Abstract
Background and Purpose
Subarachnoid hemorrhage (SAH) is associated with a temporal pattern of stroke incidence. We hypothesized that natural oscillations in gene expression controlling circadian rhythm impact the severity of neuronal injury. We moreover predict that heme oxygenase-1 (HO-1/Hmox1) and its product carbon monoxide (CO) contribute to restoration of rhythm and neuroprotection.
Methods
Murine SAH model where blood was injected at various time points of the circadian cycle. Readouts included circadian clock gene expression, locomotor activity, vasospasm, neuroinflammatory markers, and apoptosis. Additionally, cerebrospinal fluid and peripheral blood leukocytes from SAH patients and controls were analyzed for ‘clock gene’ expression.
Results
Significant elevations in the clock genes Per-1, Per-2 and NPAS-2 were observed in the hippocampus, cortex and suprachiasmatic nucleus (SCN) in mice subjected to SAH at zeitgeber time (ZT) 12 when compared to ZT2. Clock gene expression amplitude correlated with basal expression of HO-1, which was also significantly greater at ZT12. SAH animals showed a significant reduction in cerebral vasospasm, neuronal apoptosis, and microglia activation at ZT12 compared to ZT2. In animals with myeloid-specific HO-1 deletion (Lyz-Cre-Hmox1fl/fl) Per-1, Per-2, and NPAS-2 expression was reduced in the SCN, which correlated with increased injury. Treatment with low dose CO rescued Lyz-Cre-Hmox1fl/fl mice, restored Per-1, Per-2 and NPAS-2 expression and reduced neuronal apoptosis.
Conclusions
Clock gene expression regulates, in part, the severity of SAH and requires myeloid HO-1 activity to clear the erythrocyte burden and inhibit neuronal apoptosis. Exposure to CO rescues the loss of HO-1 and thus merits further investigation in patients with SAH.
Keywords: Circadian Rhythm, Subarachnoid Hemorrhage, Carbon Monoxide, Brain Injury
Subject Codes: [130] Animal models of human disease; [50] Cerebral Aneurysm, AVM, & Subarachnoid hemorrhage
Introduction
There is a striking rhythm in hemorrhagic stroke onset across the 24-hour day.1, 2 We questioned whether the response to SAH would also vary as a function of time of day. Whether this temporal pattern of occurrence has implications for stroke severity and outcome remains unclear. This daily rhythm of stroke occurrence cannot be attributed to external factors like physical activity, which suggests that the central circadian clock may be implicated in the pathophysiology of stroke.3 The central circadian clock is located in the hypothalamic suprachiasmatic nuclei (SCN) and provides temporal organization for virtually all biochemical, neurobiologic and physiologic processes.4 By tracking the solar day, the SCN is able to synchronize rhythmicity throughout the body with the external environment.5,6
This robustness of circadian rhythms can be explained, in part, by the oscillating expression of genes that form transcriptional, translational, and post-translational negative feedback loops that govern rhythmicity.7 This family of clock genes consists of several transcription factors including CLOCK, BMAL-1, RevErb-α, and NPAS-2 as well as regulatory proteins including Period (Per) 1–3 and Cryptochrome (Cry) 1–2. Disturbance or deficiency in the activity of these transcription factors leads to pathophysiology in the cardiovascular system8 and the brain,9 whereas their stabilization is protective.8 Since there are distinct gene expression cycles that occur throughout the course of a day, it is tempting to speculate that these differences in expression levels represent a key determinant of the likelihood for a stroke occurrence as well as the severity of tissue injury.
A remarkable link has been shown to exist between the circadian auto-regulatory feedback loop and the endogenous bioactive gas carbon monoxide (CO), which is produced ubiquitously by the body, including the brain, by the heme oxygenase (HO). Indeed, NPAS-2 and RevErb-α contain heme, thus their turnover involves the degradation of heme but also results in the generation of CO and the bile pigments, biliverdin and bilirubin as breakdown products. We have shown that the transcription factor RevErb-α plays an important role in regulation of endothelial cell migration and is modulated in part by CO.10 Transcriptional activity of NPAS-211 and CLOCK12, two of the transcription factors that control expression of Per and Cry, have been shown to be CO-dependent by a heme-based gas sensor mechanism.
We therefore hypothesized that SAH would lead to disturbances of both central and peripheral rhythmicity in mice. We further predicted that the time of day of SAH occurrence would impact overall sensitivity to neuronal injury. Finally, we asked whether the inducible isoform of HO HO-1, and CO are critical determinants of the severity of neuronal injury after SAH, and if so, does this link to an influence on clock gene expression.
Materials and Methods
Animals
Wild-type male C57BL/6, Lyz-Cre, and Per-2−/− mice were obtained from the Jackson Laboratory. Myeloid specific HO-1 knockout (Lyz-Cre-Hmox1fl/fl) was achieved by crossing Hmox1fl/fl mice (Riken) with mice expressing Cre recombinase under the lysozyme (Lyz) promoter.
Subarachnoid Hemorrhage (SAH) Stroke Model in Mice
SAH was modeled as described previously13 and in the online supplement. Sham treatment consisted of the same surgical procedure, without injection of blood. SAH and Sham surgery were performed at zeitgeber time (ZT)0 (the beginning of the 12h light cycle), ZT2, or ZT12.
CO Gas Treatment
After SAH, Lyz-Cre-Hmox1fl/fl and Hmox1fl/fl animals were randomly assigned to receive treatment with CO (250ppm) or air for 1h as described.14 Treatment was started immediately after the SAH procedure and repeated every 24h for 7 days.
Hematoxylin/Eosin Staining and Evaluation of Cerebral Vasospasm
Frozen brains were cut in 9μm serial coronal sections and stained with hematoxylin and eosin. Representative digital images of three consecutive middle-cerebral artery (MCA) cross-sections from each animal were obtained and the lumen radius/wall thickness ratio was quantified to assess vasospasm using ImageJ.
Immunohistochemistry, TUNEL Staining and Western blot
9μm serial coronal sections were stained for ionized calcium binding adaptor molecule 1 (Iba-1) (Wako) and conjugated with the corresponding secondary antibody for fluorescent imaging (Alexa Fluor 488 donkey anti-mouse; Invitrogen) as specified in the online supplement. Nuclear counterstain was done with Hoechst 33258 (Sigma). TdT-mediated dUTP-biotin nick end labeling (TUNEL; Roche) was done as specified in the online supplement and previously reported.15 Western Blot from mouse brains was performed as described previously15 and as described in the online supplement.
Gene expression analysis
RNA was obtained and processed as specified in the online supplement. cDNA libraries were acquired by reverse transcription (iScript cDNA synthesis kit, BioRad). Gene expression was analyzed by real-time (rt)-PCR (SYBR green master mix, Agilent Technologies) with Rplp0 as a reference gene. Primer sequences are detailed in the online supplement.
Human sample collection and analysis
After acquiring patient consent, CSF and blood samples were obtained from SAH patients admitted to the intensive care unit (ICU) on day 1 and day 7. Patients with unruptured cerebral aneurysms served as controls, and therefore CSF samples in controls were only acquired once, during surgery. RNA from cerebrospinal fluid (CSF) cells was isolated with Trizol, concentrated by spin-column purification (RNEasy Micro Kit, Qiagen) and cDNA libraries were acquired by reverse transcription (iScript cDNA synthesis kit, BioRad). Gene expression of Per-2 was analyzed by rt-PCR with Rpl13a as a reference gene. Primer sequences are detailed in the online supplement.
Statistics
Data were analyzed with a computerized statistical program (GrapPad Prism Version 5). Results are presented as means (±SD). Two groups were compared with Student’s t-test, while multiple groups were compared with one-way ANOVA with post hoc Bonferroni multiple comparison. A p-value of <0.05 was considered to be statistically significant.
Study Approval
All procedures involving the animals were approved by the Institutional Animal Care and Use Committee of Beth Israel Deaconess Medical Center, Boston, MA. Patients were studied under a protocol for acquiring CSF and blood samples that was approved by the Institutional Review Board of Beth Israel Deaconess Medical Center. Patients or their legally authorized representative provided written informed consent.
Results
Cerebrospinal fluid (CSF) from subarachnoid hemorrhage (SAH) patients shows increased and sustained Per-2 expression
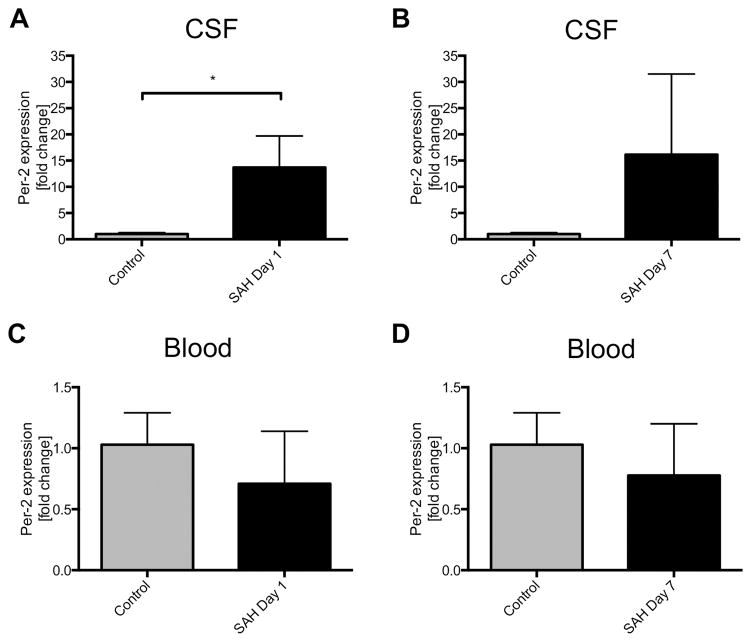
We first looked for gene expression patterns indicating disruption of normal rhythmicity in patients with SAH. On day 1 after SAH onset, Per-2 expression in cells isolated from the CSF was significantly higher as compared to control patients with unruptured aneurysm (Figure 1A). Though there was a trend for the same effect on day 7, higher variability in gene expression levels likely prevented these results from achieving statistical significance in part due to restoration of circadian rhythms in some patients (Figure 1B, p=0.09). Per-2 expression in peripheral blood leukocytes was unchanged in all groups (Figures 1C–D).
Figure 1. Per-2 expression in human subarachnoid hemorrhage (SAH) patients.
A–B: Per-2 expression in cells isolated from the cerebrospinal fluid (CSF) of SAH patients compared to control patients with unruptured cerebral aneurysms on day 1 (A) and day 7 (B) after SAH onset. [fold change vs. control]; *p=0.0072 for day 1, p=0.09 for day 7. C–D Per-2 expression in peripheral leukocytes isolated from the whole blood of SAH and control patients. Results represent mean±SD of 13 patients.
SAH disturbs circadian-dependent activity patterns
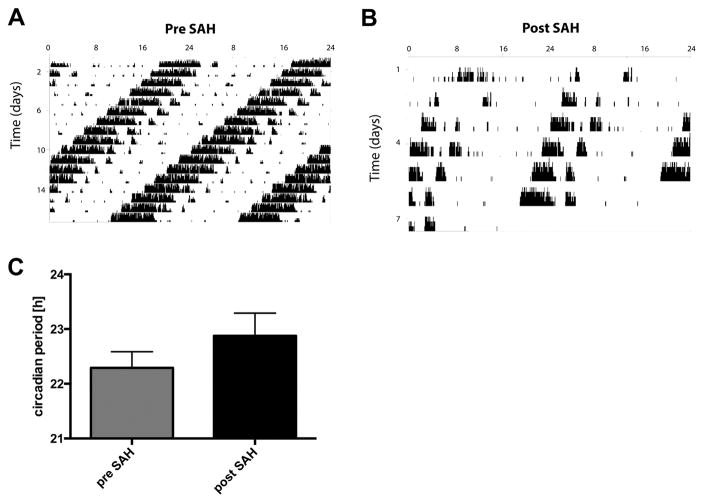
To study the effect of SAH on circadian-dependent motor activity, we chose Per-2−/− mice. These mice were housed and treated in continuous darkness (DD, free running conditions) because under these conditions, mice exhibit a shortened circadian rhythm that is easy to manipulate. DD was also used to eliminate the possibility of light entrainment that would override SAH-related changes. Per-2−/− mice housed in DD before SAH showed a shortened circadian cycle of 22.3h (Figure 2A). After SAH, the circadian period of activity pattern was approximately 30 minutes longer as compared to pre-SAH (Figure 2B–C).
Figure 2. Effects of SAH on Activity Patterns in Per2−/− mice.
A–B: Representative wheel-running actograms of Per-2−/− mice under free running conditions (constant darkness, DD) pre-SAH (A) and post-SAH (B). Results are representative of 3 independent experiments with n=3–4mice/group. C: Mean circadian period in minutes before and after SAH (pre-SAH vs. post-SAH, p=0.06).
SAH increases central and peripheral circadian rhythm gene expression
We next asked whether SAH in mice leads to disruption of clock gene expression in the brain and peripheral organs. SAH was initiated at ZT 0 and gene expression was analyzed over time. Compared to control samples taken at the same ZT times, the expression of Per-2 was induced early after SAH (Suppl. Figures IA–B). Similar effects were observed for Per-1 and NPAS-2 (data not shown). This increase in expression led to disruption of central and peripheral organ rhythmicity, as the normal cyclic change in expression of Per-2, Per-1, BMAL-1, and NPAS-2 was disrupted in the brain and kidney after SAH (Suppl. Figures IC–D, Suppl. Figure II).
Time of the day influences circadian gene and heme oxygenase (HO)-1 expression
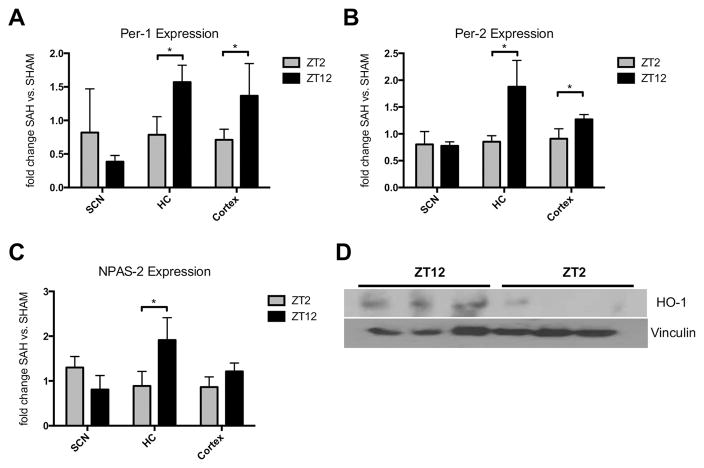
The naturally occurring oscillation of circadian rhythm gene expression as well as the fact that this rhythm is disrupted after SAH, prompted us to next study the influence of different SAH onset times on circadian gene expression. For this purpose, SAH and Sham operations were performed at either ZT2 (nadir of Per- and Cry-gene expression) or ZT12 (peak), and circadian rhythm gene expression was evaluated after 7 days. Animals with SAH occurring at ZT12 showed higher expression levels of Per-1, Per-2, NPAS-2, and CLOCK in the hippocampus (HC) and cortex as compared to animals with SAH at ZT2 (Figures 3A–C; Suppl. Figure IVA). Two transcription factors involved in the circadian rhythm feedback loop (NPAS-2, CLOCK) are modulated by the bioactive gas carbon monoxide that is generated by HO-1. Therefore, we investigated whether HO-1 expression in the brain also follows a circadian pattern. Compared to cerebral HO-1 expression at ZT2, expression at ZT12 was markedly induced (Figure 3D).
Figure 3. SAH onset time determines changes in circadian rhythm gene expression.
A–C: Changes in Per-1 (A), Per-2 (B) and NPAS-2 (C) expression in the suprachiasmatic nucleus (SCN), hippocampus (HC), and cortex of animals subjected to SAH at zeitgeber time (ZT) 2 and ZT12 compared to animals with SHAM procedures at the same time points. Samples were collected exactly 7 days after procedures to analyze the effect purely related to SAH and onset time. A: ZT2 vs. ZT12 *p=0.0212 for HC, *p=0.044 for cortex; B: *p=0.0242 for HC, *p=0.0386 for cortex; C: *p=0.0412 for HC; D: Cerebral heme oxygenase (HO)-1 protein expression analyzed by Western Blot and normalized to vinculin in animals at ZT2 and ZT12. Results represent mean ± SD with n=3–4 mice/group.
Time of day influences susceptibility to hemorrhage-induced vasospasm and neuronal injury
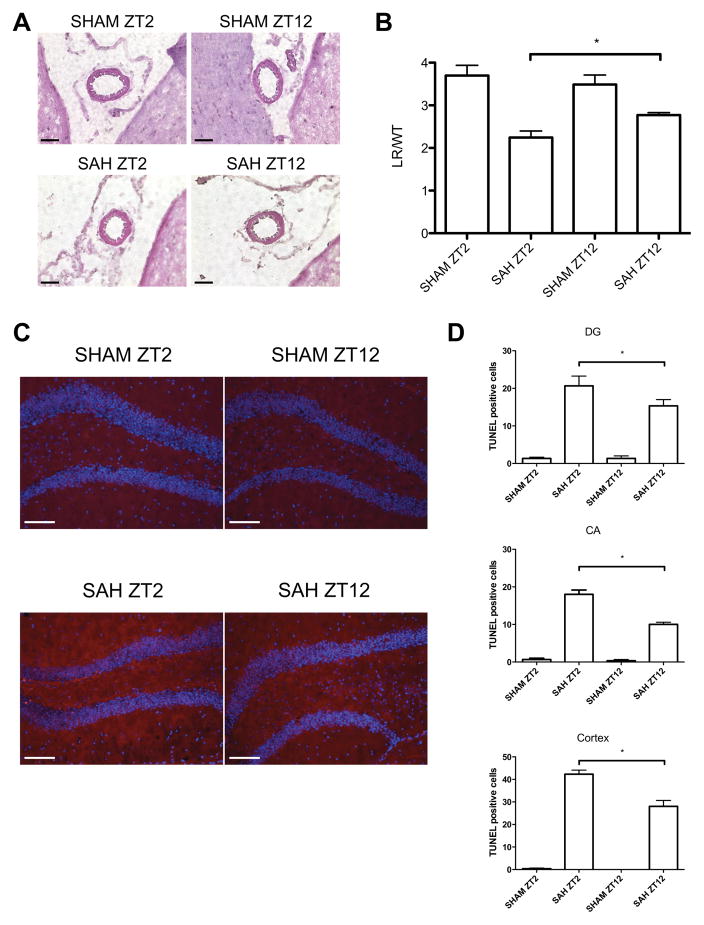
We next investigated whether the differences in circadian rhythm gene expression would alter the severity of vasospasm and neuronal injury after SAH. We measured vasospasm of the middle cerebral artery (MCA) on SAH post-operative day (POD) 7 via H&E staining (Figure 4A). SAH performed at either ZT2 or ZT12 induced vasospasm as compared to sham, but vasospasm was most severe in animals with SAH at ZT2 (Figure 4B). We also analyzed whether changes in circadian rhythm genes and the greater extent of vasospasm at ZT2 would result in increased neuronal cell death. SAH at both zeitgeber times showed increased neuronal apoptosis in the dentate gyrus (DG) and Cornu Ammonis (CA) regions of the HC as well as the cortex, when compared to sham-treated mice (Figures 4C–D and Suppl. Figures IIIA–B). . Importantly, neuronal apoptosis was markedly higher when SAH was initiated at ZT2, as compared to ZT12 (No. TUNEL-positive cells; DG 21 ±3.5 vs. 15 ±2.6; CA 18±2.0 vs. 10 ±1.0; Cortex 42 ±3.1 vs. 28 ±4.6).
Figure 4. Influence of SAH onset time on vasospasm and neuronal apoptosis.
A. Representative cross sections of the middle cerebral artery (MCA) in animals with either SHAM (upper panel) or SAH (lower panel) at ZT2 (left panel) and ZT12 (right panel), respectively, 7 days after surgery. Images are representative of 3–4 mice/group with 3 sections/brain. 40× magnification B. Quantification of MCA vasospasm was defined as the quotient of lumen radius (LR) and wall thickness (WL); results represent mean ± SD of 3 images. *p=0.0313 SAH ZT2 vs. SAH ZT12. C. TUNEL staining in the dentate gyrus (DG) 7 days after SHAM (upper panel) or SAH (lower panel) at ZT2 (left panel) and ZT12 (right panel). Representative images from 3–4 mice/group with 3 sections/brain. 20× magnification D. Quantification of TUNEL-positive cells per microscopic field (DG: top, *p=0.0387 SAH ZT2 vs. SAH ZT12; cornu ammonis (CA): middle, *p=0.0002; cortex: bottom, *p=0.0013). Results represent mean ± SD of 3 images. Scale bar = 50μM
The extent of neuroinflammation induced by SAH depends on the time of day
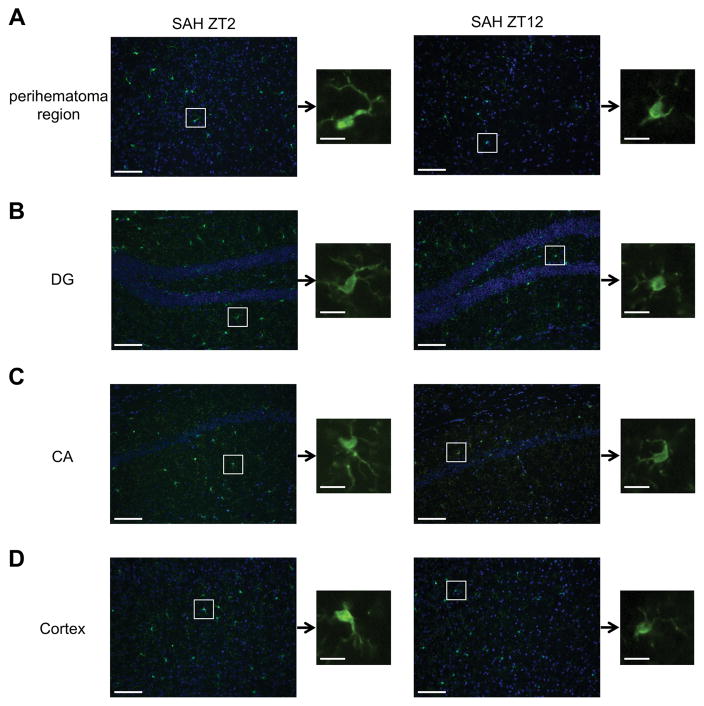
Microglia, the tissue resident macrophage of the brain, respond to injurious stimuli with changes in morphology, surface molecule expression, and migration to the site of injury. Microglial-induced neuroinflammation has been found critical to the pathology of many neurological diseases, including hemorrhagic stroke. We therefore analyzed morphological changes to microglia by immunostaining for Iba-1 surface expression in different brain regions at ZT2 and ZT12 ± SAH, which at these time points we believe are consistent for microglia. At ZT2, microglia showed a more activated morphology as compared to ZT12 with elongated cell bodies and thicker processes (Figures 5A–D). Furthermore, in the peri-hematoma region at the base of the brain, Iba-1 positive microglia were much more abundant at ZT2 compared to ZT12, indicating a higher degree of microglia migration to the site of injury. We conclude that the time of day when the injury occurs directly impacts the severity of the tissue injury.
Figure 5. The extent of neuroinflammation depends on SAH onset time.
A–D: Immunohistochemical staining for Iba-1 (green) showing the morphology and quantity of microglia cells after SAH at ZT2 and ZT12 in the perihematona region at the base of the brain (A), dentate gyrus (DG, B), cornu ammonis (CA, C) and cortex (D). Nuclei were counterstained with Hoechst 33258 (blue). Higher magnification images (20x) show the representative changes in microglia morphology. White boxes show the location of the magnified microglia. Microglia at ZT2 (left panel) show elongated cell bodies and thickened processes indicative of activation. All images are representative of 3–4 mice/group with 3 sections/brain. Scale bar = 50μM for the low magnification images and 10μM for the single cell images.
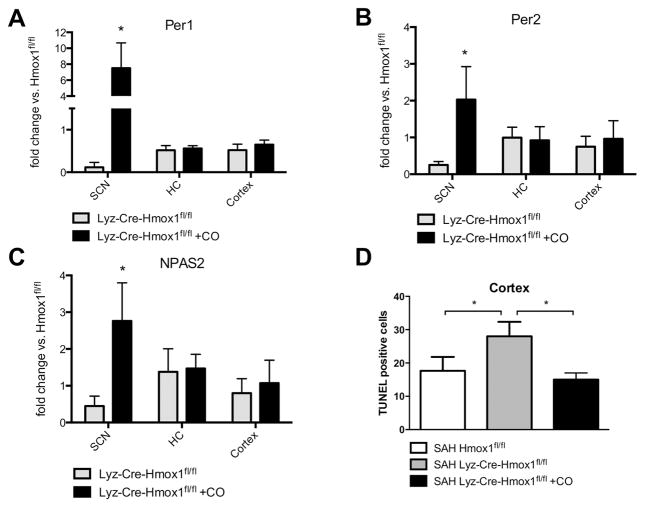
Microglial HO-1 and CO modulate circadian rhythm gene expression and neuronal injury
As shown above, increased expression of Per-1, Per-2, NPAS-2 and HO-1 were associated with less neuronal injury and neuroinflammation after SAH. We therefore investigated whether a causative link exists between CO signaling in the brain, circadian rhythm gene expression, and SAH-induced neuronal injury. Since HO-1 is predominantly upregulated in microglia following brain injury16, we utilized myeloid-specific HO-1-deficient mice (Lyz-Cre-Hmox1fl/fl) to study the role of endogenously generated CO by HO-1. Compared to Hmox1fl/fl control mice, the expression of Per-1, Per-2, NPAS-2, and CLOCK was markedly suppressed in the SCN in Lyz-Cre-Hmox1fl/fl mice (Figures 6A–C, Suppl. Figure IVB). Rescue experiments performed by exposure to CO strongly increased expression of all four circadian rhythm genes in the SCN (Figures 6A–C, Suppl. Figure IVB). In different brain regions, including the cortex, neuronal apoptosis was increased in Lyz-Cre-Hmox1fl/fl mice compared to Hmox1fl/fl controls, which was reversed when Lyz-Cre-Hmox1fl/fl mice were treated with CO (Figure 6D).
Figure 6. Myeloid HO-1 and carbon monoxide (CO) modulate circadian rhythm gene expression and susceptibility to neuronal injury.
A–D. Expression of Per-1 (A), Per-2 (B) and NPAS-2 (C) in the indicated brain regions (SCN, HC, cortex) in Lyz-Cre-Hmox1fl/fl mice ± CO treatment for 7 days after SAH compared to Hmox1fl/fl control animals. Lyz-Cre-Hmox1fl/fl vs. Lyz-Cre-Hmox1fl/fl + CO, change in the SCN: *p=0.0156 for Per-1 (A); *p=0.0267 for Per-2 (B); *p=0.0201 for NPAS-2 (C). Results represent mean ± SD from 3–4 mice/treatment group. D. Quantification of TUNEL-positive cells per microscopic field in the cortex of Hmox1fl/fl and Lyz-Cre-Hmox1fl/fl mice ±CO: Hmox1fl/fl vs. Lyz-Cre-Hmox1fl/fl *p=0.0408; Lyz-Cre- Hmox1fl/fl vs. Lyz-Cre-Hmox1fl/fl +CO *p=0.0146. Results represent mean ± SD of 3–4 mice/group.
Discussion
We show for the first time that mice subjected to SAH have disturbed molecular and behavioral circadian rhythms. Similar dysregulated clock gene expression was also observed in the CSF of SAH patients. It is clear from these studies that changes in expression levels of NPAS-2 and Period genes depend on what time of day SAH occurs. We posit that this, in turn, influences the severity of neuronal injury as measured by changes in vasoreactivity, neuronal apoptosis, and myeloid-driven neuroinflammation. Lastly, we show that the heme degradation pathway that involves HO-1 and the generation of CO, influences clock gene expression in the SCN and ultimately neuronal injury. We find that HO-1 expression is highest during the early active period for mice or ZT12 (early subjective night). Mice subjected to SAH at night showed significantly less injury and cell death when compared to ZT2 or daytime controls; this corresponded with lack of HO-1 expression. Moreover, administration of exogenous CO rescued daytime SAH mice, thus recapitulating the protection observed with increased HO-1 expression.
SAH, like other stroke subtypes, presents with a distinct temporal pattern of occurrence with the greatest frequency occurring in the early morning hours.1, 17, 18 Numerous explanations have been proposed to explain this phenomenon including changes in blood coagulation19, autonomic nervous system activation20, and blood pressure.21 However, in human stroke, disruption of circadian rhythmicity per se has not been studied as a potential etiology. This is somewhat surprising given the abundance of animal data. Reduced expression of BMAL-122, CLOCK, and particularly Per-2 increases susceptibility to multiple organ dysfunction including the brain and cardiovascular system.8, 23, 24
Only a few reports have analyzed the influence of varying clock gene expression on neuronal injury. The connection between the expression of these genes and susceptibility to injury might not be as straightforward as observed in peripheral organs due to the fact that circadian gene expression is differentially regulated depending on the specific brain region.25 Nonetheless, a connection between the quantity of Period gene expression and severity of neuronal injury after ischemic insult has been reported.9, 26 Moreover, others have demonstrated injury-dependent upregulation of Per-2 after traumatic brain injury.27 The expression pattern of circadian rhythm genes and their role in neuronal injury after SAH has not been studied. In both humans and mice, SAH leads to upregulation of clock genes in what appears to be a stress response in an effort to protect the brain from further injury. This is best supported by knockout studies, where absence of Per-2 results in enhanced injury.8
Although the auto-regulatory feedback loop controlling clock gene expression has been well described7, explicit downstream targets in specific organs remains poorly described. It is known that up to 10% of the genome participates in rhythmic patterns of expression and in a highly tissue-specific manner.28, 29 How this pattern influences susceptibility to injury and what signaling pathways and genes are expressed remains an ongoing area of study. There is likely a host of stress response genes that are involved. HO-1 is one of the more potent cytoprotective genes. When activated prior to an insult, HO-1 is highly protective in the brain and other organs; absence of HO-1 results in heightened injury. Importantly, CO, a product of HO-1 breakdown of heme, when administered exogenously, is now accepted as a protective molecule and has been shown to ameliorate hemorrhagic and ischemic stroke.15,30 CO has been shown to function as a neurotransmitter akin to nitric oxide31 and there is evidence that CO participates in long-term potentiation.32 Thus, CO is clearly involved and important in a variety of neurologic functions including inflammation and tissue stress. The mechanism by which CO exerts its neuroprotective effects in the brain is intriguing and clearly requires further study.
The role of microglia in neuronal injury is two-sided in that they can induce neuronal apoptosis via neuroinflammation13, but they can also impart neuroprotection.33 The neuronal response to activated microglia most likely depends on the etiology and degree of neuronal injury. Here, we demonstrate increased neuronal cell death, potentially due to increased microglia activation. Our data in regards to myeloid-specific HO-1 deficient mice suggests a link between severity of injury, clock gene expression, and HO-1 expression. Why HO-1 is elevated in animals at night is likely linked to their activity where we know that increases in heme-containing proteins such as those involved in metabolism, vasomotor tone, neurotransmission, and oxidative processes, all require heme turnover, and thus increased HO-1 expression. We would therefore propose that CO contributes to maintenance of proper circadian rhythms. Conversely, during the daytime when the animal sleeps, many of these same hemoproteins are not as prevalent. Collectively, we put forth a novel mechanism as to how changes in circadian rhythm might be transmitted under pathological conditions in the brain. The sudden change in clock gene expression observed after SAH may serve as a rapid signal that leads to increased HO-1 expression either due to the increased heme burden or via regulation by one or more of the clock gene transcriptional regulators such as NPAS2. It is known that HO-1 and HO-2 are involved in the activity of several transcription factors responsible for the regulation of circadian rhythm. While HO-2 is present in the brain, its expression does not change in response to external factors or stimuli. These clock gene transcription factors have been shown to be responsive to CO through their heme moieties that serve as gas-sensors.11, 12, 34 Our data show that exogenous CO modulates circadian gene expression and reduces injury. These new insights might explain the temporal pattern observed in SAH and may also help in understanding the mechanisms underlying the beneficial effects of HO-1 and CO in the brain after SAH. In the future, CO might prove to be a beneficial adjunct to stroke therapy. Understanding how the central and peripheral clocks influence the injury response may offer critical clues as to how, and perhaps more importantly, when, to intervene with therapy.
Supplementary Material
Acknowledgments
We would like to thank Drs. Chris Ogilvy and Ajith Thomas for providing control human CSF samples.
Sources of Funding
Deutsche Forschungsgemeinschaft (DFG) - SCHA1838/2-1 to NS:, NIH K08 NS078048, R21 NS099606,and BIDMC Department of Neurology to KAH; Department of Defense W81XWH-16-0464 to LEO; NS073613 to PMF, PROmote MObility in Students Deutscher Akademischer Austauschdienst (PROMOS-DAAD) to JLL.
Footnotes
Disclosures
LEO is a scientific advisor for Hillhurst Biopharmaceuticals.
References
- 1.Temes RE, Bleck T, Dugar S, Ouyang B, Mohammad Y, John S, et al. Circadian variation in ictus of aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2012;16:219–223. doi: 10.1007/s12028-011-9640-6. [DOI] [PubMed] [Google Scholar]
- 2.Elliott WJ. Circadian variation in the timing of stroke onset: A meta-analysis. Stroke. 1998;29:992–996. doi: 10.1161/01.str.29.5.992. [DOI] [PubMed] [Google Scholar]
- 3.Manfredini R, Boari B, Smolensky MH, Salmi R, la Cecilia O, Maria Malagoni A, et al. Circadian variation in stroke onset: Identical temporal pattern in ischemic and hemorrhagic events. Chronobiol Int. 2005;22:417–453. doi: 10.1081/CBI-200062927. [DOI] [PubMed] [Google Scholar]
- 4.Karatsoreos IN, Bhagat S, Bloss EB, Morrison JH, McEwen BS. Disruption of circadian clocks has ramifications for metabolism, brain, and behavior. Proc Natl Acad Sci U S A. 2011;108:1657–1662. doi: 10.1073/pnas.1018375108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Czeisler CA, Duffy JF, Shanahan TL, Brown EN, Mitchell JF, Rimmer DW, et al. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999;284:2177–2181. doi: 10.1126/science.284.5423.2177. [DOI] [PubMed] [Google Scholar]
- 6.Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, et al. Period2::Luciferase real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci U S A. 2004;101:5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shearman LP, Sriram S, Weaver DR, Maywood ES, Chaves I, Zheng B, et al. Interacting molecular loops in the mammalian circadian clock. Science. 2000;288:1013–1019. doi: 10.1126/science.288.5468.1013. [DOI] [PubMed] [Google Scholar]
- 8.Eckle T, Hartmann K, Bonney S, Reithel S, Mittelbronn M, Walker LA, et al. Adora2b-elicited per2 stabilization promotes a hif-dependent metabolic switch crucial for myocardial adaptation to ischemia. Nat Med. 2012;18:774–782. doi: 10.1038/nm.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wiebking N, Maronde E, Rami A. Increased neuronal injury in clock gene per-1 deficient-mice after cerebral ischemia. Curr Neurovasc Res. 2013;10:112–125. doi: 10.2174/1567202611310020004. [DOI] [PubMed] [Google Scholar]
- 10.Li M, Gallo D, Csizmadia E, Otterbein LE, Wegiel B. Carbon monoxide induces chromatin remodelling to facilitate endothelial cell migration. Thromb Haemost. 2014;111:951–959. doi: 10.1160/TH13-09-0748. [DOI] [PubMed] [Google Scholar]
- 11.Dioum EM, Rutter J, Tuckerman JR, Gonzalez G, Gilles-Gonzalez MA, McKnight SL. Npas2: A gas-responsive transcription factor. Science. 2002;298:2385–2387. doi: 10.1126/science.1078456. [DOI] [PubMed] [Google Scholar]
- 12.Lukat-Rodgers GS, Correia C, Botuyan MV, Mer G, Rodgers KR. Heme-based sensing by the mammalian circadian protein clock. Inorg Chem. 2010;49:6349–6365. doi: 10.1021/ic902388q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanafy KA. The role of microglia and the tlr4 pathway in neuronal apoptosis and vasospasm after subarachnoid hemorrhage. J Neuroinflammation. 2013;10:83. doi: 10.1186/1742-2094-10-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Otterbein LE, Bach FH, Alam J, Soares M, Tao Lu H, Wysk M, et al. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med. 2000;6:422–428. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- 15.Schallner N, Pandit R, LeBlanc R, 3rd, Thomas AJ, Ogilvy CS, Zuckerbraun BS, et al. Microglia regulate blood clearance in subarachnoid hemorrhage by heme oxygenase-1. J Clin Invest. 2015;125:2609–2625. doi: 10.1172/JCI78443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matz P, Turner C, Weinstein PR, Massa SM, Panter SS, Sharp FR. Heme-oxygenase-1 induction in glia throughout rat brain following experimental subarachnoid hemorrhage. Brain Res. 1996;713:211–222. doi: 10.1016/0006-8993(95)01511-6. [DOI] [PubMed] [Google Scholar]
- 17.Kelly-Hayes M, Wolf PA, Kase CS, Brand FN, McGuirk JM, D’Agostino RB. Temporal patterns of stroke onset. The framingham study. Stroke. 1995;26:1343–1347. doi: 10.1161/01.str.26.8.1343. [DOI] [PubMed] [Google Scholar]
- 18.Miranpuri AS, Akture E, Baggott CD, Miranpuri A, Uluc K, Gunes VE, et al. Demographic, circadian, and climatic factors in non-aneurysmal versus aneursymal subarachnoid hemorrhage. Clin Neurol Neurosurg. 2013;115:298–303. doi: 10.1016/j.clineuro.2012.05.039. [DOI] [PubMed] [Google Scholar]
- 19.Casetta I, Granieri E, Fallica E, la Cecilia O, Paolino E, Manfredini R. Patient demographic and clinical features and circadian variation in onset of ischemic stroke. Arch Neurol. 2002;59:48–53. doi: 10.1001/archneur.59.1.48. [DOI] [PubMed] [Google Scholar]
- 20.Panza JA, Epstein SE, Quyyumi AA. Circadian variation in vascular tone and its relation to alpha-sympathetic vasoconstrictor activity. N Engl J Med. 1991;325:986–990. doi: 10.1056/NEJM199110033251402. [DOI] [PubMed] [Google Scholar]
- 21.Castilla-Guerra L, Espino-Montoro A, Fernandez-Moreno MC, Lopez-Chozas JM. Abnormal blood pressure circadian rhythm in acute ischaemic stroke: Are lacunar strokes really different? Int J Stroke. 2009;4:257–261. doi: 10.1111/j.1747-4949.2009.00314.x. [DOI] [PubMed] [Google Scholar]
- 22.Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV, Antoch MP. Early aging and age-related pathologies in mice deficient in bmal1, the core componentof the circadian clock. Genes Dev. 2006;20:1868–1873. doi: 10.1101/gad.1432206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lefta M, Campbell KS, Feng HZ, Jin JP, Esser KA. Development of dilated cardiomyopathy in bmal1-deficient mice. Am J Physiol Heart Circ Physiol. 2012;303:H475–485. doi: 10.1152/ajpheart.00238.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Viswambharan H, Carvas JM, Antic V, Marecic A, Jud C, Zaugg CE, et al. Mutation of the circadian clock gene per2 alters vascular endothelial function. Circulation. 2007;115:2188–2195. doi: 10.1161/CIRCULATIONAHA.106.653303. [DOI] [PubMed] [Google Scholar]
- 25.Abe M, Herzog ED, Yamazaki S, Straume M, Tei H, Sakaki Y, et al. Circadian rhythms in isolated brain regions. J Neurosci. 2002;22:350–356. doi: 10.1523/JNEUROSCI.22-01-00350.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tischkau SA, Cohen JA, Stark JT, Gross DR, Bottum KM. Time-of-day affects expression of hippocampal markers for ischemic damage induced by global ischemia. Exp Neurol. 2007;208:314–322. doi: 10.1016/j.expneurol.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 27.Boone DR, Sell SL, Micci MA, Crookshanks JM, Parsley M, Uchida T, et al. Traumatic brain injury-induced dysregulation of the circadian clock. PLoS One. 2012;7:e46204. doi: 10.1371/journal.pone.0046204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, et al. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109:307–320. doi: 10.1016/s0092-8674(02)00722-5. [DOI] [PubMed] [Google Scholar]
- 29.Storch KF, Lipan O, Leykin I, Viswanathan N, Davis FC, Wong WH, et al. Extensive and divergent circadian gene expression in liver and heart. Nature. 2002;417:78–83. doi: 10.1038/nature744. [DOI] [PubMed] [Google Scholar]
- 30.Wang B, Cao W, Biswal S, Dore S. Carbon monoxide-activated nrf2 pathway leads to protection against permanent focal cerebral ischemia. Stroke. 2011;42:2605–2610. doi: 10.1161/STROKEAHA.110.607101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verma A, Hirsch DJ, Glatt CE, Ronnett GV, Snyder SH. Carbon monoxide: A putative neural messenger. Science. 1993;259:381–384. doi: 10.1126/science.7678352. [DOI] [PubMed] [Google Scholar]
- 32.Alkadhi KA, Al-Hijailan RS, Malik K, Hogan YH. Retrograde carbon monoxide is required for induction of long-term potentiation in rat superior cervical ganglion. J Neurosci. 2001;21:3515–3520. doi: 10.1523/JNEUROSCI.21-10-03515.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vinet J, Weering HR, Heinrich A, Kalin RE, Wegner A, Brouwer N, et al. Neuroprotective function for ramified microglia in hippocampal excitotoxicity. J Neuroinflammation. 2012;9:27. doi: 10.1186/1742-2094-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yin L, Wu N, Curtin JC, Qatanani M, Szwergold NR, Reid RA, et al. Rev-erbalpha, a heme sensor that coordinates metabolic and circadian pathways. Science. 2007;318:1786–1789. doi: 10.1126/science.1150179. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.