Abstract
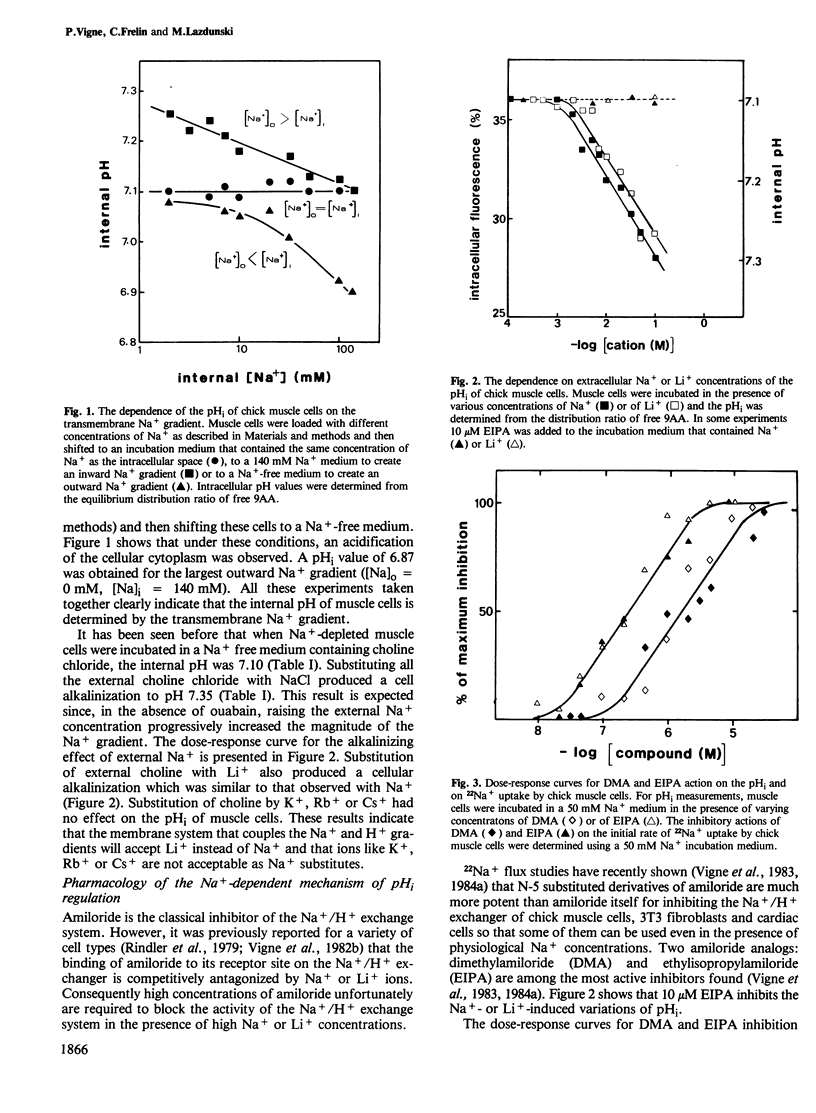
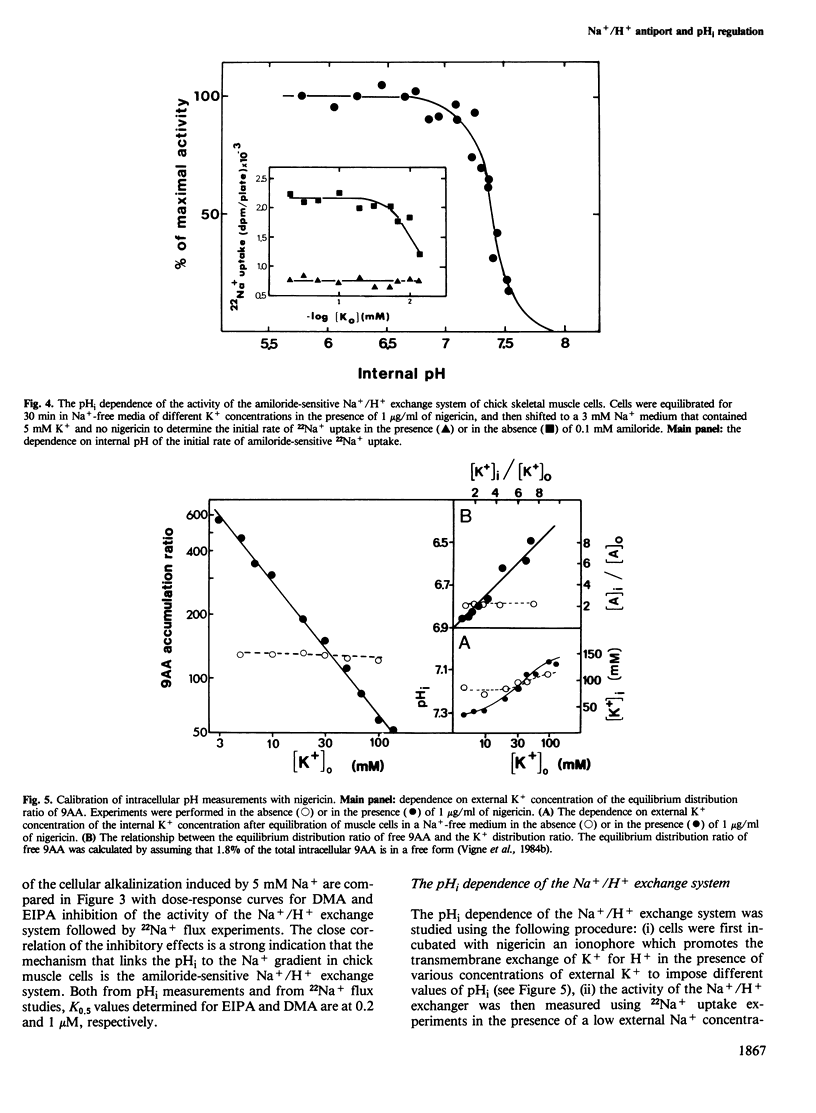
The internal pH (pHi) of chick muscle cells is determined by the transmembrane Na+ gradient. Li+, but not K+, Rb+ or Cs+, can substitute for Na+ for regulating the internal pH of chick muscle cells. Pharmacological evidence using amiloride and amiloride analogs has shown that the Na+/H+ exchange system is the membrane mechanism that couples the pHi to the transmembrane Na+ gradient. The pHi dependence of the amiloride-sensitive Na+/H+ exchange mechanism was defined. Internal H+ interacts cooperatively with the Na+/H+ exchange system, in contrast with external H+, thus indicating an asymmetrical behaviour of this exchanger. The half-maximum effect for the activation by the internal H+ of the Na+ transporting activity of the amiloride-sensitive Na+/H+ exchange was observed at pH 7.4. The Hill coefficient of the H+ concentration dependence is higher than 3. Insulin was shown to have no effect on the pHi of chick muscle cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aickin C. C., Thomas R. C. An investigation of the ionic mechanism of intracellular pH regulation in mouse soleus muscle fibres. J Physiol. 1977 Dec;273(1):295–316. doi: 10.1113/jphysiol.1977.sp012095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babcock D. F. Examination of the intracellular ionic environment and of ionophore action by null point measurements employing the fluorescein chromophore. J Biol Chem. 1983 May 25;258(10):6380–6389. [PubMed] [Google Scholar]
- Boron W. F., McCormick W. C., Roos A. pH regulation in barnacle muscle fibers: dependence on extracellular sodium and bicarbonate. Am J Physiol. 1981 Jan;240(1):C80–C89. doi: 10.1152/ajpcell.1981.240.1.C80. [DOI] [PubMed] [Google Scholar]
- Christen R., Schackmann R. W., Shapiro B. M. Elevation of the intracellular pH activates respiration and motility of sperm of the sea urchin, Strongylocentrotus purpuratus. J Biol Chem. 1982 Dec 25;257(24):14881–14890. [PubMed] [Google Scholar]
- Frelin C., Lombet A., Vigne P., Romey G., Lazdunski M. The appearance of voltage-sensitive Na+ channels during the in vitro differentiation of embryonic chick skeletal muscle cells. J Biol Chem. 1981 Dec 10;256(23):12355–12361. [PubMed] [Google Scholar]
- Frelin C., Vigne P., Lazdunski M. The amiloride-sensitive Na+/H+ antiport in 3T3 fibroblasts. J Biol Chem. 1983 May 25;258(10):6272–6276. [PubMed] [Google Scholar]
- Gross J. D., Bradbury J., Kay R. R., Peacey M. J. Intracellular pH and the control of cell differentiation in Dictyostelium discoideum. Nature. 1983 May 19;303(5914):244–245. doi: 10.1038/303244a0. [DOI] [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Johnson C. H., Epel D. Intracellular pH of sea urchin eggs measured by the dimethyloxazolidinedione (DMO) method. J Cell Biol. 1981 May;89(2):284–291. doi: 10.1083/jcb.89.2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L'Allemain G., Paris S., Pouysségur J. Growth factor action and intracellular pH regulation in fibroblasts. Evidence for a major role of the Na+/H+ antiport. J Biol Chem. 1984 May 10;259(9):5809–5815. [PubMed] [Google Scholar]
- Lee H. C., Johnson C., Epel D. Changes in internal pH associated with initiation of motility and acrosome reaction of sea urchin sperm. Dev Biol. 1983 Jan;95(1):31–45. doi: 10.1016/0012-1606(83)90004-0. [DOI] [PubMed] [Google Scholar]
- Moolenaar W. H., Boonstra J., van der Saag P. T., de Laat S. W. Sodium/proton exchange in mouse neuroblastoma cells. J Biol Chem. 1981 Dec 25;256(24):12883–12887. [PubMed] [Google Scholar]
- Moolenaar W. H., Tsien R. Y., van der Saag P. T., de Laat S. W. Na+/H+ exchange and cytoplasmic pH in the action of growth factors in human fibroblasts. Nature. 1983 Aug 18;304(5927):645–648. doi: 10.1038/304645a0. [DOI] [PubMed] [Google Scholar]
- Moore R. D. Stimulation of Na:H exchange by insulin. Biophys J. 1981 Feb;33(2):203–210. doi: 10.1016/S0006-3495(81)84881-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouysségur J., Chambard J. C., Franchi A., Paris S., Van Obberghen-Schilling E. Growth factor activation of an amiloride-sensitive Na+/H+ exchange system in quiescent fibroblasts: coupling to ribosomal protein S6 phosphorylation. Proc Natl Acad Sci U S A. 1982 Jul;79(13):3935–3939. doi: 10.1073/pnas.79.13.3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed P. W. Ionophores. Methods Enzymol. 1979;55:435–454. doi: 10.1016/0076-6879(79)55058-7. [DOI] [PubMed] [Google Scholar]
- Rindler M. J., McRoberts J. A., Saier M. H., Jr (Na+,K+)-cotransport in the Madin-Darby canine kidney cell line. Kinetic characterization of the interaction between Na+ and K+. J Biol Chem. 1982 Mar 10;257(5):2254–2259. [PubMed] [Google Scholar]
- Rindler M. J., Taub M., Saier M. H., Jr Uptake of 22Na+ by cultured dog kidney cells (MDCK). J Biol Chem. 1979 Nov 25;254(22):11431–11439. [PubMed] [Google Scholar]
- Roos A., Boron W. F. Intracellular pH. Physiol Rev. 1981 Apr;61(2):296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- Sandra A., Przybylski R. J. Ontogeny of insulin binding during chick skeletal myogenesis in vitro. Dev Biol. 1979 Feb;68(2):546–556. doi: 10.1016/0012-1606(79)90225-2. [DOI] [PubMed] [Google Scholar]
- Schuldiner S., Rozengurt E. Na+/H+ antiport in Swiss 3T3 cells: mitogenic stimulation leads to cytoplasmic alkalinization. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7778–7782. doi: 10.1073/pnas.79.24.7778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitz H., Vincent J. P., Barhanin J., Frelin C., Linden G., Hugues M., Lazdunski M. Purification and pharmacological properties of eight sea anemone toxins from Anemonia sulcata, Anthopleura xanthogrammica, Stoichactis giganteus, and Actinodendron plumosum. Biochemistry. 1981 Sep 1;20(18):5245–5252. doi: 10.1021/bi00521a023. [DOI] [PubMed] [Google Scholar]
- Thomas J. A., Buchsbaum R. N., Zimniak A., Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979 May 29;18(11):2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- Vigne P., Frelin C., Cragoe E. J., Jr, Lazdunski M. Ethylisopropyl-amiloride: a new and highly potent derivative of amiloride for the inhibition of the Na+/H+ exchange system in various cell types. Biochem Biophys Res Commun. 1983 Oct 14;116(1):86–90. doi: 10.1016/0006-291x(83)90384-4. [DOI] [PubMed] [Google Scholar]
- Vigne P., Frelin C., Cragoe E. J., Jr, Lazdunski M. Structure-activity relationships of amiloride and certain of its analogues in relation to the blockade of the Na+/H+ exchange system. Mol Pharmacol. 1984 Jan;25(1):131–136. [PubMed] [Google Scholar]
- Vigne P., Frelin C., Lazdunski M. Ontogeny of the (Na+,K+)-ATPase during chick skeletal myogenesis. J Biol Chem. 1982 May 25;257(10):5380–5384. [PubMed] [Google Scholar]
- Vigne P., Frelin C., Lazdunski M. The amiloride-sensitive Na+/H+ exchange system in skeletal muscle cells in culture. J Biol Chem. 1982 Aug 25;257(16):9394–9400. [PubMed] [Google Scholar]


