Abstract
Background
Adult mammalian hearts have a limited ability to generate new cardiomyocytes. Proliferation of existing adult cardiomyocytes (ACM) is a potential source of new cardiomyocytes. Understanding the fundamental biology of ACM proliferation could be of great clinical significance for treating myocardial infarction (MI). We aim to understand the process and regulation of ACM proliferation and its role in new cardiomyocyte formation of post-MI mouse hearts.
Methods
β-actin-GFP transgenic mice and fate-mapping Myh6-MerCreMer-tdTomato/lacZ mice were used to trace the fate of ACMs. In a co-culture system with neonatal rat ventricular myocytes (NRVMs), ACM proliferation was documented with clear evidence of cytokinesis observed with time-lapse imaging. Cardiomyocyte proliferation in the adult mouse post-MI heart was detected by cell cycle markers and EdU incorporation analysis. Echocardiography was used to measure cardiac function and histology was performed to determine infarction size.
Results
In-vitro, mononucleated and bi/multi-nucleated ACMs were able to proliferate at a similar rate (7.0%) in the co-culture. Dedifferentiation proceeded ACM proliferation, which was followed by redifferentiation. Redifferentiation was essential to endow the daughter cells with cardiomyocyte contractile function. Intercellular propagation of Ca2+ from contracting NRVMs into ACM daughter cells was required to activate the Ca2+ dependent calcineurin-nuclear factor of activated T cells signaling pathway to induce ACM redifferentiation. The properties of NRVM Ca2+ transients influenced the rate of ACM redifferentiation. Hypoxia impaired the function of gap junctions by dephosphorylating its component protein connexin 43, the major mediator of intercellular Ca2+ propagation between cardiomyocytes, thereby impairing ACM redifferentiation. In-vivo, ACM proliferation was found primarily in the MI border zone. An ischemia resistant connexin 43 mutant enhanced the redifferentiation of ACM-derived new cardiomyocytes after MI and improved cardiac function.
Conclusions
Mature ACMs can reenter the cell cycle and form new cardiomyocytes through a three-step process, dedifferentiation, proliferation and redifferentiation. Intercellular Ca2+ signal from neighboring functioning cardiomyocytes through gap junctions induces the redifferentiation process. This novel mechanism contributes to new cardiomyocyte formation in post-MI hearts in mammals.
Keywords: dedifferentiation, proliferation, redifferentiation, myocardial infarction, new cardiomyocyte formation
Introduction
Acute myocardial infarction (MI) is a leading cause of morbidity and mortality throughout the world. MI causes the rapid death of cardiomyocytes with subsequent myocardial remodeling, eventually leading to heart failure. Current clinical therapies are unable to reverse the fundamental problem of reduced cardiomyocyte number.1 Approaches that could safely increase the number of adult cardiomyocytes (ACMs) in the post MI heart would treat the actual cause of cardiac dysfunction.
Many studies have demonstrated that a very small number of new cardiomyocytes are generated in the normal adult hearts of animals and humans,1, 2 and that a few additional new myocytes are generated after cardiac injury.3 The source of these new myocytes has not been clearly defined but accumulating evidence suggests that proliferation of pre-existing cardiomyocytes is largely responsible for endogenous cardiac regeneration.3, 4 Therefore, understanding the biology of ACM proliferation could be of great clinical significance for treating MI, heart failure and other cardiac diseases in which reduced cardiomyocyte number is the principle reason for deranged cardiac function.
The mechanism by which ACMs proliferate in mammals remains poorly understood. What is clear is that most, if not all, ACMs have withdrawn from the cell cycle soon after birth.5, 6 Regeneration could be achieved by these cells reentering the cell cycle or from a small population of ACMs that have not permanently withdrawn from the cell cycle. We7 and others6, 8–11 have indicated that small mono-nucleated ACMs have greater potential to proliferate compared with bi/multi-nucleated and large-sized ACMs. However, due to the limitation of the conventional methodology, these studies did not directly examine cardiomyocyte proliferation with cytokinesis. Mostly, these studies observed the presence of markers for DNA synthesis and cell cycle activation, which could be complicated by DNA repair, polyploidy and multinucleation in ACMs. Lineage tracing and time-lapse imaging were used in the present study, to document ACM proliferation with complete cytokinesis and to explore its biological features.
In lower vertebrate species such as the zebrafish, ACMs proliferate through a process involving dedifferentiation followed by proliferation and subsequent redifferentiation.12 ACM dedifferentiation is characterized by the disassembly of sarcomeric structure, extrusion of mitochondria, electrical uncoupling and expression of regulators of cell cycle progression.12, 13 Redifferentiation is characterized by the restoration of cell morphology, sarcomeric reorganization and contractile function.14 It has been postulated that increased myocyte proliferation is linked to increased myocyte dedifferentiation.15 However, there is little direct evidence for ACM dedifferentiation, proliferation and redifferentiation (DPR) in mammalian hearts, and the putative role of these processes in cardiac regeneration is unclear.
In the present study, we developed an in-vitro model system to observe ACM-DPR with time-lapse imaging. Genetically labeled ACMs (to reliably track their fate) were isolated from adult mouse hearts and cultured with neonatal rat ventricular myocytes (NRVMs). ACMs dedifferentiated, proliferated and then connexin 43 (Cx43) mediated coupling between isolated ACMs and NRVMs was found to be required for ACM redifferentiation. Our data suggest that Ca2+ signals that propagate from NRVMs into electrically coupled and dedifferentiated ACMs, influences ACM redifferentiation. To determine the in-vivo relevance of these mechanisms we used fate-mapping mice with ACM genetic labeling, to study ACM-DPR after MI. These results support the hypothesis that ACM-DPR is an importance source of new ACMs after MI and suggest that enhancing Cx43-mediated cell-cell coupling after MI could increase new ACM formation and improve cardiac pump function.
Methods
Genetic b-actin-GFP and fate-mapping Myh6-MerCreMer-lacZ or Myh6-MerCreMer-tdTomato mice (4 months of age) were used in the present study. Animal care and all experimental procedures were performed in strict accordance with the approved protocols and animal welfare regulations of the Animal Care and Use Committee at Third Military Medical University. An in-vitro co-culture system was developed to mimic the in-vivo ischemia environment in which ACM undergoes dedifferentiate, proliferate and redifferentiate in rodent heart. ACMs were co-cultured onto NRVMs at a ratio of 1:20 and cultured for up to 7 days. A long-term time-lapse imaging analysis was performed to capture ACM division events in the co-culture system with an Olympus IX83 inverted microscope. DRAQ5™ Fluorescent Probe was applied to label ACM nuclei. The time-lapse images were taken at intervals of one hour for 7 days. Calcium transients of NRVMs and redifferentiated ACMs were measured using a protocol similar to our previous studies.16 Permanent ligation of left anterior descending (LAD) coronary artery was performed in these mice to induce MI injury.17 Immunostaining, echocardiography, and infarct size analysis were performed as previously reported.18 Mouse Cx43 mutant with serines 325/328/330 replaced by phosphomimetic glutamic acids (S3E) or by nonphosphorylatable alanines (S3A) were a gift from Dr. Glenn I. Fishman at New York University School of Medicine.19 Cx43-S3E were resistant to pathological gap junction remodeling induced by cardiac ischemia.19 AAV9-Cx43-S3E was administrated to treat MI heart.
Statistical analysis: Statistical analyses were performed using GraphPad Prism 6.0 for Windows (GraphPad Software, San Diego, CA). All values were normally distributed (Kolmogorov-Smirnov test, p>0.10 for each data set). Student’s t-test was performed for comparisons of two groups. One-way or two-way ANOVA (with or without repeated measures) followed by Bonferroni’s correction was performed for multiple group comparisons. Data are given as mean ± SEM, and a p-value of <0.05 was considered significant.
Results
1. ACM dedifferentiation, proliferation and redifferentiation (DPR) in-vitro
Isolated mouse ACMs are subject to [Ca2+] overload induced cell death in primary culture with media containing normal [Ca2+]. Their high [Na+]i favors persistent Ca2+ entry via the Na+/Ca2+ exchanger (NCX) and this Ca2+ entry leads to sarcoplasmic reticulum, and eventually mitochondrial Ca2+ overload, causing cell death.16 In the present study we developed an in-vitro system in which mouse ACMs are co-cultured with neonatal rat ventricular myocytes (NRVMs), to promote ACMs survival for long time periods without using drug treatments to reduce cell Ca2+ (such as 2,3-butanedione monoximine). Cytosolic Ca2+ overload is one of the key mechanisms of ACM death during ischemia and/or reperfusion.20 Thus, this co-culture system, containing Ca2+ overloaded mouse ACMs together with electrically coupled spontaneously beating NRVMs, in some ways simulates the MI border zone where there are ischemic ACMs which have uncoupled from the surviving myocytes and are at high risk of necrotic and/or apoptotic cell death. Since the MI border zone is the region where newly formed cardiomyocytes have been most frequently observed,21 our hypothesis is that ACMs that uncouple from their neighbors might be a source of new cardiomyocytes in the post MI heart.
ACMs from 4-month-old β-actin-GFP transgenic mice were co-cultured with NRVMs at a ratio of 1:20. GFP+/trypan blue− cells were counted as live cells. When cultured alone, mouse ACMs hyper-contracted and more than 84% were trypan blue+ within 3 days. Less than 0.1% of ACMs survived more than 7 days under these conditions.22, 23 In contrast, nearly 50% of mouse ACMs in co-culture with NRVMs were alive through day 7 (Supplemental Figure 1).
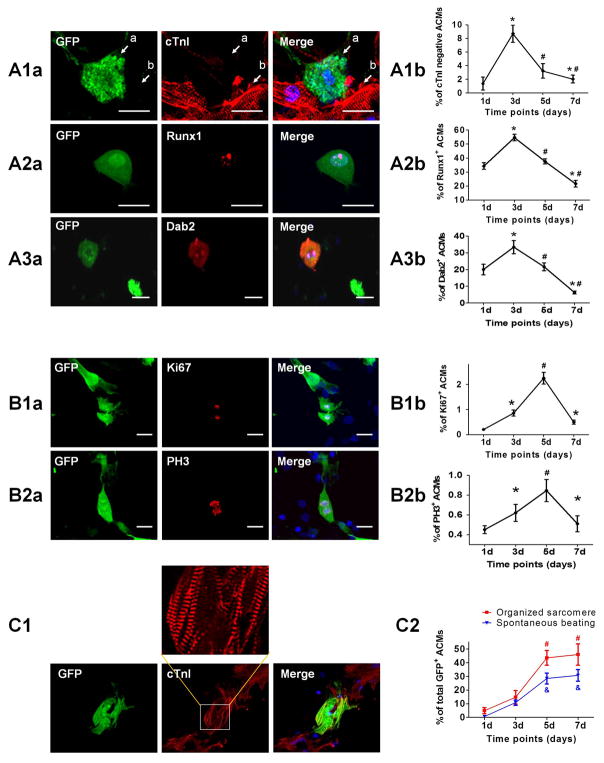
We investigated the properties of surviving ACMs during the first week in co-culture. After 3 days almost all (98.5%) ACMs lost organized sarcomeric structure and among these surviving ACMs, 8.7% did not have cardiac troponin I (cTnI) present, suggesting a greater extent of dedifferentiation (Figure 1A). During this time, some ACMs were immuno-positive for the dedifferentiation markers Runx1 and Dab2. The presence of these markers was observed as early as day 1 in co-culture, reached a peak on day 3 (54.6±2.3% of Runx1+ ACMs and 33.4±4.0% of Dab2+ ACMs) and decreased thereafter (Figure 1A). Collectively these results support the idea that some ACMs actively dedifferentiate in co-culture with NRVMs.
Figure 1. ACM dedifferentiation, proliferation and redifferentiation in-vitro.
Freshly isolated ACMs from β-actin-GFP mice were co-cultured with NRVMs for 7 days, and the ACMs remodeled over time. A: Identification of ACM dedifferentiation. A1: Representative images (A1a) and quantification (A1b) of ACMs that lost the contractile protein cTnI at day 3 after co-culture. Arrow “a” indicates an ACM without cTnI expression, while arrow “b” indicates a neighboring NRVM. A2: Representative images (A2a) and quantification (A2b) of ACMs expressing dedifferentiation marker Runx1. A3: Representative images (A3a) and quantification (A3b) of ACMs expressing dedifferentiation marker Dab2. B: Identification of ACM proliferation. B1: Representative images (B1a) and quantification (B1b) of ACMs expressing proliferation marker Ki67. B2: Representative images (B2a) and quantification (B2b) of ACMs expressing proliferation marker PH3. C: Identification of ACM redifferentiation. C1: Representative images of an ACM regained organized sarcomere at day 7 after co-culture. C2: Quantification of ACMs with organized sarcomeres or spontaneous beating out of total survived ACMs. N=12; * p<0.05 vs. ACMs at day 1; # p<0.05 vs. ACMs at day 3; & p<0.05 vs. % of spontaneous beating at day 3. Scale bars represent 20μm.
Some ACMs in co-culture were immuno-positive for proliferation markers Ki67 and PH3, indicating those cells had reentered the cell cycle (Figure 1B). The expression of these cell cycle indicators has been also recognized as a feature associated with ACM dedifferentiation.12 The expression of Ki67 and PH3 were not detected in the freshly isolated ACMs (day 0), but first seen on day 3 in the co-culture, reached a peak by day 5 (2.25±0.23% and 0.85±0.11%, respectively) and decreased thereafter (Figure 1B).
Dedifferentiated ACMs that had reenterd the cell cycle were mechanically quiescent, without organized contractile proteins. However, with time these ACMs appeared to develop an organized contractile apparatus and were contractile (began at day 3). As shown in Figures 1C, the percentages of sarcomere+ ACMs and beating ACMs were significant on day 5, and reached a peak on day 7 (45.9±7.7% with organized sarcomeres and 30.7±4.3% were beating). These data support the hypothesis that ACMs dedifferentiate, proliferate and then redifferentiate (DPR) in this in-vitro co-culture system.
2. Terminally differentiated mouse ACMs can proliferate and generate new cardiomyocytes in-vitro
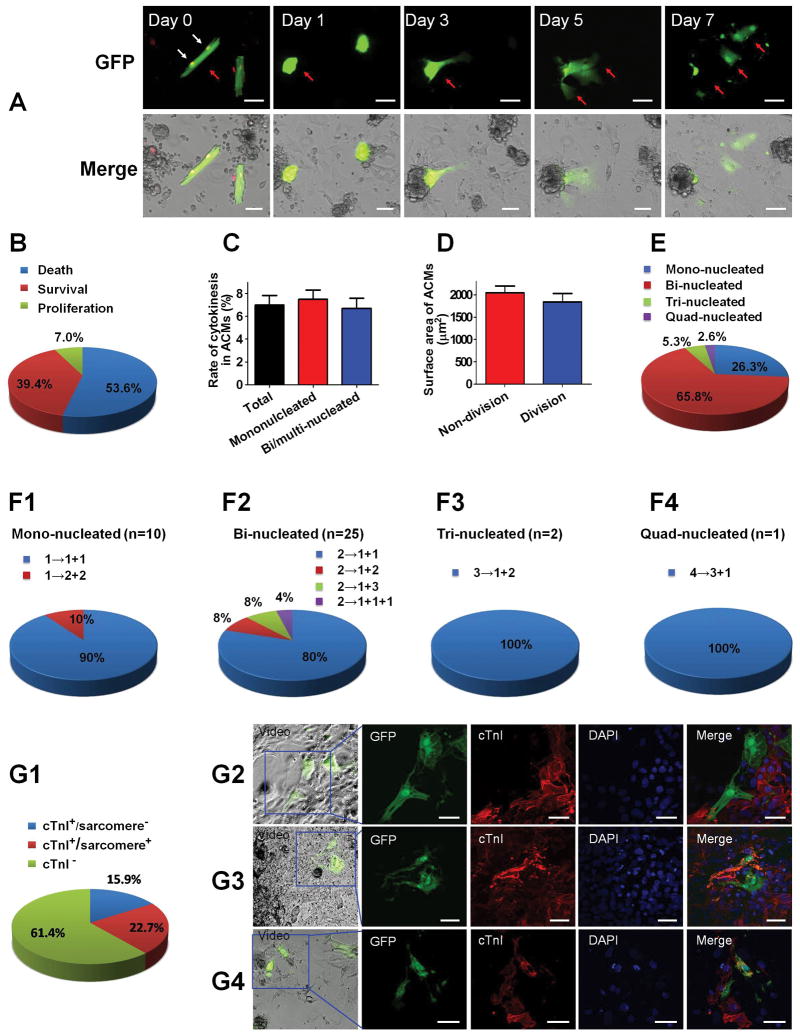
The results above were obtained in different ACM/NRVM co-culture plates that were fixed and studied at specific time points after co-culture. We next used time-lapse video microscopy to more definitively show the DPR process in individual ACMs. Mouse ACMs in co-culture transformed from their normal rod-shape to a spherical, hypercontracted form within 1 day. Over the next few days these cells dismantled their contractile apparatus (dedifferentiation) and assumed a flattened cell shape (Figure 2A). Proliferation was clearly documented with ACM cytokinesis into multiple progeny (Supplemental Videos 1–4). Only events with completed cytokinesis were counted as ACM proliferation, and a live cell nucleus labeling dye was used to label the nuclear number of ACMs. These studies showed that 7.0% of ACMs (out of 863 ACMs which were followed) proliferated (Figure 2B). Meanwhile, we repeated the time-lapse imaging experiments with ACMs isolated from fate-mapping transgenic Myh6-MerCreMer-tdTomato mice, with a red fluorescent reporter driven by a cardiomyocyte-specific promoter. The proliferation events with complete cytokinesis of these ACMs were also documented in co-culture with NRVMs (Supplemental Figures 2A–B and Supplemental Video 5). The proliferation rate was similar to that of ACMs isolated from β-actin-GFP mice. These results unambiguously demonstrate that ACMs can proliferate in co-culture with NRVMs.
Figure 2. ACM proliferation with cytokinesis in-vitro.
Freshly isolated ACMs from β-actin-GFP transgenic mice were co-cultured with NRVMs for 7 days. Every ACM was traced with a time-lapse video microscopy, and only the division events with completed cytokinesis were counted as ACM proliferation. A: The morphological remodeling of ACMs in the co-culture system observed with a time-lapse video microscopy. A bi-nucleated ACM indicated with red arrow became spherical and lost organized contractile apparatus during the first 3 days of co-culture, and then proliferated into several daughter cells that assumed a NRVM-like shape over the next few days. White arrow indicates the nuclei (labeled with DRAQ5™ Fluorescent Probe) of the ACM that underwent cytokinesis at the beginning of co-culture. The proliferation process of this ACM can be found in Supplemental Video 1. B: The cell fate of ACMs during the 7 days co-culture with NRVMs. The rates of cell death, survival and proliferation were quantified. C: The proliferation rates of mononucleated and bi/multi-nucleated ACMs. D: The surface area of ACMs that underwent proliferation and those without proliferation. E: The percent composition of proliferated ACMs. A total number of 38 ACM proliferation events with clearly visible nuclei were quantified. F: The cell division patterns of ACMs with different nuclei number. G: The characteristics of the daughter cells after ACM cytokinesis immunostained with cTnI. G1: The percentage of ACM progeny with different properties. G2: Representative images showing both of the ACM derived daughter cells lost cTnI expression. G3: Representative images showing one ACM derived daughter cell maintained sarcomeric structure while the other lost cTnI. G4: Representative images showing both of the ACM derived daughter cells regained sarcomeric structure. The proliferation process of the ACMs shown in G2-4 can be found in Supplemental Videos 2–4. For A–G, scale bars represent 50μm.
Previous studies suggested that proliferating mammalian cardiomyocytes were predominantly mononucleated,6–11 and only mononucleated cardiomyocytes could complete cytokinesis.10 However, in our system both mononucleated and bi/multi-nucleated ACMs were shown to proliferate with completed cytokinesis. Mononucleated ACMs had a tendency for higher proliferation rate than bi/multi-nucleated ACMs but the difference was not significant (Figure 2C). Moreover, the average original cell size of the proliferated ACMs was comparable with other ACMs (Figure 2D). It is known that the majority of ACMs are bi/multi-nucleated in adult mouse heart (mononucleated: 20.1%; bi-nucleated: 70.9%; multi-nucleated: 9.0% in our system). The majority of the proliferated ACMs were bi/multi-nucleated (mononucleated: 26.3%; bi-nucleated: 65.8%; multi-nucleated: 7.9%) (Figure 2E). Multiple patterns of ACM proliferation were observed. Bi-nucleated ACMs could divide into two mononucleated cells, three mononucleated cells, or one mononucleated cell and one bi/multi-nucleated cell (Figure 2F). Collectively, these findings indicate that terminally differentiated ACMs are able to reenter the cell cycle and proliferate in certain conditions, and the ACM proliferation can take place in mono and bi-nucleated myocytes.
Upon completion of time-lapse studies, i.e., 7 days after co-culture, the preparations were fixed and immunostained for cTnI to determine if the ACM progeny had organized sarcomeric structures. Among the ACM derived daughter cells, 22.7% were cTnI positive with organized sarcomere, an additional 15.9% were cTnI positive but without organized sarcomeres and 61.4% were cTnI negative (Figure 2G and Supplemental Videos 2–4). These results indicate that dedifferentiated ACMs can proliferate and some but not all of the progeny form new functional cardiomyocytes. The fact that after ACM proliferation most of the daughter cells were negative for cTnI suggests that redifferentiation is necessary for these cells to achieve functional regeneration.
To exclude cell fusion events, ACMs isolated from β-actin-GFP mice were co-cultured with NRVMs infected with Adeno-RFP (Supplemental Figure 3A). Approximately 8,500 GFP+ cells were counted and no GFP+/RFP+ cells were found during the 7-days co-culture period, excluding the possibility that fusion of ACMs and NRVMs explains our results. To confirm these results, ACMs isolated from Myh6-MHC-MerCreMer-tdTomato mice were mixed with cardiac non-myocytes isolated from β-actin-GFP mice, which were then co-cultured with NRVMs (Supplemental Figure 3B). More than 15,000 tdTomato+ cells from three mice were counted, and 4 GFP+/tdTomato+ ACMs were found, indicating a fusion rate of <0.03% between mouse ACMs and non-myocytes. This level is consistent with the previous in-vivo study with Rosa26-mTmG mice, which reported cell fusion rates <0.005%.24, 25 Collectively, these results show that cell fusion does not explain ACM proliferation and the ACM-DPR process.
3. The role of gap junction mediated intercellular Ca2+ signal propagation in ACM redifferentiation
The determinants of ACM redifferentiation are not well known. In the co-culture system, we found that ACMs needed to make direct contact with neighboring NRVMs to redifferentiate. When ACMs were physically separated from NRVMs in a transwell system, they dedifferentiated but did not proliferate and redifferentiate (Supplemental Figure 4A), suggesting the importance of physical contact for ACM proliferation and redifferentiation. We next tested the ability of NRVMs, fetal cardiomyocytes (FCMs) and cardiac fibroblasts to induce DPR. FCMs induced ACM-DPR similar to that seen with NRVMs. However, very rare ACM redifferentiation was observed in co-culture with cardiac fibroblasts (Supplemental Figures 4B–C). With time-lapse imaging observation, the ACM proliferation rate (1.7±0.2%) and re-differentiation rate (re-organization of sarcomere: 3.3±0.5%) were both significantly lower when co-cultured with non-myocytes, compared with those co-cultured with NRVMs (Supplemental Figures 4D–E). These results indicated that making contact with cardiomyocytes with spontaneous contractions (fetal or neonatal cardiomyocytes) rather than non-myocytes is important for ACM proliferation and redifferentiation.
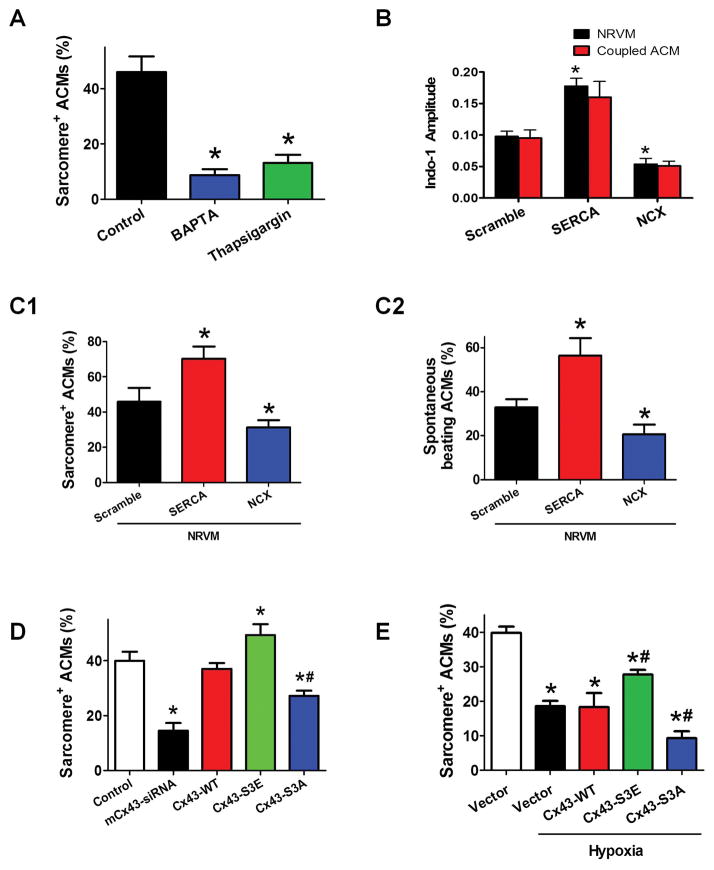
Cardiomyocyte contraction is triggered by Ca2+ influx through L type Ca2+ channels and the release of Ca2+ from the sarcoplasmic reticulum. These and other Ca2+ signals are also known to regulate cardiomyocyte growth, gene expression, differentiation and development.26 We determined if the properties of the Ca2+ transients in NRVMs influence redifferentiation. NRVMs were pretreated with the Ca2+ chelator BAPTA or the sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA2a) inhibitor thapsigargin to change the properties and frequencies of Ca2+ transients. Both treatments significantly decreased sarcomere re-organization of dedifferentiated ACMs (Figure 3A). The [Ca2+]i transient amplitudes are influenced by Ca2+ regulatory proteins including SERCA2a and NCX1. NRVMs had increased [Ca2+]i transient amplitude when infected with SERCA2a and decreased amplitude when infected with NCX1 (Figure 3B). Compared with control treatment, ACMs co-cultured with NRVMSERCA2a had a significantly higher redifferentiation rates while ACMs co-cultured with NRVMNCX1 showed a lower redifferentiation rate (Figure 3C). These results suggest that the properties of the Ca2+ transient in NRVMs influence the redifferentiation of ACMs.
Figure 3. Cx43 mediated intercellular transmission of Ca2+ influence the ACM redifferentiation.
A: The percentage of sarcomere+ ACMs in co-culture at day 7 with Ca2+ blocker BAPTA-AM and thapsigargin. B: The property of Ca2+ transients in ACMs cocultured with NRVMs over-expressing Ca2+ regulating protein SERCA2a or NCX1. The graph bars represent [Ca2+]i transient amplitude (change from diastolic [Ca2+]i to peak systolic [Ca2+]i) of redifferentiated ACMs and their coupled NRVMs. C: The percentage of ACMs with sarcomere+ (C1) or spontaneous beating (C2) in co-cultures with NRVMs overexpressing SERCA2a or NCX1 at day 7. D: The percentage of sarcomere+ ACMs in co-cultures at day 7 with Cx43 manipulation. E: The percentage of sarcomere+ ACMs in co-cultures at day 7 with Cx43 manipulation under hypoxia. N=8–10; * p<0.05 vs. control or scramble or vector; # p<0.05 vs. vector+hypoxia.
4. Hypoxia hampers connexin 43 (Cx43) function thereby inhibiting ACM redifferentiation, which is reversed by ischemia-resistant Cx43-S3E mutant
As a direct cell-to-cell cytoplasmic pathway, gap junctions allow propagation of electrical activity and other small molecules between cardiomyocytes.27 Intercellular transfer of cytoplasmic contents through gap junction between ACMs and NRVMs was demonstrated by fluorescence recovery after photo bleaching analysis (Supplemental Figure 5A). Furthermore, a Ca2+ imaging video showed the fluo-4 labeled spontaneous Ca2+ transients in a NRVM can propagate into a neighboring ACM (Supplemental Video 6). These results were also confirmed by a line-scan image analysis (Supplemental Figure 5B). Connexin 43 (Cx43) is the major isoform of gap junction proteins expressed in mammalian ventricular myocytes.27 A mouse specific Cx43-siRNA, designed to inhibit Cx43 expression in ACMs, reduced mRNA expression and decreased immunostaining of Cx43 in mouse ACMs but not in NRVMs (Supplemental Figures 5C–D). Cx43-siRNA significantly blocked the gap junction function (Supplemental Figure 5A) and reduced ACM redifferentiation (Figure 3D). These results support the idea that gap junctions allow Ca2+ transients to propagate from NRVMs into electrically coupled, dedifferentiated, ACMs to induce ACM redifferentiation.
Hypoxia/ischemia is known to cause cardiomyocyte electrical uncoupling in-vivo.19 Phosphorylation at casein kinase 1δ sites at serines 325/328/330 on Cx43 is crucial for maintaining the function of gap junctions.28 Hypoxia/ischemia induces dephosphorylation of these sites thereby leading to a reduced activity of gap junction, relocation of Cx43 proteins and enhanced degradation.29 Cx43 mutants with these sites replaced by phosphomimetic glutamic acids (Cx43-S3E) are resistant to pathological gap junction remodeling induced by cardiac ischemia.19 As shown in Figure 3E, hypoxia reduced ACM redifferentiation and infection of NRVM with Cx43-S3E rescued the inhibitory effect of hypoxia on ACM redifferentiation. Conversely, a Cx43 mutant with serines 325/328/330 replaced by nonphosphorylatable alanines (Cx43-S3A) enhanced the hypoxia induced decrease of ACM redifferentiation. These data suggest that hypoxia reduced Cx43 function thereby inhibiting ACM redifferentiation, which was reversed by ischemia-resistant Cx43-S3E mutant.
5. Ca2+-dependent calcineurin-NFAT signaling is activated during ACM redifferentiation
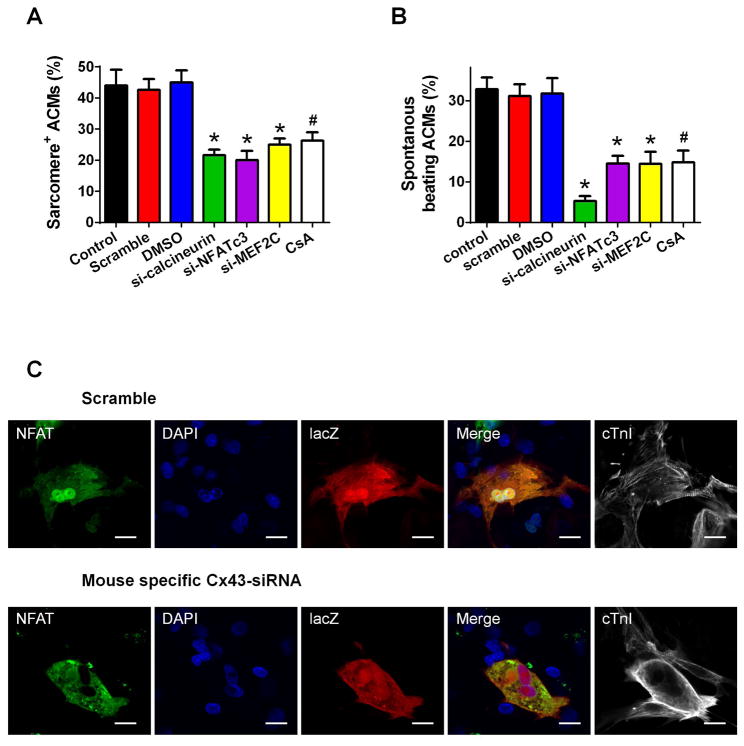
Changes in cytosolic Ca2+ can activate and regulate genes involved in cardiac growth and hypertrophy and can thereby induce changes in ACM phenotype.30 Ca2+ dependent activation of the phosphatase calcineurin/nuclear factor of activated T cells (NFAT)/myocyte-specific enhancer factor 2C (MEF2C) signaling pathway are centrally involved many of in these processes.19, 31 NFAT is also associated with myocardial remodeling,32 including myofibrillar re-organization. Knockdown of calcineurin, NFATc3 or MEF2C in ACMs with siRNAs or the calcineurin inhibitor cyclosporin A (CsA) significantly reduced ACMs with re-organized sarcomeres and spontaneous beating (Figures 4A–B). These results suggest the involvement of these signaling molecules in ACM redifferentiation. In addition, knockdown of ACM Cx43 blocked the nuclear translocation of NFATc3 and the formation of organized sarcomeres (Figure 4C). Collectively, these data suggest that Ca2+ transients propagating into dedifferentiated ACMs from spontaneously beating NRVMs via Cx43 gap junction induce redifferentiation by activating calcineurin-NFAT signaling.
Figure 4. Intracellular Ca2+ dependent calcineurin-NFAT signaling pathways regulate ACM redifferentiation.
A–B: The role of Ca2+ dependent calcineurin-NFAT signaling pathway in ACM redifferentiation. The percentage of ACMs with organized sarcomere (A) and spontaneous beating (B) were quantified at 7 days post-co-culture. The cells were pretreated with mouse specific siRNAs against calcineurin, NFATc3 and MEF2C, or calcineurin inhibitor CsA, respectively. N=6; * p<0.05 vs. Scramble; # p<0.05 vs. DMSO. C: Representative images of nuclear translocation of NFATc3 in ACMs at day 5 post-co-culture. The cells were pretreated with mouse specific siRNAs of Cx43 or scramble sequence as a control. Scale bars represent 20 μm.
6. DPR of ACMs occurs in the post-MI adult mammalian heart
Myh6-MerCreMer-lacZ mice were used to determine the origin of any myocytes regenerated after MI. Before MI, the majority of ACMs (>90%) were specifically and irreversibly marked with lacZ after 4-OH-tamoxifen treatment (Supplemental Figure 6). Labelled ACMs express lacZ even if they lose the expression of α-myosin heavy chain while original non-cardiomyocytes are lacZ negative (Supplemental Figure 7A). Nuclear EdU incorporation was used to evaluate cellular proliferation. EdU+/tropomyosin+ cells were assumed to be newly formed cardiomyocytes. These cells were primarily found in the infarct border zone (Supplemental Figure 7B). Over 2 million isolated tropomyosin+ myocytes from 7 hearts were examined and 0.091±0.022% of them were EdU+. Importantly, 35% of these EdU+/tropomyosin+ cells were mononucleated and all of these EdU+ ACMs were lacZ+. These results strongly support the idea that the new cardiomyocytes in the post-MI myocardium are derived from pre-existing ACMs.
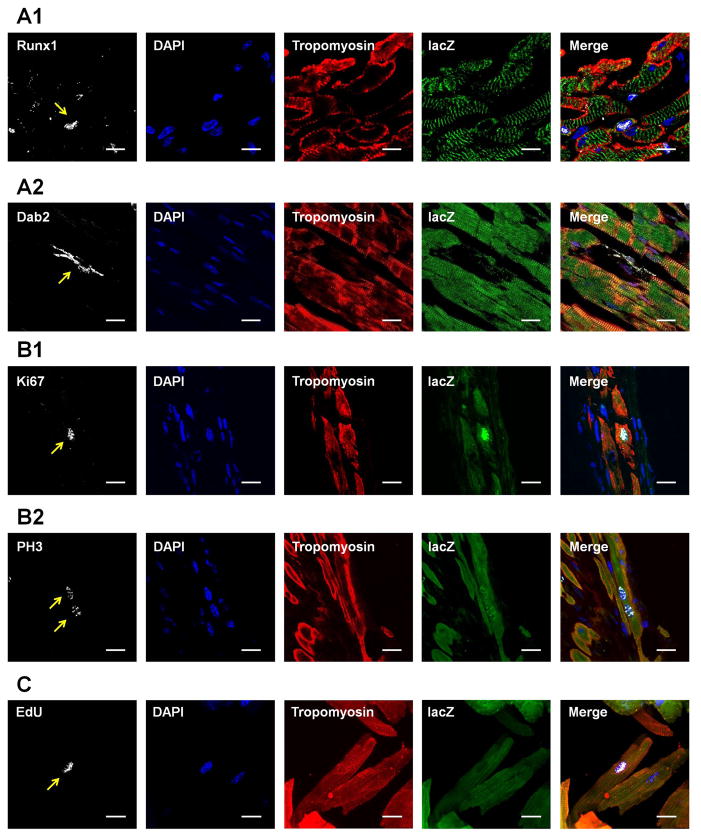
We next examined if any evidence for cardiomyocyte DPR could be shown in post-MI Myh6-MerCreMer-lacZ mice. The dedifferentiation markers Runx1 and Dab2 were found in a few lacZ+ ACMs in the infarct border zone (Figures 5A). Some lacZ+/tropomyosin+ myocytes in the infarct border zone were also positive for the proliferation markers Ki67 and PH3 (Figures 5B). Mononucleated lacZ+/EdU+ ACMs with clear sarcomeric structure, indicating new ACMs formed after proliferation and redifferentiation, were found 3 weeks post-MI (Figure 5C). Among the EdU+ cardiomyocytes (trypomyosin+) isolated from post-MI mice, both sarcomere negative and positive subsets were identified (Supplemental Figure 8A). Only 35.2±4.5% of these EdU+ cardiomyocytes were sarcomere positive with typical rod-shaped morphology. The remainder of the EdU+ cardiomyocytes had poorly organized or no detectable sarcomeric structure (Supplemental Figure 8B). It indicates that a large portion of the cells derived from ACM proliferation were still in a dedifferentiated state without significant contractile function. These results are consistent with the findings of ACM progeny in the co-culture experiments (Figure 2E). Collectively these data suggest that the few newly formed cardiomyocytes seen in the MI border zone are derived from ACMs that have undergone DPR, and that the poor re-differentiation rate of ACM progeny hinders the ACM-DPR mediated new cardiomyocyte formation.
Figure 5. Dedifferentiation, proliferation and redifferentiation of ACMs in Myh6-MerCreMer-lacZ mice subjected to MI.
A: Representative images of ACMs expressing dedifferentiation markers Runx1 (A1) or Dab2 (A2) in the infarct border zone. B: Representative images of ACMs expressing proliferation markers Ki67 (B1) and PH3 (B2). C: Representative images of a mononuclear lacZ+/EdU+ ACM isolated from hearts 3 weeks after MI. The organized sarcomere was clearly seen in the ACM. For A–C, scale bars represent 20 μm.
7. Ischemia resistant mutant AAV9-Cx43 promotes ACM re-differentiation and improves cardiac function in post-MI hearts
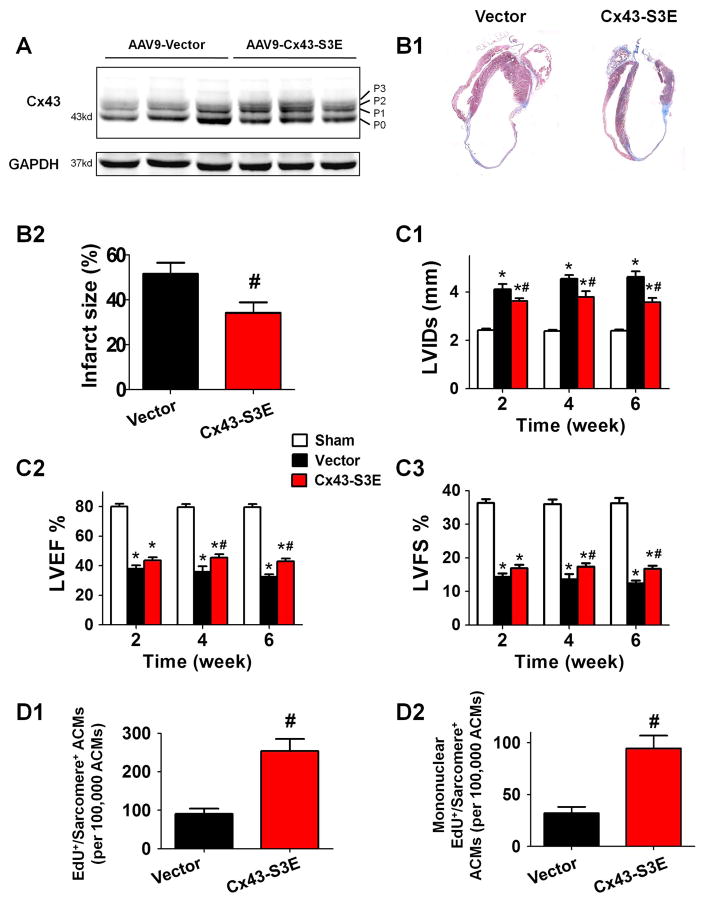
Ischemia induced gap junctional uncoupling occurs in the MI border zone and this protects the surviving regions of the heart by reducing the spread of proarrhythmic membrane depolarization and signals from dying myocytes into the surviving myocardium.33 Our in-vitro results suggest that ACMs that uncouple from their surviving neighbors can undergo DPR, but redifferentiation into functional myocytes requires re-coupling to beating myocytes. If true, uncoupled myocytes that undergo dedifferentiation in-vivo would need to recouple to myocytes that are part of the functional myocardium. To test this idea an adeno-associated virus serotype 9 (AAV9) carrying ischemia resistant Cx43-S3E mutant was used to enhance cell-cell electrical coupling between the infarct border zone and surviving myocardium. AAV9-Cx43-S3E was injected directly into the MI border zone at 3 days post-MI. Cx43 phosphorylation assessed by Western blot analysis of total protein lysates from the post-MI hearts with empty AAV-vector displayed a prominent P0 band and a faint P1 band, whereas those from the post-MI hearts with AAV-Cx43-S3E showed all (P1, P2 and P3) isoforms at a significantly higher abundance (Figure 6A). These results indicate that AAV9-Cx43-S3E administration mimicked Cx43 phosphorylation after MI.
Figure 6. Cardiac AAV9-Cx43-S3E (ischemia resistant Cx43 mutant) therapy promotes ACM proliferation and redifferentiation and improves cardiac function in the post-MI heart.
AAV9-Cx43-S3E or AAV9-vector was administrated 3 days after MI. A: Altered Cx43 phosphorylation in post-MI mice with AAV9-Cx43-S3E treatment. Western blot analysis of whole-cell lysates prepared from left ventricles of mice 6 weeks post-MI. Cx43 lysates are shown in various major phosphorylated forms of Cx43 (P0, P1, P2, P3) with the indicated treatments, probed with polyclonal pan-Cx43 antibody. B: The representative images (B1) and quantification (B2) of heart infarct size 6 weeks post-MI. C: The cardiac structure and function were assessed by serially echocardiography. Left ventricle internal diameter in systole (LVIDs, C1), left ventricular ejection fraction (LVEF, C2) and fraction shortening (LVFS, C3) at 2–6 weeks post-MI were presented. D: ACM proliferation and redifferentiation detected by EdU incorporation analysis at 3 weeks post-MI. Total EdU+/sarcomere+/lacZ+ cells (D1) and mononuclear EdU+/sarcomere+/lacZ+ cells (D2) were quantified. N=10–14; * p<0.05 vs. Sham; # p<0.05 vs. AAV9-vector.
MI caused left ventricular fractional shortening (LVFS) and ejection fraction (LVEF) to decrease in all groups of post-MI hearts. Hearts treated with AAV9-Cx43-S3E 3 days after MI had significantly decreased infarction size (Figure 6B), improved cardiac function (LVEF and LVFS) and less ventricular dilation 6 weeks post-MI (Figure 6C).
EdU+ cardiomyocytes were predominantly found in the infarct border zone at 3 weeks post-MI (EdU+ cardiomyocyte nuclei per total nuclei: infarct border zone: 1.181% vs. remote area: 0.109%) (Supplemental Figure 9). Since mis-identification of cardiomyocyte nuclei is common with conventional histology techniques, EdU incorporation into cardiomyocytes was also measured in enzymatically isolated ACMs from hearts with or without AAV9-Cx43-S3E administration. This method can unequivocally determine if an EdU+ nucleus resides within a cardiomyocyte.34 As shown in Supplemental Figure 8B, Cx43-S3E did not change the number of EdU+ cardiomyocytes, but significantly increased the sarcomere positive proportion within the total EdU+ cardiomyocytes. Conversely, Cx43-siRNA and calcineurin inhibitor CsA decreased the proportion. Significantly more lacZ+/EdU+/sarcomere+ ACMs were found in AAV9-Cx43-S3E treated hearts (Cx43-S3E: 0.254% vs. Vector: 0.091%)(Figure 6D). Mononucleated lacZ+/EdU+/sarcomere+ ACMs were also found in significantly greater abundance in AAV9-Cx43-S3E treated hearts than control (Cx43-S3E: 0.095% vs. Vector: 0.032%). Given the distinct distribution of EdU+ cardiomyocytes in post-MI heart, the percentage of lacZ+/EdU+/sarcomere+ ACMs in the infarct border zone could be much greater than the number obtained from isolated cardiomyocytes of the whole heart. These data show that ischemia resistant Cx43 reduces infarct size and improves cardiac function and increases the number of newly formed cardiomyocytes at the MI border zone.
Discussion
What appears common to all post MI studies is the presence of a few new cardiomyocytes in the infarct border zone.4, 6 Consistent with previous studies,3 our data with a fate-mapping strategy supports the view that the newly formed cardiomyocytes found in the post-MI heart are predominately derived from pre-existing ACMs. It is unclear if these new myocytes are derived from a small pool of ACMs that retain their ability to proliferate or derived from mature ACMs. Our in-vitro and in-vivo data support the hypothesis that mature ACMs can dedifferentiate, and then proliferate and redifferentiate into new cardiomyocytes.
ACM Dedifferentiation
After an MI, injured myocytes at the infarct border uncouple from their neighbors.33 These ACMs usually die over the next few days/weeks and the infarct zone expands. Our studies suggest that some of these myocytes dedifferentiate after uncoupling from the parent myocardium, and they may be the source of newly forming myocytes in the post-MI heart. To test our idea, we established an in-vitro system in which single and uncoupled ACMs are isolated from the adult heart and placed in co-culture with NRVMs. This preparation contains spontaneously beating NRVMs together with isolated and Ca2+ overloaded, electrically uncoupled ACMs.16 This co-culture system may mimic the in-vivo ischemia environment where certain surviving myocytes in the infarct border zone have uncoupled from the myocardium and are at high risk of cell death. These uncoupled myocytes are neighboring healthy myocytes contracting in synchrony with the normal heartbeat.
First our studies show that co-culture of ACMs with NRVMs promotes mouse ACM survival (Supplemental Figure 1). ACMs that survived in co-culture expressed dedifferentiation markers and dismantled their contractile apparatus. Dedifferentiation might serve as a preprogrammed survival mechanism for stressed ACMs,35 protecting them from ischemia by reduction of ATP consumption due to changes in energy metabolism and/or inactivation of energy-intensive functions.36 The dedifferentiation of mouse ACMs also might be induced by loss of cell-cell contact or by Ca2+ overload stress.
ACM proliferation
We used a variety of approaches to document ACM proliferation in co-culture. Genetically modified or fate-mapping ACMs were used so that they and their progeny expressing the genetic marker could be easily followed. The strongest data supporting the proliferation of dedifferentiated ACMs were obtained by using time-lapse imaging followed by immunostaining of those regions of the co-cultures that were observed over time. These experiments clearly showed that some ACMs proliferate during the first week in co-culture with NRVMs. Cytokinesis of proliferating ACMs was directly observed to document that dedifferentiated ACMs can form committed progeny. Visualization of nuclei during live imaging showed that some cardiomyocytes may go through the whole mitosis process, i.e., DNA replication, chromosome/nuclei separation and cytokinesis, which is a significant finding. It also gave us the opportunity to determine if mononucleated ACMs are more likely to proliferate than bi-nucleated ACMs. Interestingly, our data showed that mono- and bi/multi-nucleated myocytes are almost equally able to proliferate and form committed progeny. In addition, cell size did not appear to be a significant determinant for the potential of ACM proliferation. These findings suggest that proliferation, at least under the conditions used, is not limited to a small pool of so-called “immature”, small mononucleated ACMs as previously suggested6–11 Binucleated ACMs are the majority of cardiac muscle cells within the adult heart, and our results suggest that they are equally capable of DPR. Therefore, they are likely to be the major source of new myocytes in the adult heart. In addition, we are the first to define the dividing patterns of ACMs with different nucleation patterns. Bi-nucleated ACMs could divide into two or three mononucleated cells, or one mononucleated cell and one bi/multi-nucleated cells. Further, our data also suggest that cell-to-cell contact is important for ACM proliferation. Cx43 has been reported as a regeneration gene in zebrafish heart.37 Additional mechanisms regulating ACM proliferation were not studied in detail and need further investigation.
Redifferentiation of ACMs
Redifferentiation of dedifferentiated ACMs into contractile myocytes is essential if they are to contribute to the pump function of the heart.38 The co-culture experiments document DPR and directly demonstrate new myocyte formation after ACM dedifferentiation and proliferation. In addition, our in-vitro data show that less than half of the cells derived from dedifferentiated and proliferated ACMs go on to acquire a cardiomyocyte phenotype, at least during the time period of the current study. An important aspect of our results is that ACM redifferentiation were not observed unless the dedifferentiated ACMs made gap junction (Cx43) mediated cell-cell connections with NRVMs (Figure 3 and Supplemental Figures 4–5).
Role of Cx43 and Ca2+ transients in ACM redifferentiation
To our knowledge, there is currently no report in the literature on the regulation of ACM redifferentiation from a dedifferentiated state. Previous studies show that Ca2+ signaling is involved in embryonic cardiogenesis and stem cell cardiomyogenic differentiation.39 Our in-vitro data showed that the Ca2+ transients in NRVMs transiently elevate cytosolic Ca2+ in dedifferentiated ACMs via gap junctions to initiate redifferentiation. The entry of Ca2+ into ACMs appears to induce redifferentiation via a process that includes calcineurin-NFAT signaling pathways. In addition, the properties of the Ca2+ transient in NRVMs influenced ACM redifferentiation. Over-expression of SERCA2a (which increased the size of the Ca2+ transient) increased ACM redifferentiation. Over-expression of NCX1 (to decrease the amplitude of the Ca2+ transients) reduced ACM redifferentiation. In failing human hearts, the cardiomyocyte Ca2+ transient is reduced and prolonged due to impaired SERCA2a activity and increased expression of NCX1.40, 41 These changes might reduce ACM redifferentiation and reduce the small amount of new myocyte formation present in cardiac disease. We confirmed that the NRVM Ca2+ that influences ACM redifferentiation travels into ACM via gap junctions.42 Ischemia impaired gap junction function and blocked Ca2+ induced ACM redifferentiation. An ischemia-resistant mutant of Cx43 with stable phosphorylation at serine residues 325/328/330 rescued these effects.
Evidence for DPR after MI
The DPR process observed in our in-vitro system may not exactly represent the DPR process in-vivo. Our in-vivo experiments using fate-mapping mouse models allowed for identification of new myocytes that were derived from preexisting ACMs (lacZ+). Evidence of ACM dedifferentiation in the infarct border zone was observed at early times after MI and LacZ+/EdU+/sarcomere+ ACMs were found in the infarct border zone at later times. These results support the idea that ACMs having undergone dedifferentiation, proliferation and redifferentiation. The ratio of lacZ+/EdU+/sarcomere+ ACMs out of total ACMs isolated from the whole heart post-MI injury was about 0.1%, consistent with previous reports that have found evidence for new myocyte formation in the adult heart after ischemic injury.34, 43–45 Consistent with the findings of ACM progeny in the co-culture experiments (Figure 2E), the in-vivo data also suggests most of the cells derived from ACM proliferation were in a dedifferentiation state without contractile function, which supported the DPR phenomenon in infracted hearts and the importance of re-differentiation (Supplemental Figure 8). ACM redifferentiation requires Cx43 mediated re-coupling of dedifferentiated ACMs to neighboring cardiomyocytes that have survived the MI and are beating in synchrony with the parent myocardium. Stabilization of Cx43 phosphorylation against ischemia with Cx43-S3E enhanced ACM redifferentiation and improved cardiac function in post-MI heart. Others have found that hypoxia (especially a certain concentration of O2) enhances cardiomyocyte proliferation in adult post-MI hearts.46 Therefore, hypoxia in the MI border zone could also be an important trigger for new myocyte generation. This is a complex issue that requires much more study.
In conclusion, the present study suggests that mouse ACMs, including bi/multi-nucleated and large-sized ACMs, can proliferate and form new myocytes through a DPR process. After MI, some ACMs in the infarct border zone uncouple from the surviving myocardium, dedifferentiate, proliferate, re-couple to the surviving myocardium and redifferentiate into functional ACMs (Supplemental Figure 10), explaining the new cardiomyocytes observed in many previous studies.3, 10, 15, 21 Approaches that could safely enhance this process would be a significant step in regenerating those myocytes killed by ischemic injury.
Supplementary Material
Clinical Perspective.
1) What is new?
Terminally differentiated ACMs in co-culture with neonatal rat ventricular myocytes (NRVMs) can reenter the cell cycle and form new cardiomyocytes through a three-step process, dedifferentiation, proliferation and redifferentiation.
ACMs of all sizes and nucleation are capable of proliferating and forming committed progeny.
Dedifferentiated ACMs require electrical connections with NRVMs and gain Ca2+ signals from NRVMs to redifferentiate into functional myocytes with sarcomeres.
Enhancing electrical coupling between ACMs in the border zone of a post-MI heart increases the number of newly formed cardiomyocytes and enhances cardiac function.
2) What are the clinical implications?
Increasing the generation of new cardiomyocytes in the MI border zone could reduce infarct size and improve post MI cardiac remodeling.
New cardiomyocytes can be generated in the adult heart from pre-existing ACMs.
Understanding the fundamental aspects of this process could lead to new strategies to repair the injured heart.
Acknowledgments
Funding Sources
This study was supported by research grants from the National Natural Science Foundation of China (81470409, 81470475), National Basic Research Program of China (2013CB531104), and National Institutes of Health (NIH) of the United States (HL113598 to DDD, HL 088243 to XC, HL033921 to SRH).
Footnotes
Disclosures
None.
References
- 1.Lin Z, Pu WT. Strategies for cardiac regeneration and repair. Sci Transl Med. 2014;6:239rv231. doi: 10.1126/scitranslmed.3006681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Canseco DC, Kimura W, Garg S, Mukherjee S, Bhattacharya S, Abdisalaam S, Das S, Asaithamby A, Mammen PP, Sadek HA. Human ventricular unloading induces cardiomyocyte proliferation. J Am Coll Cardiol. 2015;65:892–900. doi: 10.1016/j.jacc.2014.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Senyo SE, Steinhauser ML, Pizzimenti CL, Yang VK, Cai L, Wang M, Wu TD, Guerquin-Kern JL, Lechene CP, Lee RT. Mammalian heart renewal by pre-existing cardiomyocytes. Nature. 2013;493:433–436. doi: 10.1038/nature11682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Berlo JH, Kanisicak O, Maillet M, Vagnozzi RJ, Karch J, Lin SC, Middleton RC, Marban E, Molkentin JD. C-kit+ cells minimally contribute cardiomyocytes to the heart. Nature. 2014;509:337–341. doi: 10.1038/nature13309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alkass K, Panula J, Westman M, Wu TD, Guerquin-Kern JL, Bergmann O. No evidence for cardiomyocyte number expansion in preadolescent mice. Cell. 2015;163:1026–1036. doi: 10.1016/j.cell.2015.10.035. [DOI] [PubMed] [Google Scholar]
- 6.Puente BN, Kimura W, Muralidhar SA, Moon J, Amatruda JF, Phelps KL, Grinsfelder D, Rothermel BA, Chen R, Garcia JA, Santos CX, Thet S, Mori E, Kinter MT, Rindler PM, Zacchigna S, Mukherjee S, Chen DJ, Mahmoud AI, Giacca M, Rabinovitch PS, Aroumougame A, Shah AM, Szweda LI, Sadek HA. The oxygen-rich postnatal environment induces cardiomyocyte cell-cycle arrest through DNA damage response. Cell. 2014;157:565–579. doi: 10.1016/j.cell.2014.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen X, Wilson RM, Kubo H, Berretta RM, Harris DM, Zhang X, Jaleel N, MacDonnell SM, Bearzi C, Tillmanns J, Trofimova I, Hosoda T, Mosna F, Cribbs L, Leri A, Kajstura J, Anversa P, Houser SR. Adolescent feline heart contains a population of small, proliferative ventricular myocytes with immature physiological properties. Circ Res. 2007;100:536–544. doi: 10.1161/01.RES.0000259560.39234.99. [DOI] [PubMed] [Google Scholar]
- 8.Mollova M, Bersell K, Walsh S, Savla J, Das LT, Park SY, Silberstein LE, Dos Remedios CG, Graham D, Colan S, Kuhn B. Cardiomyocyte proliferation contributes to heart growth in young humans. Proc Natl Acad Sci U S A. 2013;110:1446–1451. doi: 10.1073/pnas.1214608110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahmoud AI, Kocabas F, Muralidhar SA, Kimura W, Koura AS, Thet S, Porrello ER, Sadek HA. Meis1 regulates postnatal cardiomyocyte cell cycle arrest. Nature. 2013;497:249–253. doi: 10.1038/nature12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bersell K, Arab S, Haring B, Kuhn B. Neuregulin1/erbb4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell. 2009;138:257–270. doi: 10.1016/j.cell.2009.04.060. [DOI] [PubMed] [Google Scholar]
- 11.Kuhn B, del Monte F, Hajjar RJ, Chang YS, Lebeche D, Arab S, Keating MT. Periostin induces proliferation of differentiated cardiomyocytes and promotes cardiac repair. Nat Med. 2007;13:962–969. doi: 10.1038/nm1619. [DOI] [PubMed] [Google Scholar]
- 12.Jopling C, Sleep E, Raya M, Marti M, Raya A, Izpisua Belmonte JC. Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature. 2010;464:606–609. doi: 10.1038/nature08899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jopling C, Boue S, Izpisua Belmonte JC. Dedifferentiation, transdifferentiation and reprogramming: Three routes to regeneration. Nat Rev Mol Cell Biol. 2011;12:79–89. doi: 10.1038/nrm3043. [DOI] [PubMed] [Google Scholar]
- 14.Kubin T, Poling J, Kostin S, Gajawada P, Hein S, Rees W, Wietelmann A, Tanaka M, Lorchner H, Schimanski S, Szibor M, Warnecke H, Braun T. Oncostatin m is a major mediator of cardiomyocyte dedifferentiation and remodeling. Cell Stem Cell. 2011;9:420–432. doi: 10.1016/j.stem.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 15.D’Uva G, Aharonov A, Lauriola M, Kain D, Yahalom-Ronen Y, Carvalho S, Weisinger K, Bassat E, Rajchman D, Yifa O, Lysenko M, Konfino T, Hegesh J, Brenner O, Neeman M, Yarden Y, Leor J, Sarig R, Harvey RP, Tzahor E. Erbb2 triggers mammalian heart regeneration by promoting cardiomyocyte dedifferentiation and proliferation. Nat Cell Biol. 2015;17:627–638. doi: 10.1038/ncb3149. [DOI] [PubMed] [Google Scholar]
- 16.Wang W, Zhang H, Gao H, Kubo H, Berretta RM, Chen X, Houser SR. {beta}1-adrenergic receptor activation induces mouse cardiac myocyte death through both l-type calcium channel-dependent and -independent pathways. Am J Physiol Heart Circ Physiol. 2010;299:H322–331. doi: 10.1152/ajpheart.00392.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang WE, Yang D, Li L, Wang W, Peng Y, Chen C, Chen P, Xia X, Wang H, Jiang J, Liao Q, Li Y, Xie G, Huang H, Guo Y, Ye L, Duan DD, Chen X, Houser SR, Zeng C. Prolyl hydroxylase domain protein 2 silencing enhances the survival and paracrine function of transplanted adipose-derived stem cells in infarcted myocardium. Circ Res. 2013;113:288–300. doi: 10.1161/CIRCRESAHA.113.300929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pu J, Yuan A, Shan P, Gao E, Wang X, Wang Y, Lau WB, Koch W, Ma XL, He B. Cardiomyocyte-expressed farnesoid-x-receptor is a novel apoptosis mediator and contributes to myocardial ischaemia/reperfusion injury. Eur Heart J. 2013;34:1834–1845. doi: 10.1093/eurheartj/ehs011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Remo BF, Qu J, Volpicelli FM, Giovannone S, Shin D, Lader J, Liu FY, Zhang J, Lent DS, Morley GE, Fishman GI. Phosphatase-resistant gap junctions inhibit pathological remodeling and prevent arrhythmias. Circ Res. 2011;108:1459–1466. doi: 10.1161/CIRCRESAHA.111.244046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aurora AB, Mahmoud AI, Luo X, Johnson BA, van Rooij E, Matsuzaki S, Humphries KM, Hill JA, Bassel-Duby R, Sadek HA, Olson EN. Microrna-214 protects the mouse heart from ischemic injury by controlling ca(2)(+) overload and cell death. J Clin Invest. 2012;122:1222–1232. doi: 10.1172/JCI59327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiang FL, Guo M, Yutzey KE. Overexpression of tbx20 in adult cardiomyocytes promotes proliferation and improves cardiac function after myocardial infarction. Circulation. 2016;133:1081–1092. doi: 10.1161/CIRCULATIONAHA.115.019357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen X, Zhang X, Kubo H, Harris DM, Mills GD, Moyer J, Berretta R, Potts ST, Marsh JD, Houser SR. Ca2+ influx-induced sarcoplasmic reticulum ca2+ overload causes mitochondrial-dependent apoptosis in ventricular myocytes. Circ Res. 2005;97:1009–1017. doi: 10.1161/01.RES.0000189270.72915.D1. [DOI] [PubMed] [Google Scholar]
- 23.O’Connell TD, Rodrigo MC, Simpson PC. Isolation and culture of adult mouse cardiac myocytes. Methods Mol Biol. 2007;357:271–296. doi: 10.1385/1-59745-214-9:271. [DOI] [PubMed] [Google Scholar]
- 24.Kimura W, Xiao F, Canseco DC, Muralidhar S, Thet S, Zhang HM, Abderrahman Y, Chen R, Garcia JA, Shelton JM, Richardson JA, Ashour AM, Asaithamby A, Liang H, Xing C, Lu Z, Zhang CC, Sadek HA. Hypoxia fate mapping identifies cycling cardiomyocytes in the adult heart. Nature. 2015;523:226–230. doi: 10.1038/nature14582. [DOI] [PubMed] [Google Scholar]
- 25.Morikawa Y, Zhang M, Heallen T, Leach J, Tao G, Xiao Y, Bai Y, Li W, Willerson JT, Martin JF. Actin cytoskeletal remodeling with protrusion formation is essential for heart regeneration in hippo-deficient mice. Sci Signal. 2015;8:ra41. doi: 10.1126/scisignal.2005781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ibarra C, Vicencio JM, Estrada M, Lin Y, Rocco P, Rebellato P, Munoz JP, Garcia-Prieto J, Quest AF, Chiong M, Davidson SM, Bulatovic I, Grinnemo KH, Larsson O, Szabadkai G, Uhlen P, Jaimovich E, Lavandero S. Local control of nuclear calcium signaling in cardiac myocytes by perinuclear microdomains of sarcolemmal insulin-like growth factor 1 receptors. Circ Res. 2013;112:236–245. doi: 10.1161/CIRCRESAHA.112.273839. [DOI] [PubMed] [Google Scholar]
- 27.Tirziu D, Giordano FJ, Simons M. Cell communications in the heart. Circulation. 2010;122:928–937. doi: 10.1161/CIRCULATIONAHA.108.847731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lampe PD, Cooper CD, King TJ, Burt JM. Analysis of connexin43 phosphorylated at s325, s328 and s330 in normoxic and ischemic heart. J Cell Sci. 2006;119:3435–3442. doi: 10.1242/jcs.03089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Le HT, Sin WC, Lozinsky S, Bechberger J, Vega JL, Guo XQ, Saez JC, Naus CC. Gap junction intercellular communication mediated by connexin43 in astrocytes is essential for their resistance to oxidative stress. J Biol Chem. 2014;289:1345–1354. doi: 10.1074/jbc.M113.508390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nam YJ, Song K, Luo X, Daniel E, Lambeth K, West K, Hill JA, DiMaio JM, Baker LA, Bassel-Duby R, Olson EN. Reprogramming of human fibroblasts toward a cardiac fate. Proc Natl Acad Sci U S A. 2013;110:5588–5593. doi: 10.1073/pnas.1301019110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harada M, Luo X, Murohara T, Yang B, Dobrev D, Nattel S. Microrna regulation and cardiac calcium signaling: Role in cardiac disease and therapeutic potential. Circ Res. 2014;114:689–705. doi: 10.1161/CIRCRESAHA.114.301798. [DOI] [PubMed] [Google Scholar]
- 32.Sankar N, Detombe PP, Mignery GA. Calcineurin-nfatc regulates type 2 inositol 1,4,5-trisphosphate receptor (insp3r2) expression during cardiac remodeling. J Biol Chem. 2014;289:6188–6198. doi: 10.1074/jbc.M113.495242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maass K, Chase SE, Lin X, Delmar M. Cx43 ct domain influences infarct size and susceptibility to ventricular tachyarrhythmias in acute myocardial infarction. Cardiovasc Res. 2009;84:361–367. doi: 10.1093/cvr/cvp250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malliaras K, Zhang Y, Seinfeld J, Galang G, Tseliou E, Cheng K, Sun B, Aminzadeh M, Marban E. Cardiomyocyte proliferation and progenitor cell recruitment underlie therapeutic regeneration after myocardial infarction in the adult mouse heart. EMBO Mol Med. 2013;5:191–209. doi: 10.1002/emmm.201201737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thijssen VL, Ausma J, Borgers M. Structural remodelling during chronic atrial fibrillation: Act of programmed cell survival. Cardiovasc Res. 2001;52:14–24. doi: 10.1016/s0008-6363(01)00367-4. [DOI] [PubMed] [Google Scholar]
- 36.Poling J, Gajawada P, Lorchner H, Polyakova V, Szibor M, Bottger T, Warnecke H, Kubin T, Braun T. The janus face of osm-mediated cardiomyocyte dedifferentiation during cardiac repair and disease. Cell Cycle. 2012;11:439–445. doi: 10.4161/cc.11.3.19024. [DOI] [PubMed] [Google Scholar]
- 37.Yin VP, Lepilina A, Smith A, Poss KD. Regulation of zebrafish heart regeneration by mir-133. Dev Biol. 2012;365:319–327. doi: 10.1016/j.ydbio.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aguirre A, Sancho-Martinez I, Izpisua Belmonte JC. Reprogramming toward heart regeneration: Stem cells and beyond. Cell Stem Cell. 2013;12:275–284. doi: 10.1016/j.stem.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 39.Ferreira-Martins J, Rondon-Clavo C, Tugal D, Korn JA, Rizzi R, Padin-Iruegas ME, Ottolenghi S, De Angelis A, Urbanek K, Ide-Iwata N, D’Amario D, Hosoda T, Leri A, Kajstura J, Anversa P, Rota M. Spontaneous calcium oscillations regulate human cardiac progenitor cell growth. Circ Res. 2009;105:764–774. doi: 10.1161/CIRCRESAHA.109.206698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gwathmey JK, Yerevanian AI, Hajjar RJ. Cardiac gene therapy with serca2a: From bench to bedside. J Mol Cell Cardiol. 2011;50:803–812. doi: 10.1016/j.yjmcc.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kumarswamy R, Lyon AR, Volkmann I, Mills AM, Bretthauer J, Pahuja A, Geers-Knorr C, Kraft T, Hajjar RJ, Macleod KT, Harding SE, Thum T. Serca2a gene therapy restores microrna-1 expression in heart failure via an akt/foxo3a-dependent pathway. Eur Heart J. 2012;33:1067–1075. doi: 10.1093/eurheartj/ehs043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hammer KP, Ljubojevic S, Ripplinger CM, Pieske BM, Bers DM. Cardiac myocyte alternans in intact heart: Influence of cell-cell coupling and beta-adrenergic stimulation. J Mol Cell Cardiol. 2015;84:1–9. doi: 10.1016/j.yjmcc.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eulalio A, Mano M, Dal Ferro M, Zentilin L, Sinagra G, Zacchigna S, Giacca M. Functional screening identifies mirnas inducing cardiac regeneration. Nature. 2012;492:376–381. doi: 10.1038/nature11739. [DOI] [PubMed] [Google Scholar]
- 44.Lin Z, von Gise A, Zhou P, Gu F, Ma Q, Jiang J, Yau AL, Buck JN, Gouin KA, van Gorp PR, Zhou B, Chen J, Seidman JG, Wang DZ, Pu WT. Cardiac-specific yap activation improves cardiac function and survival in an experimental murine mi model. Circ Res. 2014;115:354–363. doi: 10.1161/CIRCRESAHA.115.303632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li J, Gao E, Vite A, Yi R, Gomez L, Goossens S, van Roy F, Radice GL. Alpha-catenins control cardiomyocyte proliferation by regulating yap activity. Circ Res. 2015;116:70–79. doi: 10.1161/CIRCRESAHA.116.304472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakada Y, Canseco DC, Thet S, Abdisalaam S, Asaithamby A, Santos CX, Shah AM, Zhang H, Faber JE, Kinter MT, Szweda LI, Xing C, Hu Z, Deberardinis RJ, Schiattarella G, Hill JA, Oz O, Lu Z, Zhang CC, Kimura W, Sadek HA. Hypoxia induces heart regeneration in adult mice. Nature. 2017;541:222–227. doi: 10.1038/nature20173. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.