Abstract
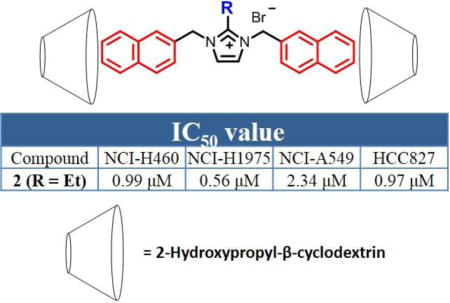
Alkyl- and N,N′-bisnaphthyl-substituted imidazolium salts were tested in vitro for their anti-cancer activity against four non-small cell lung cancer cell lines (NCI–H460, NCI–H1975, HCC827, A549). All compounds had potent anticancer activity with 2 having IC50 values in the nanomolar range for three of the four cell lines, a 17-fold increase in activity against NCI-H1975 cells when compared to cisplatin. Compounds 1–4 also showed high anti-cancer activity against nine NSCLC cell lines in the NCI-60 human tumor cell line screen. In vitro studies performed using the Annexin V and JC-1 assays suggested that NCI-H460 cells treated with 2 undergo an apoptotic cell death pathway and that mitochondria could be the cellular target of 2 with the mechanism of action possibly related to a disruption of the mitochondrial membrane potential. The water solubilities of 1–4 was over 4.4 mg/mL using 2-hydroxypropyl-β-cyclodextrin as a chemical excipient, thereby providing sufficient solubility for systemic administration.
Keywords: Imidazolium salt, Anti-cancer, Anti-tumor, Lung cancer, Cyclodextrin
Graphical abstract
Lung cancer causes more deaths than any other type of cancer, and it is estimated that there will be over 158,000 deaths in the United States due to lung cancer in 2016.1 Lung cancer is classified into two types, small cell and non-small cell lung carcinoma (NSCLC), the latter of which makes up 83% of lung cancers. The current treatments for NSCLC include surgery, radiation, and chemotherapy.2 Many chemotherapeutic strategies include the use of a combination of organic-based drugs and metal-based platinum drugs. Among the platinum-based therapeutics, cisplatin is the most commonly prescribed as a first line treatment for NSCLC.3 However, treatment with platinum-based drugs commonly elicits severe adverse side effects, including nephrotoxicity (e.g., 20% of patients treated with high doses of cisplatin undergo severe renal dysfunction).4 Patient relapse may also occur as malignant cells become resistant to treatment with cisplatin.5 The five-year survival rate for patients with NSCLC is only 21%; whereas, the average five-year survival rate for all other cancers is 68%.1 Therefore, there is a clear need for new chemotherapeutic agents that can effectively target this form of cancer while offering a more favorable side effect profile.
During our group’s investigation of the anti-tumor activity of silver carbene complexes, it was discovered that certain imidazolium salt precursors had very high anti-tumor activity against select NSCLC cell lines.6 Imidazolium salts with a naphthylmethyl at the N3 position had high anti-cancer activity as part of hybrid imidazolium salt compounds.7–9 However, imidazolium salts with naphthylmethyl substituents at both the N1 and N3 positions have yielded the highest activity observed in our research thus far.10–13 The first imidazolium salt of this class with high anti-cancer activity against several NSCLC lines was 4,5-dichloro-1,3-bis(naphthalen-2-ylmethyl)imidazolium bromide (IS23; Figure 1). Although IS23 displayed high anticancer activity, comparable to the chemotherapeutic agent cisplatin, it had extremely poor water solubility, which would impair its distribution upon systemic administration.10 The balance between water solubility and high anti-cancer activity has represented a major obstacle for the potential clinical use of lipophilic imidazolium salts.
Figure 1.
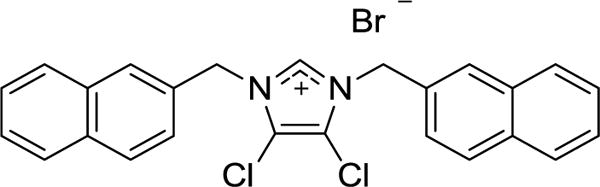
Structure of 4,5-dichloro-1,3-bis(naphthalen-2-ylmethyl)imidazolium bromide (IS23).
Although it has been shown that the quinolylmethyl substituent at the N1 or N3 position could be used to increase solubility and not severely alter anti-cancer activity,11–13 the highest anti-cancer activities were observed with the most lipophilic compounds containing two naphthylmethyl substituents. Therefore, molecular excipients such as cyclodextrins (CD), which have been used to improve drug solubility and the bioavailability of such compounds,14 were considered to aid in solubilizing highly lipophilic and highly active compounds. Cyclodextrins have a hydrophobic internal cavity that can interact with hydrophobic drugs of certain size and polarity.15 Also, cyclodextrins have two types of alcoholic functional groups that make up a hydrophilic outer shell. Together, these two properties allow a host-guest interaction to form between the cyclodextrin and the hydrophobic drug that can be solubilized in aqueous media.16 Cyclodextrins are generally regarded as safe (GRAS) by the Food and Drug Administration (FDA) for use as food additives and as a part of several major drug formulations.17
A series of imidazolium salts bearing 2-naphthylmethyl substituents at the N1 and N3 positions and varying alkyl substituents at the C2 position have been synthesized. All compounds have been tested for in vitro efficacy to create a structure-activity relationship (SAR) with and without the use of cyclodextrin. These compounds display anticancer activity comparable to that of IS23 and cisplatin, with significantly increased solubility when combined with cyclodextrin.
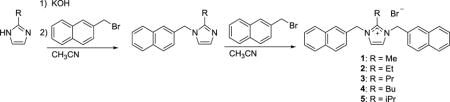
Compounds 1–5 were synthesized by the general synthetic route outlined in Equation 1 based upon previously published procedures.10,18 The starting imidazole was deprotonated with a slight excess of potassium hydroxide followed by addition of 2-(bromomethyl)naphthalene. The mixture was refluxed overnight, which resulted in formation of a white precipitate (presumed to be potassium bromide) that was removed by filtration. 2-(Bromomethyl)naphthalene was added to the filtrate and the mixture was refluxed overnight. Compounds 1 and 3 precipitated from hot acetonitrile; whereas, compounds 2, 4, and 5 precipitated after the addition of diethyl ether to the reaction mixture. All compounds were washed with diethyl ether to remove any excess 2-(bromomethyl)naphthalene. Compound 2 was recrystallized from ethanol; whereas, compounds 3, 4, and 5 were recrystallized from acetonitrile.
All compounds were characterized by 1H and 13C NMR spectroscopy, mass spectrometry, elemental analysis, and melting point determination. Additionally, compounds 1, 2, 4, 5, and the cationic portion of 3, as the perchlorate salt, were characterized by single-crystal X-ray crystallography. In the 1H NMR spectra, the chemical shifts of the methylene linkers bridging the imidazole ring and the naphthalene rings were used as evidence for the formation of the imidazolium salts. The chemical shift of the methylene linker in the mono-naphthylated intermediates ranged from 5.32 ppm to 5.35 ppm. The second naphthylation and resulting formation of the desired imidazolium salt was indicated by an increase in the integration of this methylene resonance and a downfield shift due to the deshielding effect of the cationic imidazolium ring. Compounds 1–5 had chemical shifts in the range from 5.62 ppm to 5.81 ppm for this methylene linker, which was consistent with related compounds.10,19,20 The 13C NMR spectra for compounds 1–5 were consistent with the proposed structures.
Compounds 1, 2, 4, 5, and the perchlorate salt derivative of 3 (3-ClO4) were characterized by single-crystal X-ray crystallography. A single crystal of 1 (Figure 2) was grown from a combination of methanol and ethyl acetate; whereas, a single crystal of 5 (Figure 3) was grown from acetonitrile. Single crystals of 2 and 4 suitable for single crystal X-ray analysis were obtained by slow evaporation of an ethanol solution (Figure 4 and Figure 5). Two molecules of compound 4 co-crystallized with one molecule of ethanol. Single crystals of 3 could not be obtained. Anion exchange of bromide for perchlorate was performed by dissolving compound 3 in methanol and adding silver perchlorate. A precipitate formed, presumed to be silver bromide, and was removed by filtration. A single crystal of 3-ClO4, was obtained from methanol/diethyl ether at −5 °C (Figure 6).
Figure 2.
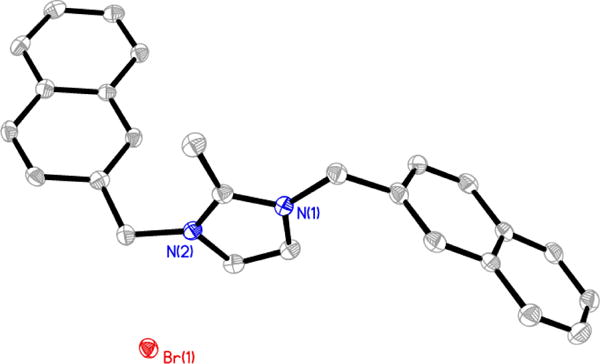
Thermal ellipsoid plot of 1 with thermal ellipsoids drawn at 50% probability. Hydrogen atoms and carbon labels have been removed for clarity.
Figure 3.
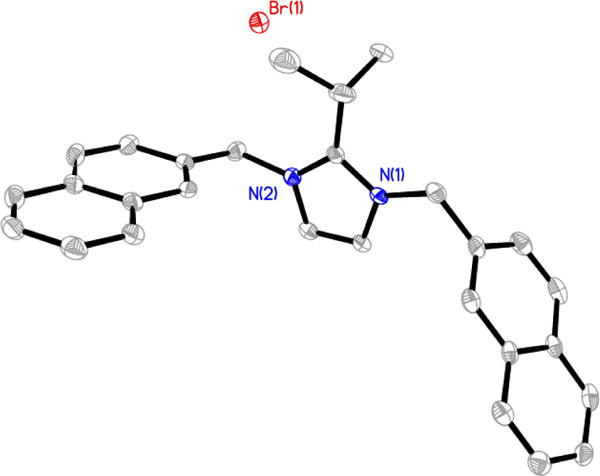
Thermal ellipsoid plot of 5 with thermal ellipsoids drawn at 50% probability. Hydrogen atoms and carbon labels have been removed for clarity.
Figure 4.
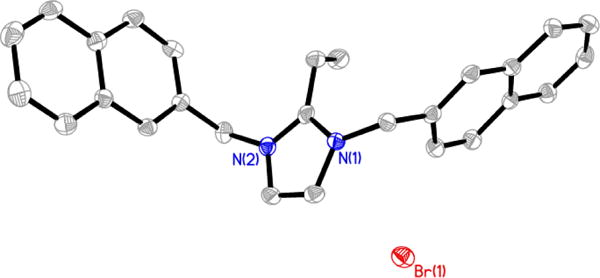
Thermal ellipsoid plot of 2 with thermal ellipsoids drawn at 50% probability. Hydrogen atoms and carbon labels have been removed for clarity.
Figure 5.
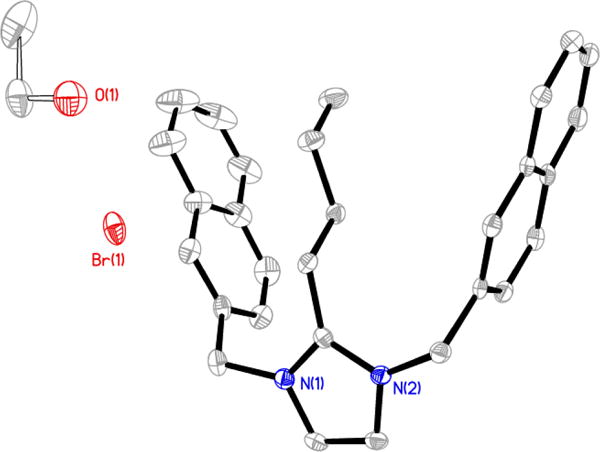
Thermal ellipsoid plot of 4-C2H6O with thermal ellipsoids drawn at 50% probability. Hydrogen atoms and carbon labels have been removed for clarity.
Figure 6.
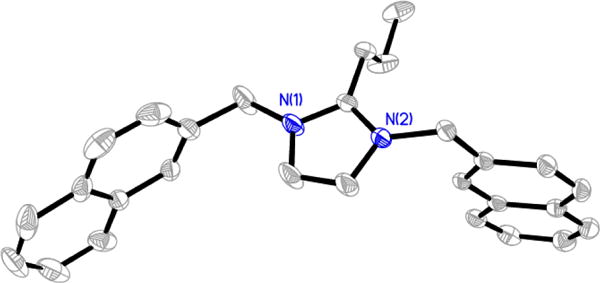
Thermal ellipsoid plot of 3-ClO4, the cationic portion of 3 with a perchlorate anion. Thermal ellipsoids are drawn at 50% probability. Hydrogen atoms, the disordered perchlorate anion, and carbon labels have been removed for clarity.
The anti-cancer activity of compounds 1 and 4 (Figure 7 and Table 1) and compounds 2, 3, and 5 (Figure 7, Figure 8, and Table 1) were evaluated against several NSCLC cell lines (NCI-H460, NCI-H1975, A549, and HCC827) to create a structure-activity relationship (SAR). Cells were exposed to compounds 1–5 or cisplatin for 72 hours, at which time the MTT assay was utilized to determine cell viability. The anti-proliferative effects of compounds 1–5 were evaluated by their IC50 values (the IC50 value denotes the drug concentration at which there was 50% inhibition in cell viability relative to control cells). The major disadvantage of imidazolium salts with naphthylmethyl substituents on both nitrogen atoms is their low water solubility.10–13,18 Compounds 1–5 continued to manifest suboptimal water solubility with aqueous solubilities of less than 0.5 mg/mL. Therefore, solutions of each compound were prepared by first dissolving the compound in DMSO and then diluting with water to a final concentration of 1% DMSO in water. Compounds were further diluted into growth medium with a maximum DMSO concentration of 0.032% that was added to the cells. A solution of cisplatin was prepared by adding the compound to water and stirring at room temperature for several hours.
Figure 7.
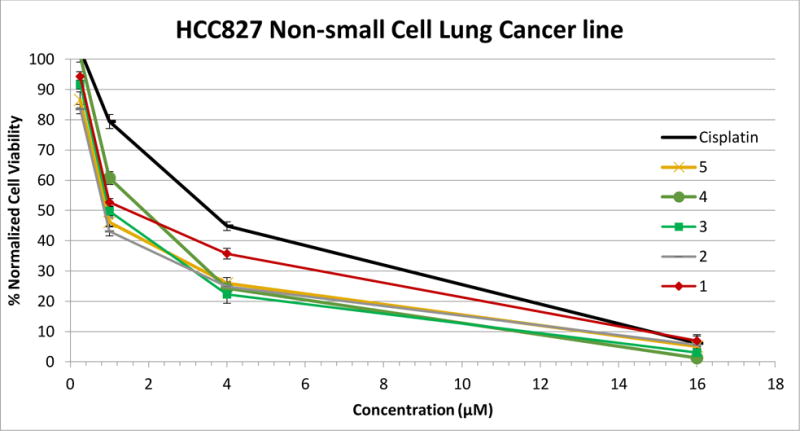
Plot of MTT results from MTT assay of compounds 1–5 and cisplatin against the HCC827 NSCLC line.
Table 1.
IC50 values of compounds 1–5, cisplatin, and IS23 dissolved in 1% DMSO/water solution and a 10% by weight solution of 2-HPβCD.
| IC50 value after 72 hours (μM) | ||||
|---|---|---|---|---|
| Compound | Cancer Cell Line | |||
| NCI-H460 | NCI-H1975 | NCI-A549 | HCC827 | |
| 1 (Me w/DMSO) | 2.53 ± 0.09 | 0.83 ± 0.02 | 3.42 ± 0.10 | 2.34 ± 0.48 |
| 1 (Me w/2-HPβCD) | 2.70 ± 0.11 | 0.75 ± 0.02 | 3.43 ± 0.19 | 1.98 ± 0.39 |
| 2 (Et w/DMSO) | 0.92 ± 0.01 | 0.66 ± 0.01 | 2.63 ± 0.07 | 1.15 ± 0.18 |
| 2 (Et w/2-HPβCD) | 0.99 ± 0.08 | 0.56 ± 0.04 | 2.34 ± 0.19 | 0.97 ± 0.17 |
| 3 (Pr w/DMSO) | 1.91 ± 0.24 | 0.85 ± 0.07 | 2.93 ± 0.03 | 1.45 ± 0.27 |
| 3 (Pr w/2-HPβCD) | 2.13 ± 0.06 | 0.63 ± 0.12 | 2.81 ± 0.11 | 1.25 ± 0.27 |
| 4 (Bu w/DMSO) | 2.62 ±0.04 | 1.74 ± 0.13 | 3.50 ± 0.06 | 2.64 ± 0.10 |
| 4 (Bu w/2-HPβCD) | 2.43 ± 0.06 | 1.02 ± 0.07 | 3.25 ± 0.14 | 2.18 ± 0.31 |
| 5 (iPr w/DMSO) | 0.92 ± 0.03 | 0.69 ± 0.01 | 2.54 ± 0.05 | 1.29 ± 0.22 |
| 5 (iPr w/2-HPβCD) | 0.86 ± 0.03 | 0.62 ± 0.03 | 2.13 ± 0.26 | 1.14 ± 0.32 |
| cisplatin | 2.92 ± 0.34 | 9.77 ± 0.41 | 5.63 ± 0.49 | 4.76 ± 0.87 |
| IS23 (DMSO)* | 5 | 5 | 9 | 6 |
Results taken from reference Wright et al. 2015
Figure 8.
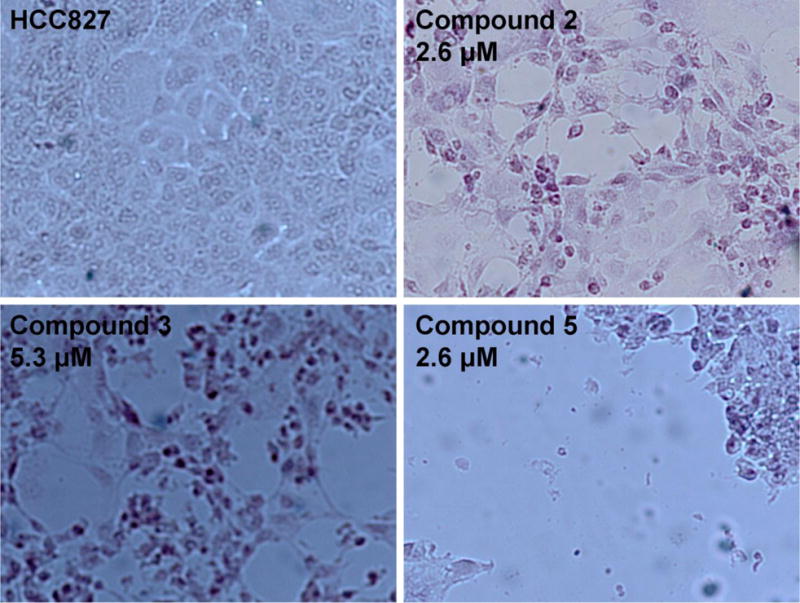
Microscopic appearance of HCC827 cells after 72 hours of exposure to medium alone (upper left panel) or to compounds 2, 3, or 5 at the indicated concentrations
Compounds 2, 3, and 5 exhibited higher solubility in the water/DMSO solution than compounds 1 and 4. Compounds 2, 3, and 5 remained soluble once water was added to the DMSO solution; whereas, slight precipitation was observed upon addition of water to the DMSO solutions of compounds 1 and 4. Although the structure of 1 would suggest it was the least lipophilic, the compound crystallizes readily from a variety of organic solvents suggesting the compound is tightly packed in its crystalline form and difficult to solubilize. Compounds 2, 3, and 5 likely have higher solubility in water due to the higher number of degrees of freedom in the alkyl chain linkers; whereas, 4 has become too lipophilic to be solubilized even with the further increase in degrees of freedom in the butyl chain. Although the solubility of compounds 1–5 in pure water was low (< 0.5 mg/mL), the anti-proliferative activity of these compounds was measured in order to evaluate the addition of alkyl groups at the C2 position, with naphthylmethyl substituents at the N1 and N3 positions.
Compounds 1–5 demonstrated high activity against all four NSCLC lines, comparable to the well-known chemotherapeutic agent cisplatin and to IS23 (Table 1).10 Although the solubilities of compounds 1–5 varied in the water/DMSO solution, the anti-tumor properties were similar for the methyl, ethyl, propyl, butyl, and the more sterically hindering isopropyl group with IC50 values ranging from 0.66 μM to 3.50 μM. When closely evaluating the IC50 values, 1 and 4 had the highest IC50 values against each of the four NCSLC cell lines. This suggests that the poor solubility may have played a role in the anti-cancer properties. In previous studies evaluating imidazolium salts with increasing alkyl chain length, the compounds with the longest alkyl chain had the highest anti-cancer activity.10,21 A clear distinction is not made with this small group of compounds and cannot be used to further confirm results seen previously. However, these compounds may be too similar to observes results seen previously with alkyl chains ranging from three to 17 carbons. All five compounds were most effective against the NCI-H1975 cell line with IC50 values ranging from 0.66 μM to 1.74 μM with 2 having 15-fold better activity than cisplatin. These compounds were more active than previously published derivatives with either one or two quinolylmethyl substituents at the N1 and/or N3 positions.13 This information greatly contributes to the SAR we have established with this class of imidazolium salts.
As discussed previously in the case of IS23, naphthylmethyl-substituted imidazolium salts have high anticancer activity towards select NSCLC cell lines. Compounds 1–5 collectively have some of lowest IC50 values of imidazolium salts with two 2-(naphthylmethyl) substituents when compared to those tested previously.10–13 Compounds 1–5 also have similar IC50 values against the A549 cell line compared to imidazolium salts with one naphthylmethyl substituent and one dibenzofuran derivative.9 However, there is no discussion of the water solubility of this compound and only a reference to a review of ionic liquids (Ranke, et al 2007) whose toxicity increased with decreased water solubility.22 These results suggest that added lipophilicity at the 2-position of these imidazolium salts was beneficial for the compound’s anti-proliferative properties when comparing these results to compounds with a proton at the C2 position. The reason for lower IC50 values for compounds 1–5 compared to previously reported analogous compounds is unknown at this time. It is possible that these alkyl substituents at the C2 position enhance lipophilicity enough to allow uptake into the cell by passive diffusion across the membrane at a faster rate, but we have no evidence to confirm this.
To our knowledge, there are no approved drugs on the market that are co-formulated with DMSO and used for the treatment of cancer. Therefore, a different vehicle would be necessary to aid in increasing the water solubility of these potent anti-cancer agents allowing them to be systemically administered. We chose to use the compound 2-hydroxypropyl-β-cyclodextrin (2-HPβCD), the product of a chemical modification of β-cyclodextrin isolated from the digestion of starch by bacteria.17,23 The solubilities of 1–5 were determined with differing amounts of 2-HPβCD in water. All compounds were soluble at amounts greater than 4.4 mg/mL (~ 5.0 mM) in a 20% by weight solution (w/v) of 2-HPβCD in water. However, to minimize the amount of 2-HPβCD used to solubilize the compounds a 10% (w/v) of 2-HPβCD in water. Compounds 1–4 were able to be solubilized at concentration of greater than 4.4 mg/mL in a 10% 2-HPβCD by weight solution in water. This was the initial concentration of 2-HPβCD the compounds were dissolved in prior to further dilution and exposure to cells. Compound 5 was mostly soluble at this concentration, yielding a clear solution with minor insoluble material. By using 2-HPβCD to solubilize compounds 1–4, the water solubilities increased from less than 0.5 mg/mL to over 4.4 mg/mL making them viable candidates for future in vivo studies and as novel chemotherapeutics for the treatment of NSCLC.
The in vitro MTT assay was conducted with compounds 1–5 dissolved in the 2-HPβCD aqueous solution to confirm there was no reduction in the anti-proliferative effects of 1–5. Cells treated with compounds 1–5 were compared to control cells treated with 2-HPβCD to account for any effect the vehicle had on the cells. The maximum concentration of 2-HPβCD in treated wells was 0.008% by weight. The concentration of 2-HPβCD in all control wells was 0.008% by weight. Cells treated with fresh medium were also compared to cells treated with 2-HPβCD and virtually no toxicity was observed (when considering the four different cell lines tested, cells treated with 2-HPβCD grew at rates of 96–106% when compared to cells treated with medium alone).
Compounds 1–5 all displayed high anti-cancer activity when dissolved in 2-HPβCD against all four NSCLC lines tested (Table 1). All IC50 values were comparable to values recorded when dissolving the compounds in DMSO suggesting the vehicle has no effect on the overall in vitro efficacy of these compounds. Although 2-HPβCD is FDA approved and GRAS, we wanted to confirm that it had no effects on the anti-cancer properties of our compounds considering an interaction was necessary for the compounds to be solubilized in water. Studies are underway to better understand the interaction between 2-HPβCD and these imidazolium salts to fine tune the anti-cancer properties as best as possible.
The National Cancer Institute’s (NCI) Developmental Therapeutics Program (DTP) tested 1–4 in their 60 human tumor cell line one-dose and five-dose assays. The 60 human tumor cell line panel consists of nine non-small cell lung cancer lines, two of which we also tested in our laboratory, the NCI-H460 and A549 lines. In the one-dose assay, each cell line is exposed to the tested compound at a single dose (10 μM). Results are given as a growth percentage relative to the initial number of cells at the beginning of the study. Briefly, cells are plated at a density relative to their doubling rate and incubated overnight. Compounds are exposed to the cells at 10 μM for 24 hours. Growth percentage is calculated by comparing the protein density at the end of the experiment to the protein density at the beginning of the experiment. Full experimental details can be found on the NCI’s DTP webpage (https://dtp.cancer.gov/discovery_development/nci-60/methodology.htm).
Results for compounds 1–4 can be found in Table 2. As described above, each compound has high anti-cancer activity against all NSCLC cell lines tested. When averaging the growth percentages for each compound against the nine NSCLC lines tested, a clear pattern can be observed with the anti-cancer properties. As the alkyl chain lengthens, the cells exhibit a lower growth percentage. This suggests that lipophilicity of these compounds is directly correlated with anticancer activity. These results are consistent with previously described naphthylmethyl-substituted imidazolium salts with a variety of substituents at the C4 and C5 positions.12
Table 2.
Growth % values for NSCLC cell lines treated with 1–4 in the NCI-60 human tumor cell line one-dose assay.
| Compound | Cell Line | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A549/ATCC | EKVX | HOP-62 | HOP-92 | NCI-H226 | NCI-H23 | NCI-H322M | NCI-H460 | NCI-H522 | Average | |
| 1 | 42.30 | 46.99 | 18.00 | −20.33 | 18.99 | 5.53 | 48.92 | 9.12 | n/a | 21.19 |
| 2 | 28.54 | 11.92 | 11.94 | −31.42 | 6.75 | −14.57 | 44.31 | 5.54 | −25.40 | 4.18 |
| 3 | 31.63 | 21.10 | 5.82 | −38.34 | 0.23 | −28.22 | 23.19 | 3.26 | n/a | 2.33 |
| 4 | 29.75 | 14.36 | −2.71 | −38.22 | −0.47 | −23.78 | 23.26 | −13.07 | n/a | −1.36 |
Compounds 1–4 were considered highly active in the one-dose assay and also tested in the five-dose assay by the NCI’s DTP. In this assay, cells are exposed to compounds at concentrations of 10 nM, 100 nM, 1 μM, 10 μM, and 100 μM. Results for this assay are given as GI50, growth inhibition of 50% of cell relative to control cells; TGI, total growth inhibition relation to control cells; and LC50, lethal concentration for 50% of cells relative to control cells. Results are summarized in Table 3 and full plots for the NSCLC cell lines exposed to each compound can be found in Figure 9. All GI50 values in are in the mid nanomolar range to low micromolar range. TGI values range from 1.44 μM to 21.3 μM. Finally, LC50 values range from the low micromolar concentration to greater than 100 μM. Only two LC50 values were given for 4 with results from the NCI-H23 and NCI-H322M cell lines. These results are not surprising with all GI50 values in the mid nanomolar to low micromolar range considering these results were similar to the IC50 values found by our MTT experiments. These results also suggest that these imidazolium salts are capable of completely inhibiting the growth of NSCLC lung cancer cells and killing NSCLC cells at higher concentrations. The results are promising from the five-dose assay and confirm that these are potent anti-cancer agents against NSCLC.
Table 3.
Results from the NCI-60 human tumor cell line five-dose assay for compounds 1–4. GI50, TGI, and LC50 values are given in μM concentrations.
| Cell Line | 1 | 2 | 3 | 4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GI50 | TGI | LC50 | GI50 | TGI | LC50 | GI50 | TGI | LC50 | GI50 | TGI | LC50 | |
| A549/ATCC | 3.81 | 21.3 | 66.1 | 2.59 | 13.9 | 57.9 | 2.67 | 12.1 | 60.3 | 1.67 | 3.24 | n/a |
| EKVZ | 2.32 | 17.1 | 52.4 | 0.766 | 5.55 | 35.8 | 1.71 | 11.4 | 60.5 | 1.35 | 3.47 | n/a |
| HOP-62 | 1.60 | 8.35 | 45.3 | 1.42 | 3.65 | 9.36 | 1.26 | 2.80 | 6.19 | 1.29 | 2.75 | n/a |
| HOP-92 | 0.673 | 3.64 | 26.4 | 0.338 | 1.77 | 1.77 | 0.863 | 2.66 | 7.58 | 0.844 | 2.35 | n/a |
| NCI-H226 | 1.85 | 10.4 | 64.1 | 1.08 | 3.82 | 3.82 | 1.31 | 3.92 | > 100 | 1.35 | 2.93 | n/a |
| NCI-H23 | 0.717 | 2.93 | 10.4 | 0.396 | 2.02 | 2.02 | 0.521 | 2.51 | 9.77 | 0.793 | 2.31 | 5.85 |
| NCI-H322M | 2.46 | 10.4 | 32.9 | 2.12 | 6.71 | 6.71 | 1.96 | 6.00 | 23.8 | 1.38 | 2.75 | 5.48 |
| NCI-H460 | 1.59 | 10.7 | 44.0 | 0.724 | 2.15 | 2.15 | 1.22 | 3.01 | 7.44 | 1.25 | 2.82 | n/a |
| NCI-H522 | 0.256 | 1.81 | 6.72 | 0.303 | 1.44 | 1.44 | 0.378 | 1.68 | 4.92 | 0.491 | 1.89 | n/a |
Figure 9.
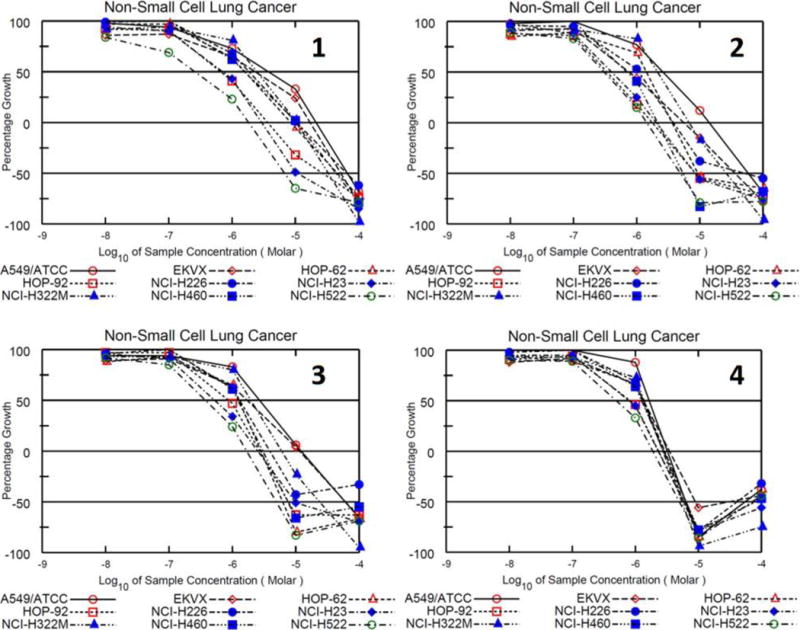
Growth percentage plot of NSCLC cell lines exposed to 1–4 in the NCI-60 cell line screen 5-dose assay.
Little is known about the mechanism of cell death upon treatment with the various imidazolium salts with naphthylmethyl substituents that have been reported thus far. It was reported previously that IS23 and a C4 and C5 hydrogen-substituted derivative were able to induce apoptosis in the NCI-H460 cell line.10,12 An Annexin V apoptosis detection kit was utilized to determine if 2 caused apoptosis or if cellular death resulted from necrosis. In cells that undergo apoptosis, phosphatidylserine (PS) is detected on the outside of the cellular membrane.24 This occurs during the early stages of apoptosis because cell membrane asymmetry and integrity is lost allowing PS to translocate to the outer leaflet of the cell membrane where it can interact with Annexin V, which is conjugated to FITC (fluorescein isothiocyanate) to give fluorescence detectable by microscopy. In order to fully distinguish apoptosis from necrosis, a secondary stain is necessary. Propidium iodide (PI), which interacts with DNA, is a cell membrane impermeable compound. It only has the ability to enter cells that have compromised membranes, which occurs later in the process of apoptosis.24 The progression in time of cells from having strictly green fluorescence to green and red fluorescence is indicative of apoptosis. On the other hand, necrotic cells would be visualized with both green and red fluorescence even at early time points.
The Annexin V assay was performed with compound 2 to determine if adding an alkyl group to the C2 position would influence the initiation of cellular death. For example, morphological changes such as cell shrinkage, rounding, and detachment occurred in H460 cells within the first hour when treated with IS23, but morphological changes and strong green fluorescence suggesting apoptosis were not apparent with the C4 and C5 hydrogen-substituted derivative until 3 hours. These results indicate that subtle changes to the imidazole scaffold can have drastic effects on anti-proliferative efficacy. Strong green fluorescence was observed for 2 when dissolved in a water/DMSO solution (the stock solution was prepared the same as in the MTT assay), at time points similar to that of IS23 (i.e., 1 hour). However, when 2 was dissolved in the 10% by weight 2-HPβCD aqueous solution, strong green fluorescence was not observed until the 12-hour time point (see supplementary data).
The presence of blebbing, is another indicator of the apoptotic mode of cell death. Compound 2 caused blebbing when administered with either DMSO or 2-HPβCD as the solubilizing vehicle. However, a stark contrast can be seen in the time frames of when this occurs. When in DMSO, blebbing occurs at 6 hours of treatment; whereas, blebbing was not seen until 14 hours of treatment when 2 was dissolved with 2-HPβCD (Figure 10 and Figure 11). This suggests that 2 not only causes apoptosis in two independent Annexin V trials, but that the vehicle used to administer it has an effect on compound availability and timely progression of cell fate.
Figure 10.
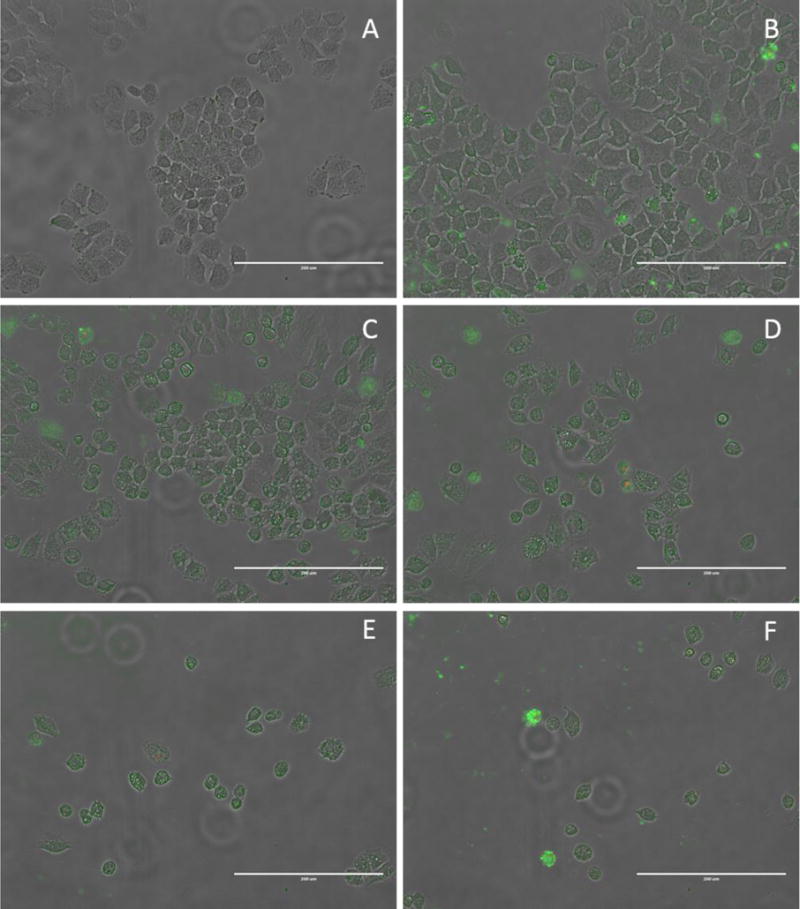
Images of the Annexin V assay on H460 cells grown in 6-well plates using compound 2 in 10% by weight 2-HPβCD aqueous solution as the compound treatment. All images taken using a 20× objective. Images are presented as a merged image of the normal transmitted light, green fluorescence and red fluorescence figures. (A) 2-HPβCD control, 20 hours. (B) Cisplatin control, 20 hours. (C) Compound 2, 12 hour. (D) Compound 2, 14 hours. (E) Compound 2, 17 hours. (F) Compound 2, 20 hours. Scale bars equal 200 μm.
Figure 11.
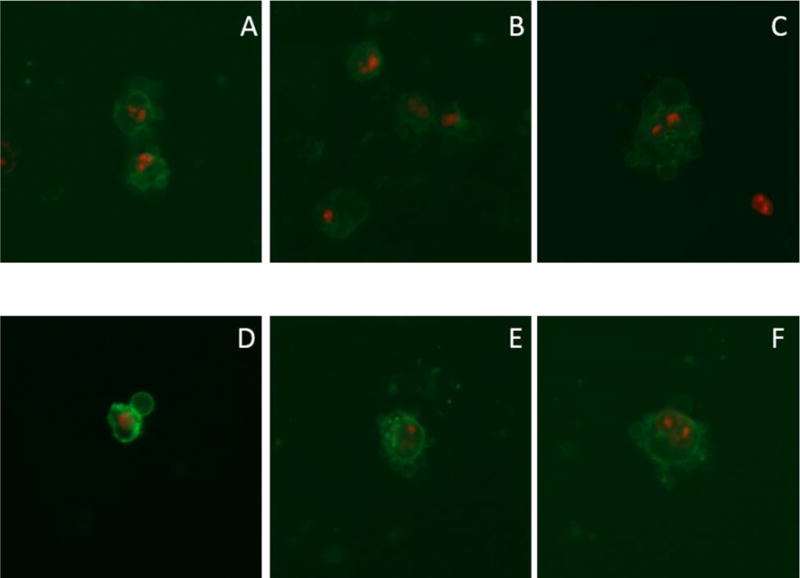
Images of the Annexin V assay on H460 cells grown in 6-well plates using compound 2 in 10% by weight 2-HPβCD aqueous solution as the compound treatment. All images taken using a 20× objective. Images are presented as a merged image of the green fluorescence and red fluorescence figures, omitting the normal transmitted light image for blebbing clarity. Images AC were taken at the 14-hour time point and D–F were taken at the 17-hour time point.
For potential chemotherapeutics, the apoptotic cell death pathway is desirable considering there are increased levels of inflammation and other issues when cells undergo death by a necrotic pathway. It is known that when compounds are dissolved in cyclodextrin solutions there is equilibrium between the freely dissolved compound and the compound in complex with the cyclodextrin. Therefore, the delay in detecting the early stages of apoptosis visualized in cells treated with 2 dissolved in 2-HPβCD compared to 2 dissolved with DMSO was not surprising, and as mentioned above, further studies are underway to better understand this interaction.
Compound 2’s interaction with DNA was investigated by viscosity and Fluroscent interacaltior displacement assays. Results from this assay suggested that 2 had little to no interaction with DNA proposing that DNA in an unlikely intracellular target for 2. Results from these experiments can be found in the supplementary data section. After determining that DNA was not a viable cellular target for 2, the mitochondria was considered as an intracellular target considering it has been previously reported that delocalized lipophilic cations (DLCs) can target the mitochondria of cells.25,26 Imidazolium salts containing naphthylmethyl substituents at the N1 and N3 positions can be classified as DLCs, and thus mitochondria were studied for their potential role in the mechanism of action. The JC-1 assay was utilized to determine if mitochondrial disruption was a potential mechanism of action for 2. JC-1 is a cationic dye that can accumulate in the mitochondria with intact membrane potentials (Figure 12). This mitochondrial membrane potential, or MMP, is caused by a proton gradient across the inner membrane and is important for the production of ATP by the enzyme ATP synthase.27 The formation of J-aggregates by a high concentration of JC-1 in the matrix of mitochondria produces red fluorescence. However, when MMP is disrupted, J-aggregates do not congregate in the matrix and fluorescence is shifted from red to green as the monomer disperses in the cell. Interestingly, the structure of JC-1 shows some similarities to the imidazolium salts presented here.28 Healthy cells with intact MMP will display red fluorescence in the mitochondria with green fluorescence in the cytosol, and cells with disrupted MMP will show green fluorescence with a decrease in the amount of red fluorescence or no red fluorescence at all.
Figure 12.
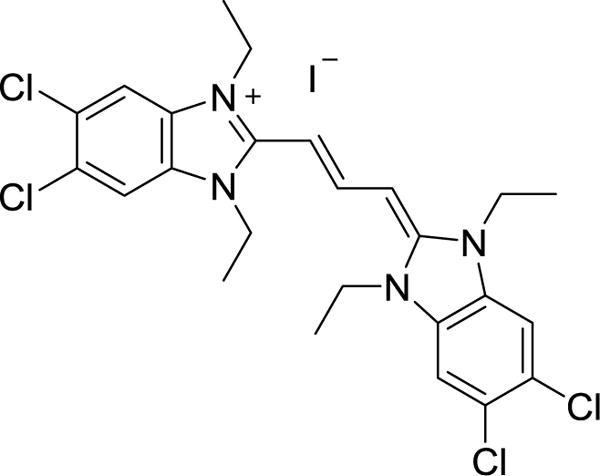
Structure of JC-1.
The H460 cell line was treated with 2 at a concentration of 40 μM (the same as for the Annexin V assay) for 1 hour and 2.5 hours (Annexin V showed blebbing at 3 hours when using DMSO for solubilization). There was minimal red fluorescence observed at either time point. However, cells exposed to the media and vehicle control solutions had intact MMP. This suggested that at a concentration of 40 μM, 2 had disrupted the MMP of the majority of mitochondria by 1 hour. It was presumed that a shorter time frame was necessary to observe the progression of fluorescence from normal MMP to disrupted MMP. In an attempt to observe the progression of fluorescence from normal MMP to disrupted MMP, the H460 cells were treated with 2 at 40 μM for 30 minutes and 1 hour prior to adding JC-1. Unfortunately, the 30-minute time point also showed minimal red fluorescence; whereas, media control cells had red fluorescence suggesting the mitochondria were still intact. Figure 13 shows a comparison of the 30-minute and 1-hour treatment of cells with 2 demonstrating the lack of red fluorescence at both time points.
Figure 13.
Images of the JC-1 assay on H460 cells grown in 35 mm glass bottom dishes using compound 2 at 40 μM in a 1% DMSO aqueous solution as the compound treatment. All images were taken using a 100× objective. Images are presented as a merged image of the normal transmitted light, green fluorescence, blue fluorescence, and red fluorescence figures (merged column) or the red fluorescence alone (J-aggregates column) at the specified time frames. Scale bars equal 50 μm.
In order to visualize the progression of red fluorescence to the lack of red fluorescence at time points feasible for cellular treatment and imaging, the concentration of 2 was decreased from 40 μM to 20 μM. Compound 2 was exposed to cells for 15 minutes and 1 hour at this concentration. Red fluorescence was observed at 15 minutes and the 1 hour time point with an apparent decrease in intensity for cells treated with 2 for 1 hour. A positive control, carbonylcyanide m-chlorophenylhydrazone (CCCP), was used to verify the assay. Similarly to the cells treated with 2, mitochondria potential was decreased compared to the media control cells, as shown by a decrease in red fluorescence signal. Results of this assay suggest that 2 may have disrupted the MMP, visualized by the decrease in observed red fluorescence of cells (Figure 14). To our knowledge, this is the first report to indicate that N,N′-bisnaphthylmethyl imidazolium salts may target the mitochondria of H460 NSCLC cells as part of their mechanism of action. These results, in accordance with the Annexin V assay, give important information regarding the cellular target and mechanism of action for 2 against the NCI-H460 NSCLC cell line. In the JC-1 assay, cells treated with 2 at a concentration of 40 μM were visualized to have completely disrupted MMP by the 30-minute time point. Images from the Annexin V assay, at the same concentration of 2, show green fluorescence of cells with normal morphology at 1 hour and cellular blebbing at 3 hours indicating that MMP disruption occurs prior to the cells going through the process of apoptosis. Further studies to confirm these results include quantification of fluorescence from the JC-1 assay and utilizing other cellular assays to determine mitochondrial reactive oxygen species (ROS) production.
Figure 14.
Images of the JC-1 assay on H460 cells grown in 35 mm glass bottom dishes using compound 2 at 20 μM in 1% DMSO aqueous solution as the compound treatment. All images were taken using a 100× objective. Images are presented as the individual fluorescence images followed by the merged image of the normal transmitted light, green fluorescence, blue fluorescence, and red fluorescence figures. Scale bars equal 50 μm.
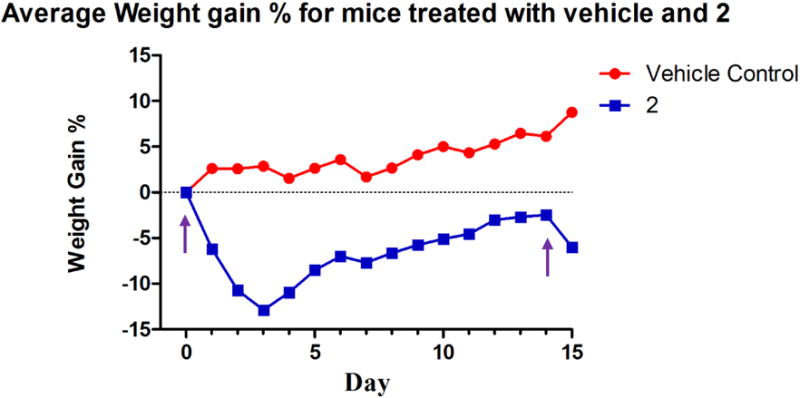
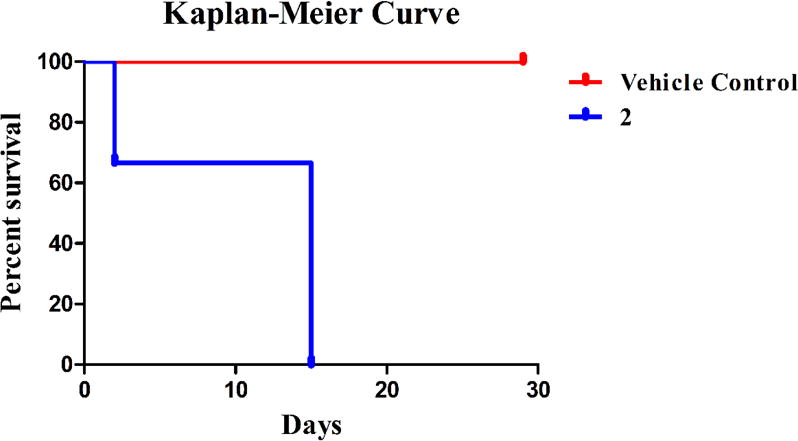
Considering the high anti-cancer activity of 2, the Annexin V results. and the JC-1 results obtained, 2 was chosen for a preliminary in vivo toxicity study using C57BL/6 mice. A 20% 2-HPβCD solution was used to fully solubilize 2 and was used as the vehicle control solution. On day zero, vehicle control animals were injected with 100 μL of 2-HPβCD (20% w/v) solution by intraperitoneal (IP) injection; whereas, the experimental group was injected with 100 μL of a 20 mg/kg dose (assuming an average mass of 20 g for each mouse) of 2 dissolved in the 2-HPβCD (20% w/v) solution by IP injection. Vehicle control mice were also injected on days seven, fourteen, twenty-one, twenty-five, and twenty-nine. Experimental mice were only injected again on day fourteen with a 15 mg/kg dose of 2. Each animal’s behavior and weight were closely monitored for the duration of the study (Figure 15). All mice in the vehicle control group gained weight at a steady pace and survived over the entire course of the study (Figure 16).
Figure 15.
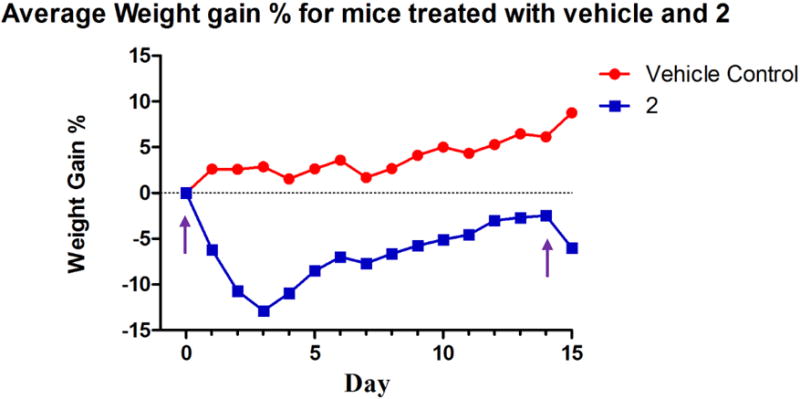
Weight chart for C57BL/6 mice injected with the vehicle control and 2. The purple arrows signify the days mice were injected with 2.
Figure 16.
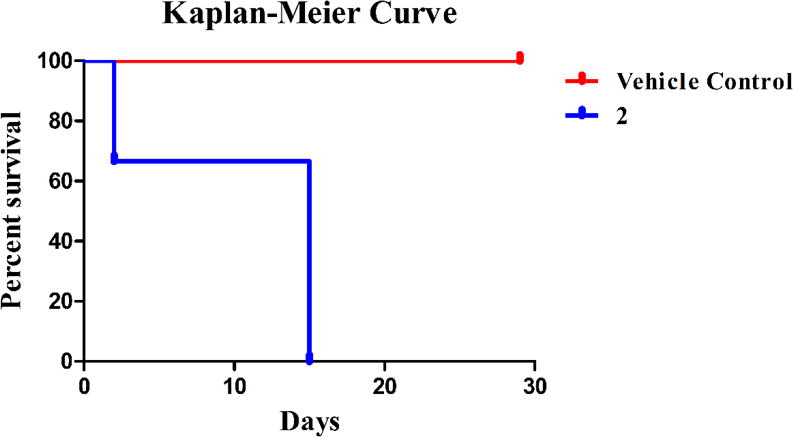
Kaplan-Meier curve describing survival of C57BL/6 mice treated with the vehicle control and 2.
A sharp decrease in weight was observed for all mice treated with 2 after the first injection. On day two, one mouse from the experimental group was found deceased. Both surviving mice treated with 2 began to regain weight on day four and continued this trend until injected with a 15 mg/kg dose on day fourteen. Although average weight loss was around 6%, both animals were sacrificed due to lethargic movement the day after the second injection. Compound 2 proved to be quite toxic at the doses administered. However, this preliminary study results in a starting point for future in vivo studies with 2 involving either a more intensive toxicity study or a lung xenograft model to determine the ability of 2 to inhibit the growth of a tumor and reduce the size of a tumor mass in vivo.
In conclusion, compounds 1–5 all displayed high anti-cancer activity towards several NSCLC cell lines, as determined by the MTT assay, comparable to cisplatin and previously published imidazolium salts with naphthylmethyl substituents. Compounds 1–4 also displayed high anti-proliferative effects in the NCI-60 human tumor cell line screen one-dose assay. Considering the compounds were active in the one dose assay the NCI’s DTP also tested 1–4 in their five-dose assay. All compounds were again highly active in the five-dose assay.
Compound 2 was further tested in an Annexin V assay to determine the mode of cell death. Images from the Annexin V assay suggest an apoptotic mode of cell death similar to related N,N′-bisnapthylmethyl imidazolium salts.10,12 Mechanism of action studies using 2 included in vitro DNA interaction studies and a JC-1 assay. Compound 2 did not show any interaction with DNA by a viscosity or fluorescent intercalator displacement assay. However, a lack of red fluorescence in cells treated with 2 in the JC-1 assay suggested a disruption of the MMP. Understanding the mechanism of action is essential to the progression into clinical settings and suggesting the mitochondria as the cellular target is vital for the progression and use of 2 in future studies.
Unfortunately, these lipophilic imidazolium salts have limited aqueous solubility that would prevent the ability to systemically administer them. However, a chemical excipient, 2-HPβCD, was used to solubilize 1–4 at concentrations of 4.4 mg/mL making them clinically relevant. 2-HPβCD is FDA approved and used in drug formulations. The MTT assay for 1–5 and the Annexin V assay for 2 were also performed with stock solutions of each compound dissolved in a 2-HPβCD solution versus using DMSO. Results from the MTT assay were strikingly similar when using DMSO or 2-HPβCD to solubilize each compound suggesting the excipient does not cause any difference in the anti-cancer activity of the compound. The Annexin V assay using 2 dissolved in 2-HPβCD also suggested an apoptotic mode of cell death, although the time frame was extended when compared to results taken from the compound initially dissolved with DMSO.
A preliminary in vitro toxicity study using 2 suggested the compound was quite toxic at the dose tested causing mortality days after the first injection in one animal. However, this toxicity study provides a starting point for future studies considering this compound is highly cytotoxic towards NSCLC, it could inhibit the growth of a tumor at lower doses without causing severe side effects. Therefore, since 2 could be dissolved at a high concentration with 2-HPβCD and considering we now have some in vitro mechanistic data for 2, it is a prime candidate for future studies including further in vivo toxicity and lung xenograft models.
Supplementary Material
Highlights.
A series of N,N′-bisnaphthylmethyl-2-alkyl imidazolium salts was synthesized
Compounds were characterized by 1H and 13C NMR and X-ray crystallography
All compounds reported have high anti-cancer activity comparable to cisplatin
NCI-60 cell line one-dose and five-dose assays
Mechanism of action studies and in vivo toxicity study
Acknowledgments
The authors would like to thank to NCI’s DTP for performing the 60 human tumor cell line screen with their one-dose and five-dose assays for 1–4. This project has been funded by The University of Akron, the Akron Research Commercialization Corporation, the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (R01-DK082546), and the OMNOVA Foundation. We thank the National Science Foundation (NSF) for providing funds for the purchase of the NMR instruments (Nos. CHE-0341701 and DMR-0414599), mass spectrometers (CHE-0821313 and CHE-1012636) and X-ray diffractometers (CHE-0116041 and CHE-0840446) used in this work.
Abbreviations
- NCI
National Cancer Institute
- IC50
inhibitory concentration 50 %
- NSCLC
non-small cell lung cancer
- IC23
4,5-dichloro-1,3-bis(naphthalen-2-ylmethyl)imidazolium bromide
- MTT
3-(45-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- CD
cyclodextrin
- 2-HPβCD
2-hydroxypropyl-β-cyclodextrin
- FDA
Food and Drug Administration
- PS
phosphatidylserine
- PI
propidium iodide
- FID
fluorescent interacalator displacement
- CT-DNA
calf thymus DNA
- DTP
Developmental Therapeutics Program
- IP
intraperitoneal
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary data
Full details of the Materials and Methods can be found in the supplementary data. The 1H and 13C NMR for 1–5, full plots of the five-dose assays against the nine NSCLC cell lines tested for 1–4, and details of the DNA interaction studies are also included in the supplementary data. Crystallographic Information Files (CIF) for compounds 1, 2, 3-ClO4, 4, and 5 (CCDC #’s 1044144-1044148) can be found on the Cambridge Crystallographic Data Center website. This material is available free of charge via the Internet at http://www.ccdc.cam.ac.uk/pages/Home.aspx.
References
- 1.American Cancer Society. Cancer Facts Fig 2016. Atlanta: American Caner Society; 2016. [Google Scholar]
- 2.Hamilton M, Wolf JL, Drolet DW, Fettner SH, Rakhit AK, Witt K, Lum BL. Cancer Chemother Pharmacol. 2014;73:613–621. doi: 10.1007/s00280-014-2390-3. [DOI] [PubMed] [Google Scholar]
- 3.Wangari-Talbot J, Hopper-Borge E. J Can Res Updat. 2014;2:265–282. doi: 10.6000/1929-2279.2013.02.04.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yao X, Panichpisal K, Kurtzman N, Nugent K. Am J Med Sci. 2007;334:115–124. doi: 10.1097/MAJ.0b013e31812dfe1e. [DOI] [PubMed] [Google Scholar]
- 5.Katano K, Kondo A, Safaei R, Holzer A, Samimi G, Mishima M, Kuo YM, Rochdi M, Howell SB. Cancer Res. 2002;62:6559–6565. [PubMed] [Google Scholar]
- 6.Youngs WJ, Panzner MJ, Deblock MC, Tessier CA, Wright BD, Wagers PO, Robishaw NK. Azolium and purinium salt anticancer and antimicrobial agents 2015. US20140142307. US patent. 2014 May 22;:A1.
- 7.Zhou B, Liu L-X, Deng G-G, Chen W, Li M-Y, Yang L-J, Li Y, Yang X-D, Zhang H-B. Org Biomol Chem. 2016;14:9423–9430. doi: 10.1039/c6ob01495j. [DOI] [PubMed] [Google Scholar]
- 8.Xu X, Yu C, Chen W, Li Y, Yang L-J, Li Y, Zhang H-B, Yang X-D. Org Biomol Chem. 2015;13:1550–1557. doi: 10.1039/c4ob02385d. [DOI] [PubMed] [Google Scholar]
- 9.Sun C-J, Chen W, Li Y, Liu L-X, Wang X-Q, Li L-J, Zhang H-B, Yang X-D. RSC Adv. 2014;4:16312–16319. [Google Scholar]
- 10.Wright BD, Deblock MC, Wagers PO, Duah E, Robishaw NK, Shelton KL, Southerland MR, DeBord MA, Kersten KM, McDonald LJ, Stiel JA, Panzner MJ, Tessier CA, Paruchuri S, Youngs W. Med Chem Res. 2015;24:2838–2861. doi: 10.1007/s00044-015-1330-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shelton KL, Debord MA, Wagers PO, Southerland MR, Williams TM, Robishaw NK, Shriver LP, Tessier CA, Panzner MJ, Youngs WJ, Williams TM, Robishaw NK, Shriver LP, Tessier CA, Panzner MJ, Youngs WJ. Bioorg Med Chem. 2016;25(1):421–439. doi: 10.1016/j.bmc.2016.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shelton KL, Debord MA, Wagers PO, Southerland MR, Taraboletti A, Robishaw NK, Jackson DP, Tosanovic R, Kofron WG, Tessier CA, Paruchuri S, Shriver LP, Panzner MJ, Youngs WJ. Tetrahedron. 2016;72:5729–5743. [Google Scholar]
- 13.Debord MA, Wagers PO, Crabtree SR, Tessier CA, Panzner MJ, Youngs WJ. Bioorg Med Chem Lett. 2016 doi: 10.1016/j.bmcl.2016.11.075. http://dx.doi.org/10.1016/j.bmcl.2016.11.075. [DOI] [PMC free article] [PubMed]
- 14.Mizusako H, Tagami T, Hattori K, Ozeki T. J Pharm Sci. 2015;104:2934–2940. doi: 10.1002/jps.24428. [DOI] [PubMed] [Google Scholar]
- 15.Zarrabi A, Vossoughi M. J Mol Liq. 2015;208:145–150. [Google Scholar]
- 16.Jelić R, Tomović M, Stojanović S, Joksović L, Jakovljević I, Djurdjević P. Monatshefte für Chemie - Chem Mon. 2015;146:1621–1630. [Google Scholar]
- 17.Brewster ME, Loftsson T. Adv Drug Deliv Rev. 2007;59:645–666. doi: 10.1016/j.addr.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 18.Wagers PO, Tiemann KM, Shelton KL, Kofron WG, Panzner MJ, Wooley KL, Youngs WJ, Hunstad DA. Antimicrob Agents Chemother. 2015;59:AAC.00881-15. doi: 10.1128/AAC.00881-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Medvetz DA, Hindi KM, Panzner MJ, Ditto AJ, Yun YH, Youngs WJ. Met Based Drugs. 2008:1–7. doi: 10.1155/2008/384010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ornelas-Megiatto C, Shah PN, Wich PR, Cohen JL, Tagaev JA, Smolen JA, Wright BD, Panzner MJ, Youngs WJ, Fréchet JMJ, Cannon CL. Mol Pharm. 2012;9:3012–3022. doi: 10.1021/mp3004379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malhotra SV, Kumar V. Bioorganic Med Chem Lett. 2010;20:581–585. doi: 10.1016/j.bmcl.2009.11.085. [DOI] [PubMed] [Google Scholar]
- 22.Ranke J, Stolte S, Störmann R, Aming J, Jastorff B. Chem Rev. 2007;107:2183–2206. doi: 10.1021/cr050942s. [DOI] [PubMed] [Google Scholar]
- 23.Loftsson T, Duchêne D. Int J Pharm. 2007;329:1–11. doi: 10.1016/j.ijpharm.2006.10.044. [DOI] [PubMed] [Google Scholar]
- 24.van Engeland M, Nieland LJW, Ramaekers FCS, Schutte B. Reutelingsperger CPM. 1998;31:1–9. doi: 10.1002/(sici)1097-0320(19980101)31:1<1::aid-cyto1>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 25.Kelley SO, Stewart KM, Mourtada R. Pharm Res. 2011;28:2808–2819. doi: 10.1007/s11095-011-0530-6. [DOI] [PubMed] [Google Scholar]
- 26.Kurtoglu M, Lampidis TJ. Mol Nutr Food Res. 2009;53:68–75. doi: 10.1002/mnfr.200700457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wadia JS, Chalmers-Redman RM, Ju WJ, Carlile GW, Phillips JL, Fraser AD, Tatton WG. J Neurosci. 1998;18:932–947. doi: 10.1523/JNEUROSCI.18-03-00932.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smiley ST, Reers M, Mottola-Hartshorn C, Lin M, Chen A, Smith TW, Steele GD, Chen LB. Proc Natl Acad Sci USA. 1991;88:3671–3675. doi: 10.1073/pnas.88.9.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.