Abstract
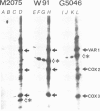
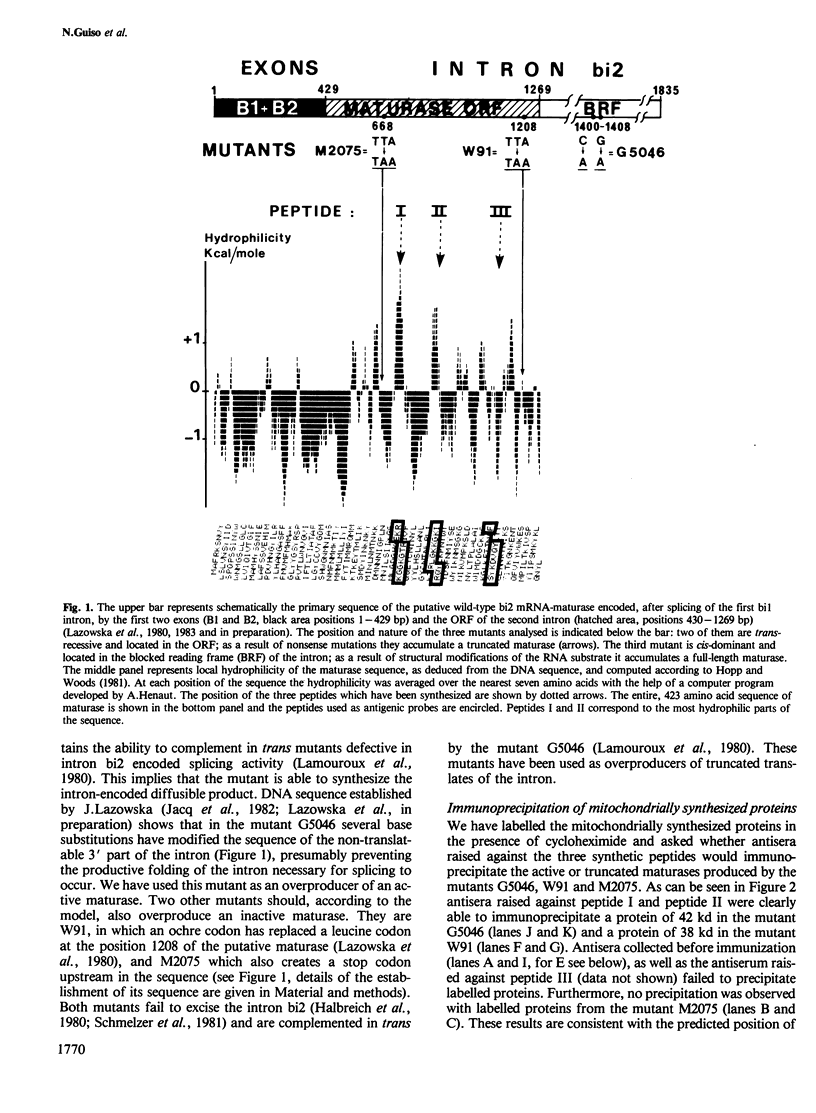
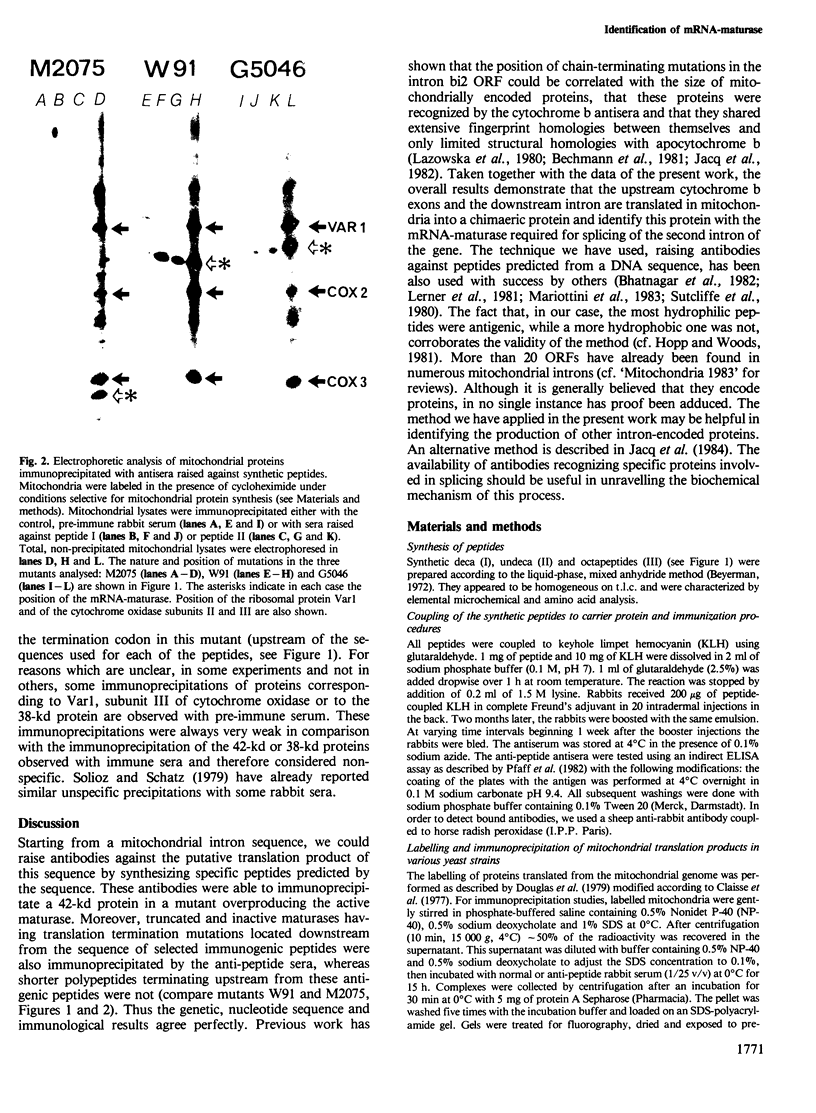
Genetic and biochemical evidence has strongly suggested that several introns located in yeast mitochondrial genes specifying apocytochrome b or cytochrome oxidase encode trans-acting proteins (termed mRNA-maturases) responsible for splicing the cognate intron and maturation of the mRNA. We have chemically synthesized three oligopeptides, predicted from the DNA sequence of the open reading frame (ORF) present in the second intron of the cob-box gene, and raised antibodies against them. These antibodies have allowed us to identify a protein of 42 kd as the product translated from the ORF of the wild-type intron. In two splicing-deficient mutants this protein is replaced by shorter polypeptides whose lengths and antigenic properties are in full agreement with the positions of TAA codons established by the DNA sequence of the intron's ORF.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anziano P. Q., Hanson D. K., Mahler H. R., Perlman P. S. Functional domains in introns: trans-acting and cis-acting regions of intron 4 of the cob gene. Cell. 1982 Oct;30(3):925–932. doi: 10.1016/0092-8674(82)90297-5. [DOI] [PubMed] [Google Scholar]
- Bechmann H., Haid A., Schweyen R. J., Mathews S., Kaudewitz F. Expression of the "split gene" COB in yeast mtDNA. Translation of intervening sequences in mutant strains. J Biol Chem. 1981 Apr 10;256(7):3525–3531. [PubMed] [Google Scholar]
- Bhatnagar P. K., Papas E., Blum H. E., Milich D. R., Nitecki D., Karels M. J., Vyas G. N. Immune response to synthetic peptide analogues of hepatitis B surface antigen specific for the a determinant. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4400–4404. doi: 10.1073/pnas.79.14.4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church G. M., Slonimski P. P., Gilbert W. Pleiotropic mutations within two yeast mitochondrial cytochrome genes block mRNA processing. Cell. 1979 Dec;18(4):1209–1215. doi: 10.1016/0092-8674(79)90233-2. [DOI] [PubMed] [Google Scholar]
- De La Salle H., Jacq C., Slonimski P. P. Critical sequences within mitochondrial introns: pleiotropic mRNA maturase and cis-dominant signals of the box intron controlling reductase and oxidase. Cell. 1982 Apr;28(4):721–732. doi: 10.1016/0092-8674(82)90051-4. [DOI] [PubMed] [Google Scholar]
- Douglas M., Finkelstein D., Butow R. A. Analysis of products of mitochondrial protein synthesis in yeast: genetic and biochemical aspects. Methods Enzymol. 1979;56:58–66. doi: 10.1016/0076-6879(79)56009-1. [DOI] [PubMed] [Google Scholar]
- Groudinsky O., Dujardin G., Slonimski P. P. Long range control circuits within mitochondria and between nucleus and mitochondria. II. Genetic and biochemical analyses of suppressors which selectively alleviate the mitochondrial intron mutations. Mol Gen Genet. 1981;184(3):493–503. doi: 10.1007/BF00352529. [DOI] [PubMed] [Google Scholar]
- Haid A., Schweyen R. J., Bechmann H., Kaudewitz F., Solioz M., Schatz G. The mitochondrial COB region in yeast codes for apocytochrome b and is mosaic. Eur J Biochem. 1979 Mar;94(2):451–464. doi: 10.1111/j.1432-1033.1979.tb12913.x. [DOI] [PubMed] [Google Scholar]
- Halbreich A., Pajot P., Foucher M., Grandchamp C., Slonimski P. A pathway of cytochrome b mRNA processing in yeast mitochondria: specific splicing steps and an intron-derived circular DNA. Cell. 1980 Feb;19(2):321–329. doi: 10.1016/0092-8674(80)90506-1. [DOI] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudspeth M. E., Ainley W. M., Shumard D. S., Butow R. A., Grossman L. I. Location and structure of the var1 gene on yeast mitochondrial DNA: nucleotide sequence of the 40.0 allele. Cell. 1982 Sep;30(2):617–626. doi: 10.1016/0092-8674(82)90258-6. [DOI] [PubMed] [Google Scholar]
- Jacq C., Banroques J., Becam A. M., Slonimski P. P., Guiso N., Danchin A. Antibodies against a fused 'lacZ-yeast mitochondrial intron' gene product allow identification of the mRNA maturase encoded by the fourth intron of the yeast cob-box gene. EMBO J. 1984 Jul;3(7):1567–1572. doi: 10.1002/j.1460-2075.1984.tb02012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazowska J., Jacq C., Slonimski P. P. Sequence of introns and flanking exons in wild-type and box3 mutants of cytochrome b reveals an interlaced splicing protein coded by an intron. Cell. 1980 Nov;22(2 Pt 2):333–348. doi: 10.1016/0092-8674(80)90344-x. [DOI] [PubMed] [Google Scholar]
- Lazowska J., Jacq C., Slonimski P. P. Splice points of the third intron in the yeast mitochondrial cytochrome b gene. Cell. 1981 Nov;27(1 Pt 2):12–14. doi: 10.1016/0092-8674(81)90355-x. [DOI] [PubMed] [Google Scholar]
- Lerner R. A., Green N., Alexander H., Liu F. T., Sutcliffe J. G., Shinnick T. M. Chemically synthesized peptides predicted from the nucleotide sequence of the hepatitis B virus genome elicit antibodies reactive with the native envelope protein of Dane particles. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3403–3407. doi: 10.1073/pnas.78.6.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariottini P., Chomyn A., Attardi G., Trovato D., Strong D. D., Doolittle R. F. Antibodies against synthetic peptides reveal that the unidentified reading frame A6L, overlapping the ATPase 6 gene, is expressed in human mitochondria. Cell. 1983 Apr;32(4):1269–1277. doi: 10.1016/0092-8674(83)90308-2. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Pfaff E., Mussgay M., Böhm H. O., Schulz G. E., Schaller H. Antibodies against a preselected peptide recognize and neutralize foot and mouth disease virus. EMBO J. 1982;1(7):869–874. doi: 10.1002/j.1460-2075.1982.tb01262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelzer C., Haid A., Grosch G., Schweyen R. J., Kaudewitz F. Pathways of transcript splicing in yeast mitochondria. Mutations in intervening sequences of the split gene COB reveal a requirement for intervening sequence-encoded products. J Biol Chem. 1981 Jul 25;256(14):7610–7619. [PubMed] [Google Scholar]
- Solioz M., Schatz G. Mutations in putative intervening sequences of the mitochondrial cytochrome b gene of yeast produce abnormal cytochrome b polypeptides. J Biol Chem. 1979 Oct 10;254(19):9331–9334. [PubMed] [Google Scholar]
- Sutcliffe J. G., Shinnick T. M., Green N., Liu F. T., Niman H. L., Lerner R. A. Chemical synthesis of a polypeptide predicted from nucleotide sequence allows detection of a new retroviral gene product. Nature. 1980 Oct 30;287(5785):801–805. doi: 10.1038/287801a0. [DOI] [PubMed] [Google Scholar]
- Weiss-Brummer B., Rödel G., Schweyen R. J., Kaudewitz F. Expression of the split gene cob in yeast: evidence for a precursor of a "maturase" protein translated from intron 4 and preceding exons. Cell. 1982 Jun;29(2):527–536. doi: 10.1016/0092-8674(82)90169-6. [DOI] [PubMed] [Google Scholar]