Abstract
A nuclease-treated plastid extract from mustard (Sinapis alba L.) allows efficient transcription of cloned plastid DNA templates. In this in vitro system, the major runoff transcript of the truncated gene for the 32 000 mol. wt. photosystem II protein was accurately initiated from a site close to or identical with the in vivo start site. By using plasmids with deletions in the 5'-flanking region of this gene as templates, a DNA region required for efficient and selective initiation was detected ˜28-35 nucleotides upstream of the transcription start site. This region contains the sequence element TTGACA, which matches the consensus sequence for prokaryotic `−35' promoter elements. In the absence of this region, a region ˜13-27 nucleotides upstream of the start site still enables a basic level of specific transcription. This second region contains the sequence element TATATAA, which matches the consensus sequence for the `TATA' box of genes transcribed by RNA polymerase II (or B). The region between the `TATA'-like element and the transcription start site is not sufficient but may be required for specific transcription of the plastid gene. This latter region contains the sequence element TATACT, which resembles the prokaryotic `−10' (Pribnow) box. Based on the structural and transcriptional features of the 5' upstream region, a `promoter switch' mechanism is proposed, which may account for the developmentally regulated expression of this plastid gene.
Keywords: chloroplast gene, 32 000 mol. wt. photosystem II protein, plastid promoter, plastid transcription extract
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bedbrook J. R., Link G., Coen D. M., Bogorad L. Maize plastid gene expressed during photoregulated development. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3060–3064. doi: 10.1073/pnas.75.7.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Blackburn P., Wilson G., Moore S. Ribonuclease inhibitor from human placenta. Purification and properties. J Biol Chem. 1977 Aug 25;252(16):5904–5910. [PubMed] [Google Scholar]
- Bogorad L. Evolution of organelles and eukaryotic genomes. Science. 1975 May 30;188(4191):891–898. doi: 10.1126/science.1138359. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Bottomley W., Smith H. J., Bogorad L. RNA polymerases of maize: partial purification and properties of the chloroplast enzyme. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2412–2416. doi: 10.1073/pnas.68.10.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Briat J. F., Dron M., Loiseaux S., Mache R. Structure and transcription of the spinach chloroplast rDNA leader region. Nucleic Acids Res. 1982 Nov 11;10(21):6865–6878. doi: 10.1093/nar/10.21.6865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambon P. Eukaryotic nuclear RNA polymerases. Annu Rev Biochem. 1975;44:613–638. doi: 10.1146/annurev.bi.44.070175.003145. [DOI] [PubMed] [Google Scholar]
- Dron M., Rahire M., Rochaix J. D. Sequence of the chloroplast DNA region of Chlamydomonas reinhardii containing the gene of the large subunit of ribulose bisphosphate carboxylase and parts of its flanking genes. J Mol Biol. 1982 Dec 25;162(4):775–793. doi: 10.1016/0022-2836(82)90547-2. [DOI] [PubMed] [Google Scholar]
- Garoff H., Ansorge W. Improvements of DNA sequencing gels. Anal Biochem. 1981 Aug;115(2):450–457. doi: 10.1016/0003-2697(81)90031-2. [DOI] [PubMed] [Google Scholar]
- Grosveld G. C., Shewmaker C. K., Jat P., Flavell R. A. Localization of DNA sequences necessary for transcription of the rabbit beta-globin gene in vitro. Cell. 1981 Jul;25(1):215–226. doi: 10.1016/0092-8674(81)90246-4. [DOI] [PubMed] [Google Scholar]
- Gruissem W., Greenberg B. M., Zurawski G., Prescott D. M., Hallick R. B. Biosynthesis of chloroplast transfer RNA in a spinach chloroplast transcription system. Cell. 1983 Dec;35(3 Pt 2):815–828. doi: 10.1016/0092-8674(83)90114-9. [DOI] [PubMed] [Google Scholar]
- Gruissem W., Prescott D. M., Greenberg B. M., Hallick R. B. Transcription of E. coli and Euglena chloroplast tRNA gene clusters and processing of polycistronic transcripts in a HeLa cell-free system. Cell. 1982 Aug;30(1):81–92. doi: 10.1016/0092-8674(82)90014-9. [DOI] [PubMed] [Google Scholar]
- Hartley S. E., McBeath S. Cytogenetic follow-up in chronic myeloid leukemia. Cancer Genet Cytogenet. 1981 Jan;3(1):37–46. doi: 10.1016/0165-4608(81)90054-6. [DOI] [PubMed] [Google Scholar]
- Hawley D. K., McClure W. R. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 1983 Apr 25;11(8):2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschberg J., McIntosh L. Molecular Basis of Herbicide Resistance in Amaranthus hybridus. Science. 1983 Dec 23;222(4630):1346–1349. doi: 10.1126/science.222.4630.1346. [DOI] [PubMed] [Google Scholar]
- Johnson W. C., Moran C. P., Jr, Losick R. Two RNA polymerase sigma factors from Bacillus subtilis discriminate between overlapping promoters for a developmentally regulated gene. Nature. 1983 Apr 28;302(5911):800–804. doi: 10.1038/302800a0. [DOI] [PubMed] [Google Scholar]
- Jolly S. O., Bogorad L. Preferential transcription of cloned maize chloroplast DNA sequences by maize chloroplast RNA polymerase. Proc Natl Acad Sci U S A. 1980 Feb;77(2):822–826. doi: 10.1073/pnas.77.2.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly S. O., McIntosh L., Link G., Bogorad L. Differential transcription in vivo and in vitro of two adjacent maize chloroplast genes: The large subunit of ribulosebisphosphate carboxylase and the 2.2-kilobase gene. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6821–6825. doi: 10.1073/pnas.78.11.6821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd G. H., Bogorad L. Peptide maps comparing subunits of maize chloroplast and type II nuclear DNA-dependent RNA polymerases. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4890–4892. doi: 10.1073/pnas.76.10.4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch W., Edwards K., Kössel H. Sequencing of the 16S-23S spacer in a ribosomal RNA operon of Zea mays chloroplast DNA reveals two split tRNA genes. Cell. 1981 Jul;25(1):203–213. doi: 10.1016/0092-8674(81)90245-2. [DOI] [PubMed] [Google Scholar]
- Koller B., Gingrich J. C., Stiegler G. L., Farley M. A., Delius H., Hallick R. B. Nine introns with conserved boundary sequences in the Euglena gracilis chloroplast ribulose-1,5-bisphosphate carboxylase gene. Cell. 1984 Feb;36(2):545–553. doi: 10.1016/0092-8674(84)90247-2. [DOI] [PubMed] [Google Scholar]
- Krebbers E. T., Larrinua I. M., McIntosh L., Bogorad L. The maize chloroplast genes for the beta and epsilon subunits of the photosynthetic coupling factor CF1 are fused. Nucleic Acids Res. 1982 Aug 25;10(16):4985–5002. doi: 10.1093/nar/10.16.4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumano M., Tomioka N., Sugiura M. The complete nucleotide sequence of a 23S rRNA gene from a blue-green alga, Anacystis nidulans. Gene. 1983 Oct;24(2-3):219–225. doi: 10.1016/0378-1119(83)90082-3. [DOI] [PubMed] [Google Scholar]
- Link D. P., Lantz B. M. Vasodilator response in the lower extremity induced by contrast medium. I Canine model. Acta Radiol Diagn (Stockh) 1982;23(2):81–86. doi: 10.1177/028418518202300201. [DOI] [PubMed] [Google Scholar]
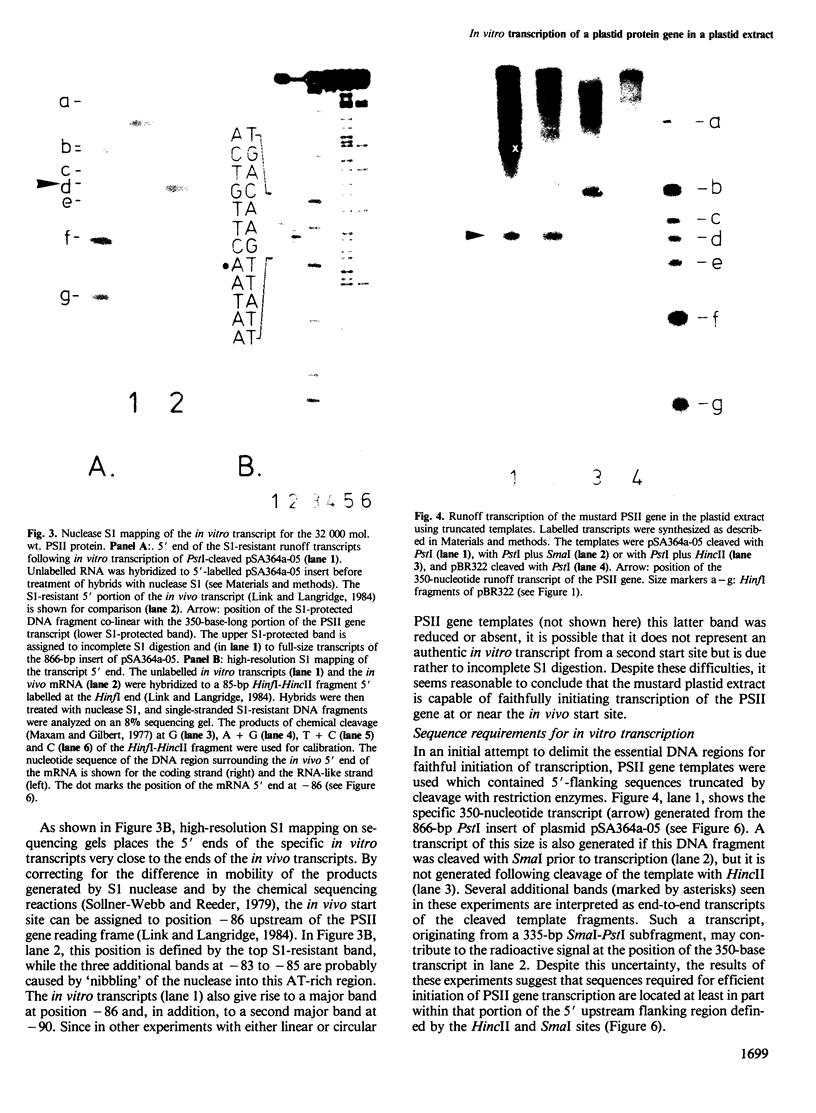
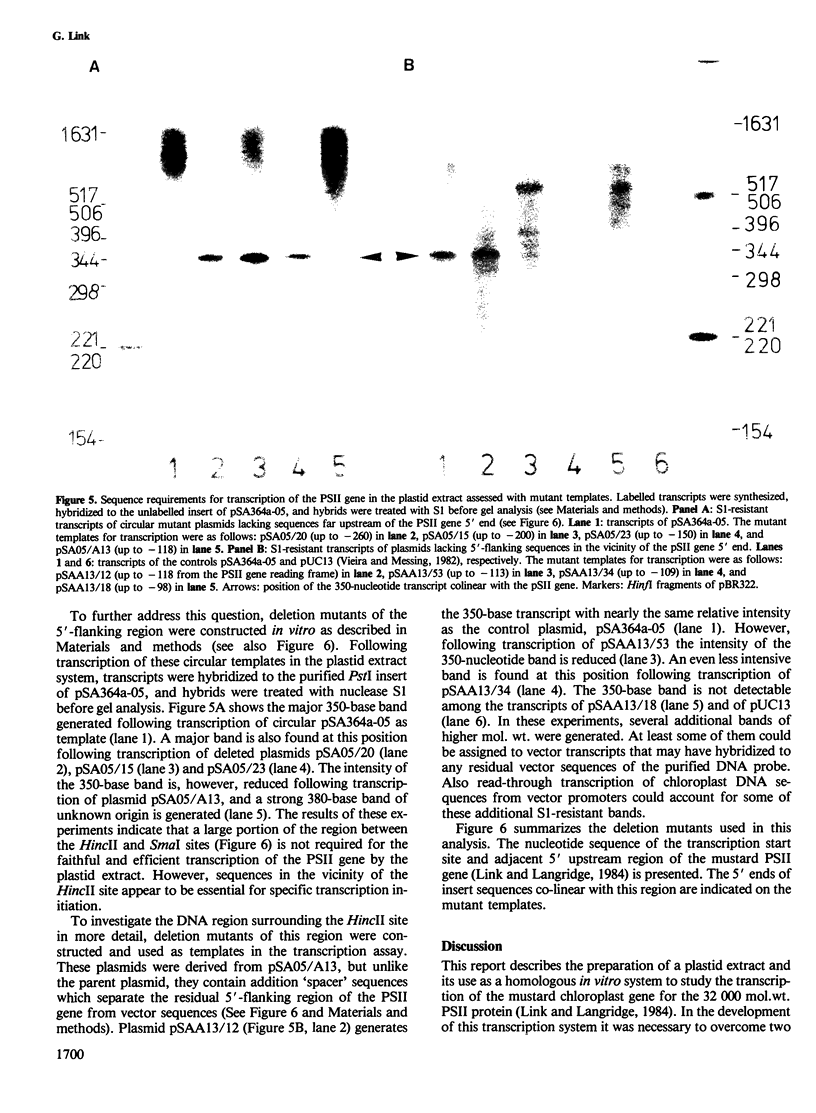
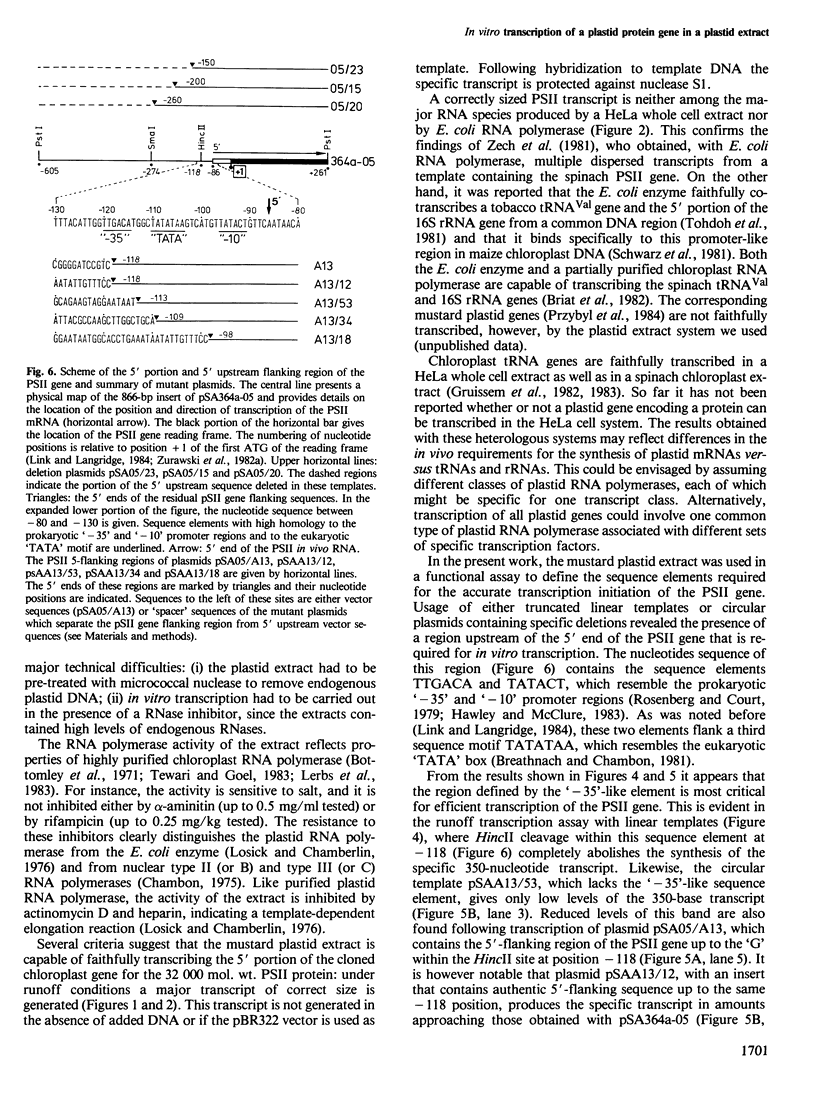
- Link G., Langridge U. Structure of the chloroplast gene for the precursor of the Mr 32,000 photosystem II protein from mustard (Sinapis alba L.). Nucleic Acids Res. 1984 Jan 25;12(2):945–958. doi: 10.1093/nar/12.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonsdale D. M., Hodge T. P., Howe C. J., Stern D. B. Maize mitochondrial DNA contains a sequence homologous to the ribulose-1,5-bisphosphate carboxylase large subunit gene of chloroplast DNA. Cell. 1983 Oct;34(3):1007–1014. doi: 10.1016/0092-8674(83)90558-5. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., van deSande H. Chain length determination of small double- and single-stranded DNA molecules by polyacrylamide gel electrophoresis. Biochemistry. 1975 Aug 26;14(17):3787–3794. doi: 10.1021/bi00688a010. [DOI] [PubMed] [Google Scholar]
- Manley J. L., Fire A., Cano A., Sharp P. A., Gefter M. L. DNA-dependent transcription of adenovirus genes in a soluble whole-cell extract. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3855–3859. doi: 10.1073/pnas.77.7.3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattoo A. K., Pick U., Hoffman-Falk H., Edelman M. The rapidly metabolized 32,000-dalton polypeptide of the chloroplast is the "proteinaceous shield" regulating photosystem II electron transport and mediating diuron herbicide sensitivity. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1572–1576. doi: 10.1073/pnas.78.3.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlino G. T., Tyagi J. S., de Crombrugghe B., Pastan I. Transcription of the chicken alpha 2 (Type I) collagen gene by homologous cell-free extracts. J Biol Chem. 1982 Jun 25;257(12):7254–7261. [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Rochaix J. D., Malnoe P. Anatomy of the chloroplast ribosomal DNA of Chlamydomonas reinhardii. Cell. 1978 Oct;15(2):661–670. doi: 10.1016/0092-8674(78)90034-x. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Sakonju S., Bogenhagen D. F., Brown D. D. A control region in the center of the 5S RNA gene directs specific initiation of transcription: I. The 5' border of the region. Cell. 1980 Jan;19(1):13–25. doi: 10.1016/0092-8674(80)90384-0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. The use of thin acrylamide gels for DNA sequencing. FEBS Lett. 1978 Mar 1;87(1):107–110. doi: 10.1016/0014-5793(78)80145-8. [DOI] [PubMed] [Google Scholar]
- Schwarz Z., Kössel H., Schwarz E., Bogorad L. A gene coding for tRNA is located near 5' terminus of 16S rRNA gene in Zea mays chloroplast genome. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4748–4752. doi: 10.1073/pnas.78.8.4748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki K., Sugiura M. Sequence of the intercistronic region between the ribulose-1, 5-bisphosphate carboxylase/oxygenase large subunit and coupling factor beta subunit gene. Nucleic Acids Res. 1982 Aug 25;10(16):4923–4934. doi: 10.1093/nar/10.16.4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. J., Bogorad L. The polypeptide subunit structure of the DNA-dependent RNA polymerase of Zea mays chloroplasts. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4839–4842. doi: 10.1073/pnas.71.12.4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollner-Webb B., Reeder R. H. The nucleotide sequence of the initiation and termination sites for ribosomal RNA transcription in X. laevis. Cell. 1979 Oct;18(2):485–499. doi: 10.1016/0092-8674(79)90066-7. [DOI] [PubMed] [Google Scholar]
- Spielmann A., Stutz E. Nucleotide sequence of soybean chloroplast DNA regions which contain the psb A and trn H genes and cover the ends of the large single copy region and one end of the inverted repeats. Nucleic Acids Res. 1983 Oct 25;11(20):7157–7167. doi: 10.1093/nar/11.20.7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinback K. E., McIntosh L., Bogorad L., Arntzen C. J. Identification of the triazine receptor protein as a chloroplast gene product. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7463–7467. doi: 10.1073/pnas.78.12.7463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J. G. pBR322 restriction map derived from the DNA sequence: accurate DNA size markers up to 4361 nucleotide pairs long. Nucleic Acids Res. 1978 Aug;5(8):2721–2728. doi: 10.1093/nar/5.8.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohdoh N., Shinozaki K., Sugiura M. Sequence of a putative promoter region for the rRNA genes of tobacco chloroplast DNA. Nucleic Acids Res. 1981 Oct 24;9(20):5399–5406. doi: 10.1093/nar/9.20.5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil P. A., Luse D. S., Segall J., Roeder R. G. Selective and accurate initiation of transcription at the Ad2 major late promotor in a soluble system dependent on purified RNA polymerase II and DNA. Cell. 1979 Oct;18(2):469–484. doi: 10.1016/0092-8674(79)90065-5. [DOI] [PubMed] [Google Scholar]
- Weinbaum S. A., Gressel J., Reisfeld A., Edelman M. Characterization of the 32,000 Dalton Chloroplast Membrane Protein: III. Probing Its Biological Function in Spirodela. Plant Physiol. 1979 Nov;64(5):828–832. doi: 10.1104/pp.64.5.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawski G., Bohnert H. J., Whitfeld P. R., Bottomley W. Nucleotide sequence of the gene for the M(r) 32,000 thylakoid membrane protein from Spinacia oleracea and Nicotiana debneyi predicts a totally conserved primary translation product of M(r) 38,950. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7699–7703. doi: 10.1073/pnas.79.24.7699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawski G., Bottomley W., Whitfeld P. R. Structures of the genes for the beta and epsilon subunits of spinach chloroplast ATPase indicate a dicistronic mRNA and an overlapping translation stop/start signal. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6260–6264. doi: 10.1073/pnas.79.20.6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawski G., Perrot B., Bottomley W., Whitfeld P. R. The structure of the gene for the large subunit of ribulose 1,5-bisphosphate carboxylase from spinach chloroplast DNA. Nucleic Acids Res. 1981 Jul 24;9(14):3251–3270. doi: 10.1093/nar/9.14.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]







