littermate‐controlled experiments were performed.
Systemic TF targeting by antibody does not affect inflammation but increases the risk of bleeding during acid‐induced ALI
To elucidate a possible systemic role of TF in the progression of acid‐induced ALI, we administered the TF‐blocking antibody 1H1 intraperitoneally in C57BL/6J littermates 33. We observed no adverse events such as fatal bleeding during the 8‐h observation period after intratracheal acid instillation. When we analyzed ALI‐induced leukocyte influx in BALF, we found no differences for CD45+ leukocytes, Ly6G+ neutrophils, or F4/80+ macrophages (Fig. 7A–C). In contrast to what was seen in cell type‐specific TF‐deficient mice, systemic blockade of TF led to a significant increase in erythrocyte numbers in BALF (Fig. 7D), indicating that TF is required to maintain bronchoalveolar barrier function. Accordingly, we did not observe gross changes in IL‐6 (Fig. 7E) or KC (Fig. 7F) levels.
Figure 7.
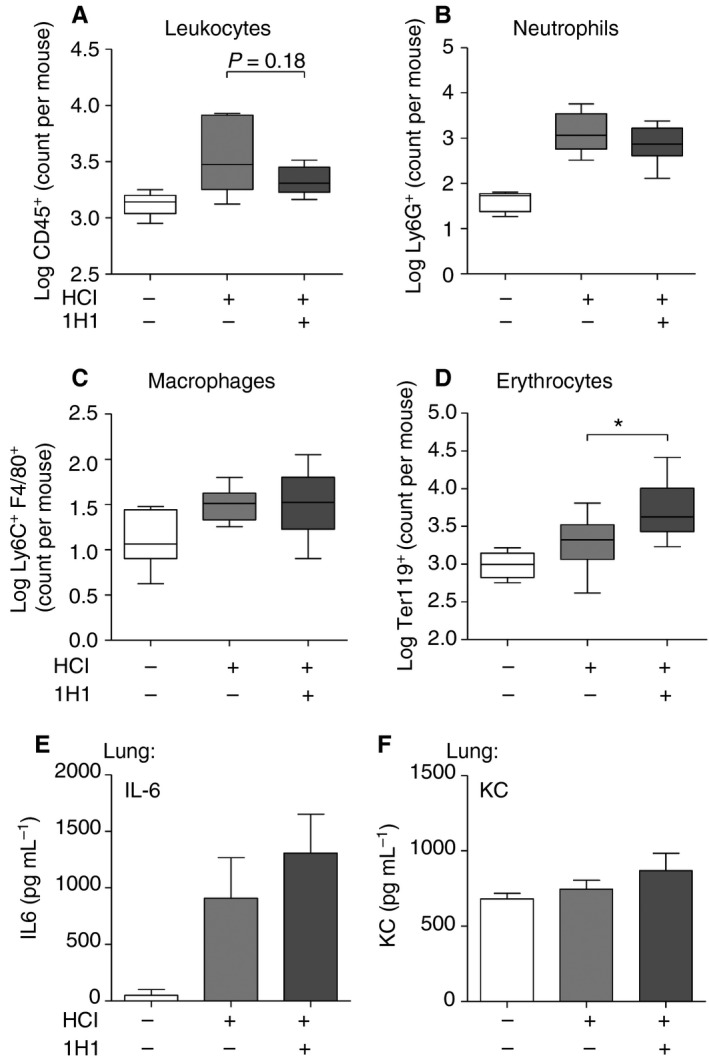
Systemic tissue factor (TF) inhibition in acid‐induced acute lung injury (ALI). Wild‐type mice were intraperitoneally injected with anti‐TF blocking antibody (1H1) or vehicle prior to HCl treatment, and parameters were analyzed 8 h after HCl treatment. (A–D) Extravasation of (A) leukocytes (CD45+), (B) neutrophils (CD45+ Ly6G+ F4/80−), (C) macrophages (CD45+ F4/80+) and (D) erythrocytes (Ter119+) in the bronchoalveolar lavage fluid (BALF) was determined by flow cytometry. (E, F) Interleukin (IL)‐6 (E) and chemokine (C‐X‐C‐motif) ligand‐1 (KC) (F) levels in BALF were determined by ELISA. (A) n control WT = 5; n HC l WT = 12; n HC l 1H1 = 9. (B) n control WT = 5; n HC l WT = 8; n HC l 1H1 = 8. (C) n control WT = 7; n HC l WT = 8; n HC l 1H1 = 8. (D) n control WT = 7; n HC l WT = 10; n HC l 1H1 = 10. (E, F) n control WT = 3; n HC l WT = 6; n HC l 1H1 = 6. For statistical analysis, unpaired Student's t‐tests were performed; *P < 0.05. Littermate‐controlled experiments were performed.
Discussion
TF and the extrinsic coagulation cascade are intricately involved in the disease mechanisms underlying ALI. For instance, exacerbated fibrin deposition is detrimental to the function of the alveolar–capillary system by causing barrier dysfunction and hampering gas exchange 34. We found that myeloid TF deficiency exacerbated the pathology of acid‐induced ALI. Surprisingly, TF deficiency of lung epithelial cells resulted in an unusually long‐lasting (24 h) disease pathology of acid‐induced ALI, accompanied by modest intra‐alveolar hemorrhage. This is in line with previous findings showing that epithelial TF exerts protective effects, e.g. supporting the alveolar–capillary barrier, in various infectious and non‐infectious ALI models 18, 35, 36. For instance, in an LPS‐induced lung inflammation model, the presence of epithelial cell TF determines the severity of the lung pathology 18, 33. Whereas, in LPS‐induced ALI, myeloid TF is of minor importance 36, we provide evidence that, in acid‐induced ALI, deficiency of myeloid TF promoted the inflammatory response and thereby significantly exacerbated disease progression. Increased inflammation may be the reason for increased TF expression in whole lung tissue, as observed in TFΔmye mice as compared with wild‐type mice during ALI. Previous studies have indicated that the effect of myeloid TF depends significantly on the disease model. Whereas, in low‐TF mice, bacterial burden and cytokine release were unaltered as compared with wild‐type mice during tuberculosis 37, myeloid TF deficiency augmented the pulmonary growth of Mycobacterium tuberculosis, most likely because of reduced fibrin generation and shifting macrophage polarization 38.
Such disease model‐dependent differences could explain our observations regarding the role of myeloid TF in ALI. In contrast to LPS‐induced ALI, which induces apoptosis in epithelial and endothelial cells 39, 40, acid‐induced ALI creates a direct cell‐damaging disease pattern. Intratracheal instillation of HCl mainly affects epithelial cells, leading to impaired epithelial cell function and fluid transport, and indirect damage to the capillaries 41. Epithelial cell injury was also exemplified by damage‐associated release of IL‐33 into the bronchoalveolar space. IL‐33 is abundantly expressed in lung epithelial cells 42. However, we did not observe any differences in IL‐33 levels in BALF between TFΔmye mice and wild‐type mice, indicating comparable initial insults by acid aspiration in both groups. However, during acid‐induced ALI, TFΔmye mice showed increased pulmonary TF expression, indicating enhanced activation of non‐hematopoietic cells, e.g. type I and type II lung epithelial cells, upon acid injury. The most apparent differences in inflammation were significant increases in proinflammatory IL‐6 levels and leukocyte/neutrophil influx. These occurred independently of coagulation activation or the presence of TF on epithelial cells, and was solely dependent on myeloid TF. Although there was a tendency for there to be increased cytokine expression in TFΔmye mice during LPS‐induced ALI, no significant differences were observed 36.
In line with elevated cytokine levels, we also observed increased recruitment of leukocytes into the bronchoalveolar space of TFΔmye mice, comprising inflammatory macrophages and, particularly, neutrophils; however, total MPO levels were only slightly increased. These findings indicate that, although coagulation is not affected by the lack of TF on myeloid cells and, in particular, on alveolar macrophages, the proinflammatory environment created by acid‐induced ALI further substantiates the damage. This idea is supported by the significant upregulation of proinflammatory cytokines, e.g. IL‐6, by isolated TF‐deficient alveolar macrophages upon LPS stimulation in vitro.
However, neutrophils can also upregulate TF expression during inflammation 5, 43. Infection with influenza virus A/H1N1 induces TF expression on various cell types, including neutrophils 44. Thus, we cannot exclude the possibility that lack of TF on neutrophils contributes to the observed phenotype during ALI.
TF upregulation during ALI results not only in a mild hypercoagulable state but also in an inflammatory state, as FVIIa, FXa, and activated FII can activate protease‐activated receptors, which induce the expression of various cytokines 45. However, we provide evidence that myeloid TF itself dampens inflammatory reactions via downregulation of macrophage IL‐6 expression. Our data indicate that there is TF cell type‐specific and disease‐specific regulation of inflammatory responses, suggesting that TF acts as a double‐edged sword in the cross‐talk between inflammation and coagulation.
Taking our findings together, we conclude that myeloid TF has roles in addition to activating the extrinsic coagulation cascade, as TF clearly shows immunomodulatory potential. Thus, myeloid TF may potently regulate the intensity of inflammatory responses to exogenous lung insults such as that caused by gastric contents.
Addendum
J. B. Kral‐Pointner designed and performed experiments, analyzed the results, and wrote the manuscript. W. C. Schrottmaier and V. Horvath planned and performed research, and analyzed the data. H. Datler was involved in data collection and animal experiments. C. Ay, B. Niederreiter, and L. Hell were involved in data collection. N. Mackman provided conditional knockout mice and supervised the study. B. Jilma and J. A. Schmid were involved in interpreting and discussing the results. A. Assinger and S. Knapp participated in study design and contributed to the discussion and interpretation of the data. G. Schabbauer designed the study, was involved in result interpretation and discussion of the research progress, and wrote the manuscript. All authors confirm that they had full access to the data and the manuscript; they revised its intellectual content and approved the final version.
Disclosure of Conflict of Interests
The authors state that they have no conflict of interest.
Supporting information
Data S1. Methods.
Fig. S1. Genotyping data of myeloid and airway epithelial TF‐deficient mice.
Fig. S2. Macrophage recruitment into the lung 8 h after acid‐induced acute lung injury (ALI).
Fig. S3. Myeloid TF does not influence leukocyte recruitment during sterile peritonitis.
Fig. S4. No significant changes in IL‐12 and IL‐1β levels between myeloid TF and wild‐type littermates 8 h after acid‐induced ALI.
Fig. S5. Effect of thrombin and FVIIa on the inflammatory response of macrophages.
Acknowledgements
We thank H. Paar, B. Birnecker, and A. Knöbl for their excellent technical assistance. Floxed TF mice crossbred with LysMcre and SPC cre mice were a kind gift from N. Mackman. We wish to thank C. T. Esmon for kindly providing the fibrin β‐monomer‐specific antibody 59D8, C. Binder and M. Schwameis for fruitful discussions, and J. S. Brunner, L. Hanzl, and T. Afonyushkin for excellent technical advice. The study was supported by the Austrian Science Fund FWF with the projects FWF‐SFB 54 and FWF‐P24978.
Kral‐Pointner JB, Schrottmaier WC, Horvath V, Datler H, Hell L, Ay C, Niederreiter B, Jilma B, Schmid JA, Assinger A, Mackman N, Knapp S, Schabbauer G. Myeloid but not epithelial tissue factor exerts protective anti‐inflammatory effects in acid aspiration‐induced acute lung injury. J Thromb Haemost 2017; 15: 1625–39.
Manuscript handled by: W. Ruf
Final decision: P. H. Reitsma, 5 May 2017
References
- 1. Mackman N, Taubman M. Tissue factor: past, present, and future. Arterioscler Thromb Vasc Biol 2009; 29: 1986–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Williams JC, Mackman N. Tissue factor in health and disease. Front Biosci (Elite Ed) 2012; 4: 358–72. [DOI] [PubMed] [Google Scholar]
- 3. Wilcox JN, Smith KM, Schwartz SM, Gordon D. Localization of tissue factor in the normal vessel wall and in the atherosclerotic plaque. Proc Natl Acad Sci USA 1989; 86: 2839–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moosbauer C, Morgenstern E, Cuvelier SL, Manukyan D, Bidzhekov K, Albrecht S. Eosinophils are a major intravascular location for tissue factor storage and exposure. Blood 2007; 109: 995–1002. [DOI] [PubMed] [Google Scholar]
- 5. Maugeri N, Brambilla M, Camera M, Carbone A, Tremoli E, Donati MB, de Gaetano G, Cerletti C. Human polymorphonuclear leukocytes produce and express functional tissue factor upon stimulation. J Thromb Haemost 2006; 4: 1323–30. [DOI] [PubMed] [Google Scholar]
- 6. Steinemann S, Ulevitch RJ, Mackman N. Role of the lipopolysaccharide (LPS)‐binding protein/CD14 pathway in LPS induction of tissue factor expression in monocytic cells. Arterioscler Thromb 1994; 14: 1202–9. [DOI] [PubMed] [Google Scholar]
- 7. Drake TA, Cheng J, Chang A, Taylor FB Jr. Expression of tissue factor, thrombomodulin, and E‐selectin in baboons with lethal Escherichia coli sepsis. Am J Pathol 1993; 142: 1458–70. [PMC free article] [PubMed] [Google Scholar]
- 8. Erlich J, Fearns C, Mathison J, Ulevitch RJ, Mackman N. Lipopolysaccharide induction of tissue factor expression in rabbits. Infect Immun 1999; 67: 2540–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cui MZ, Parry GC, Oeth P, Larson H, Smith M, Huang RP, Adamson ED, Mackman N. Transcriptional regulation of the tissue factor gene in human epithelial cells is mediated by Sp1 and EGR‐1. J Biol Chem 1996; 271: 2731–9. [DOI] [PubMed] [Google Scholar]
- 10. Mackman N, Brand K, Edgington TS. Lipopolysaccharide‐mediated transcriptional activation of the human tissue factor gene in THP‐1 monocytic cells requires both activator protein 1 and nuclear factor kappa B binding sites. J Exp Med 1991; 174: 1517–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Oeth P, Parry GC, Mackman N. Regulation of the tissue factor gene in human monocytic cells. Role of AP‐1, NF‐kappa B/Rel, and Sp1 proteins in uninduced and lipopolysaccharide‐induced expression. Arterioscler Thromb Vasc Biol 1997; 17: 365–74. [DOI] [PubMed] [Google Scholar]
- 12. Schabbauer G, Schweighofer B, Mechtcheriakova D, Lucerna M, Binder BR, Hofer E. Nuclear factor of activated T cells and early growth response‐1 cooperate to mediate tissue factor gene induction by vascular endothelial growth factor in endothelial cells. Thromb Haemost 2007; 97: 988–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Engelmann B, Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol 2013; 13: 34–45. [DOI] [PubMed] [Google Scholar]
- 14. Luo D, Szaba FM, Kummer LW, Plow EF, Mackman N, Gailani D, Smiley ST. Protective roles for fibrin, tissue factor, plasminogen activator inhibitor‐1, and thrombin activatable fibrinolysis inhibitor, but not factor XI, during defense against the gram‐negative bacterium Yersinia enterocolitica. J Immunol 2011; 187: 1866–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pawlinski R, Pedersen B, Schabbauer G, Tencati M, Holscher T, Boisvert W, Andrade‐Gordon P, Frank RD, Mackman N. Role of tissue factor and protease‐activated receptors in a mouse model of endotoxemia. Blood 2004; 103: 1342–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Welty‐Wolf KE, Carraway MS, Ortel TL, Ghio AJ, Idell S, Egan J, Zhu X, Jiao JA, Wong HC, Piantadosi CA. Blockade of tissue factor–factor X binding attenuates sepsis‐induced respiratory and renal failure. Am J Physiol Lung Cell Mol Physiol 2006; 290: L21–31. [DOI] [PubMed] [Google Scholar]
- 17. Bastarache JA, Sebag SC, Clune JK, Grove BS, Lawson WE, Janz DR, Roberts LJ, Dworski R, Mackman N, Ware LB. Low levels of tissue factor lead to alveolar haemorrhage, potentiating murine acute lung injury and oxidative stress. Thorax 2012; 67: 1032–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shaver CM, Grove BS, Putz ND, Clune JK, Lawson WE, Carnahan RH, Mackman N, Ware LB, Bastarache JA. Regulation of alveolar procoagulant activity and permeability in direct acute lung injury by lung epithelial tissue factor. Am J Respir Cell Mol Biol 2015; 53: 719–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med 2005; 353: 1685–93. [DOI] [PubMed] [Google Scholar]
- 20. Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol 2007; 81: 1–5. [DOI] [PubMed] [Google Scholar]
- 21. Imai Y, Kuba K, Neely GG, Yaghubian‐Malhami R, Perkmann T, van Loo G, Ermolaeva M, Veldhuizen R, Leung YH, Wang H, Liu H, Sun Y. Identification of oxidative stress and Toll‐like receptor 4 signaling as a key pathway of acute lung injury. Cell 2008; 133: 235–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Reiss LK, Uhlig U, Uhlig S. Models and mechanisms of acute lung injury caused by direct insults. Eur J Cell Biol 2012; 91: 590–601. [DOI] [PubMed] [Google Scholar]
- 23. Glas GJ, Van Der Sluijs KF, Schultz MJ, Hofstra JJ, Van Der Poll T, Levi M. Bronchoalveolar hemostasis in lung injury and acute respiratory distress syndrome. J Thromb Haemost 2013; 11: 17–25. [DOI] [PubMed] [Google Scholar]
- 24. Faust N, Varas F, Kelly LM, Heck S, Graf T. Insertion of enhanced green fluorescent protein into the lysozyme gene creates mice with green fluorescent granulocytes and macrophages. Blood 2000; 96: 719–26. [PubMed] [Google Scholar]
- 25. Rautou PE, Tatsumi K, Antoniak S, Owens AP III, Sparkenbaugh E, Holle LA, Wolberg AS, Kopec AK, Pawlinski R, Luyendyk JP, Mackman N. Hepatocyte tissue factor contributes to the hypercoagulable state in a mouse model of chronic liver injury. J Hepatol 2016; 64: 53–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pfaffl MW. A new mathematical model for relative quantification in real‐time RT‐PCR. Nucleic Acids Res 2001; 29: e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stojkovic S, Kaun C, Basilio J, Rauscher S, Hell L, Krychtiuk KA, Bonstingl C, de Martin R, Gröger M, Ay C, Holnthoner W, Eppel W, Neumayer C, Huk I, Huber K, Demyanets S, Wojta J. Tissue factor is induced by interleukin‐33 in human endothelial cells: a new link between coagulation and inflammation. Sci Rep 2016; 6: 25171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 2003; 19: 71–82. [DOI] [PubMed] [Google Scholar]
- 29. Garcia‐Bonilla L, Faraco G, Moore J, Murphy M, Racchumi G, Srinivasan J, Brea D, Iadecola C, Anrather J. Spatio‐temporal profile, phenotypic diversity, and fate of recruited monocytes into the post‐ischemic brain. J Neuroinflammation 2016; 13: 285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Martin NT, Martin MU. Interleukin 33 is a guardian of barriers and a local alarmin. Nat Immunol 2016; 17: 122–31. [DOI] [PubMed] [Google Scholar]
- 31. Wicks IP, Roberts AW. Targeting GM‐CSF in inflammatory diseases. Nat Rev Rheumatol 2016; 12: 37–48. [DOI] [PubMed] [Google Scholar]
- 32. Tanjore H, Xu XC, Polosukhin VV, Degryse AL, Li B, Han W, Sherrill TP, Plieth D, Neilson EG, Blackwell TS, Lawson WE. Contribution of epithelial‐derived fibroblasts to bleomycin‐induced lung fibrosis. Am J Respir Crit Care Med 2009; 180: 657–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kirchhofer D, Moran P, Bullens S, Peale F, Bunting S. A monoclonal antibody that inhibits mouse tissue factor function. J Thromb Haemost 2005; 3: 1098–9. [DOI] [PubMed] [Google Scholar]
- 34. Ware LB, Camerer E, Welty‐Wolf K, Schultz MJ, Matthay MA. Bench to bedside: targeting coagulation and fibrinolysis in acute lung injury. Am J Physiol Lung Cell Mol Physiol 2006; 291: L307–11. [DOI] [PubMed] [Google Scholar]
- 35. Antoniak S, Tatsumi K, Hisada Y, Milner JJ, Neidich SD, Shaver CM, Pawlinski R, Beck MA, Bastarache JA, Mackman N. Tissue factor deficiency increases alveolar hemorrhage and death in influenza A infected mice. J Thromb Haemost 2016; 14: 1238–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shaver CM, Grove BS, Clune JK, Mackman N, Ware LB, Bastarache JA. Myeloid tissue factor does not modulate lung inflammation or permeability during experimental acute lung injury. Sci Rep 2016; 6: 22249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kothari H, Rao LV, Vankayalapati R, Pendurthi UR. Mycobacterium tuberculosis infection and tissue factor expression in macrophages. PLoS ONE 2012; 7: e45700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Venkatasubramanian S, Tripathi D, Tucker T, Paidipally P, Cheekatla S, Welch E, Raghunath A, Jeffers A, Tvinnereim AR, Schechter ME, Andrade BB, Mackman N, Idell S, Vankayalapati R. Tissue factor expression by myeloid cells contributes to protective immune response against Mycobacterium tuberculosis infection. Eur J Immunol 2016; 46: 464–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kawasaki M, Kuwano K, Hagimoto N, Matsuba T, Kunitake R, Tanaka T, Maeyama T, Hara N. Protection from lethal apoptosis in lipopolysaccharide‐induced acute lung injury in mice by a caspase inhibitor. Am J Pathol 2000; 157: 597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang HL, Akinci IO, Baker CM, Urich D, Bellmeyer A, Jain M, Chandel NS, Mutlu GM, Budinger GR. The intrinsic apoptotic pathway is required for lipopolysaccharide‐induced lung endothelial cell death. J Immunol 2007; 179: 1834–41. [DOI] [PubMed] [Google Scholar]
- 41. McAuley DF, Frank JA, Fang X, Matthay MA. Clinically relevant concentrations of beta2‐adrenergic agonists stimulate maximal cyclic adenosine monophosphate‐dependent airspace fluid clearance and decrease pulmonary edema in experimental acid‐induced lung injury. Crit Care Med 2004; 32: 1470–6. [DOI] [PubMed] [Google Scholar]
- 42. Prefontaine D, Nadigel J, Chouiali F, Audusseau S, Semlali A, Chakir J. Increased IL‐33 expression by epithelial cells in bronchial asthma. J Allergy Clin Immunol 2010; 125: 752–4. [DOI] [PubMed] [Google Scholar]
- 43. Higure A, Okamoto K, Hirata K, Todoroki H, Nagafuchi Y, Takeda S, Martin JG, Hamid Q. Macrophages and neutrophils infiltrating into the liver are responsible for tissue factor expression in a rabbit model of acute obstructive cholangitis. Thromb Haemost 1996; 75: 791–5. [PubMed] [Google Scholar]
- 44. Prutkina EV, Sepp AV, Tsybikov NN. Lung tissue factor expression changes in relation to the stage of respiratory distress syndrome in the presence of pneumonia in A/H1N1 influenza. Arkh Patol 2013; 75: 16–21. [PubMed] [Google Scholar]
- 45. Mackman N. The many faces of tissue factor. J Thromb Haemost 2009; 7: 136–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Methods.
Fig. S1. Genotyping data of myeloid and airway epithelial TF‐deficient mice.
Fig. S2. Macrophage recruitment into the lung 8 h after acid‐induced acute lung injury (ALI).
Fig. S3. Myeloid TF does not influence leukocyte recruitment during sterile peritonitis.
Fig. S4. No significant changes in IL‐12 and IL‐1β levels between myeloid TF and wild‐type littermates 8 h after acid‐induced ALI.
Fig. S5. Effect of thrombin and FVIIa on the inflammatory response of macrophages.


