Abstract
Measurement of the serum concentration of insulin-like growth factor-1 (IGF-I) is generally used as a screening investigation for disorders of the growth hormone (GH)/IGF-I axis in children and adolescents with short stature. IGF-I concentration is sensitive to short-term and chronic alterations in the nutritional state, and the interpretation of IGF-I measurements requires knowledge of the child’s nutritional status. In this review, we summarize the effects of nutrition on the GH/IGF-I axis, and review the clinical implications of these interactions throughout childhood, both in under-nutrition and over-nutrition.
Keywords: IGF-I, Nutrition, Growth hormone, Obesity
Introduction
Faltering linear growth in childhood may be a sensitive but nonspecific sign of disease (1). Two of the diagnostic categories that can present with faltering growth are insufficient nutrition and disorders of the growth hormone (GH) / insulin-like growth factor-l (IGF-I) axis. GH stimulates IGF-I production, and measurement of IGF-I concentration is commonly used to screen for disorders of the GH/IGF-I axis (2,3). Nutritional status and GH signaling can both affect IGF-I concentrations, and an understanding of these overlapping effects on IGF-I production is essential in the appropriate interpretation of IGF-I measurements in children. The aim of this review is to provide an overview of the effects of nutrition on the GH/IGF-I axis and to summarize the clinical implications of these interactions throughout childhood, both in under-nutrition and over-nutrition.
The Growth Hormone / Insulin-like Growth Factor-l Axis and Potential Interaction with Nutrition
GH is secreted by the somatotrophic cells of the anterior pituitary gland in pulsatile fashion, with approximately five to eight peaks each day (4). This pulsatile secretion is stimulated by hypothalamic growth hormone releasing hormone (GHRH), with endocrine feedback mechanisms further regulating this system. These include somatostatin, IGF-I, and ghrelin (Figure 1). Nutritional status can interact with the GH/IGF-I axis at numerous points in the pathway, at both the hormone secretion and postreceptor signaling levels.
Figure 1. The effect of under-nutrition on GH secretion.

Leptin modifies hypothalamic regulation of GH secretion through hypothalamic receptors, and in severe under-nutrition insufficient leptin reduces GH production both directly and through the consequent increase in Neuropeptide Y. Anterior pituitary GH secretion is controlled by hypothalamic GHRH and somatostatin, as well as feedback mechanisms through IGF-I, ghrelin and GH concentrations.
GH= Growth Hormone, IGF-l = Insulin-like Growth Factor-1, IGFBP = Insulin-like Binding Protein, ALS = Acid Labile Subunit, GHRH = Growth Hormone Releasing Hormone
The Impact of Under-Nutrition on the Regulation of Growth Hormone Secretion
In chronic under-nutrition, alterations in leptin and neuropeptide Y (NPY) concentrations can reduce GH secretion. Leptin is produced by adipose tissue (5), and normal concentrations of circulating leptin are required for GH secretion. In animal studies, reduction in leptin concentrations by leptin anti-serum led to decreased GH secretion (6). The mechanism for this interaction may be through a direct effect on hypothalamic leptin receptors, or indirectly through NPY. Leptin suppresses hypothalamic NPY production, and NPY suppresses GH release (7,8). Thus, in starvation there are reduced leptin concentrations and increased hypothalamic production of NPY, which may reduce pituitary GH secretion.
The oxyntic glands of the gastric fundus secrete Ghrelin and this binds to the GH secretagogue receptor 1a, stimulating increased GH secretion by the anterior pituitary. Three days of a 50% reduction in caloric intake of prior to GH stimulation testing may increase the peak stimulated GH concentration achieved during testing of GH-sufficient children (9). Ghrelin concentrations are increased during fasting and suppressed with feeding (10) and may mediate this effect. However, ghrelin administration in chronic malnutrition seen in patients with anorexia nervosa is not associated with a rise in GH concentration (11).
The Impact of Under-Nutrition on Growth Hormone Signaling
The GH receptor is a type 1 cytokine receptor that is expressed predominantly by hepatocytes. GH binding results in rotation of one of the monomers of this dimeric receptor, and the intracellular domain binds Janus Kinase 2 (JAK2) (12) This activates numerous signaling pathways, specifically the Signal Transducer and Activator of Transcription (STAT) -1, -3, -5a, -5b, Mitogen-activated protein kinase (MAPK) and phosphoinositide-3-kinase (PI3K) pathways (13) (Figure 2). Activation of these intracellular signaling pathways, primarily STAT5b, stimulates transcription of IGF-I.
Figure 2. Nutrition and intracellular growth hormone signaling.
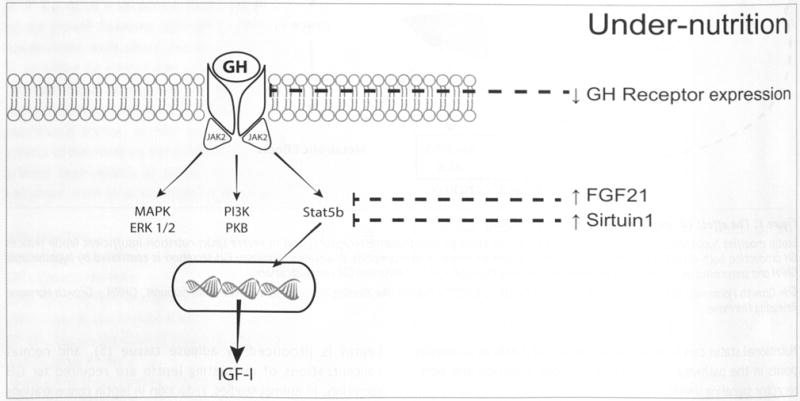
Growth hormone binds to the extracellular domain of the dimeric GH receptor, and results in activation of multiple intracellular signaling pathways. GH receptor expression is reduced in caloric restriction. FGF21 and Sirtuin-1 concentrations are increased in fasting, and both of these reduce tyrosine kinase phosphorylation of STAT5b.
JAK2 = Janus kinase 2, MAPK = Mitogen-activated protein kinase, ERK = Extracellular-signal regulated kinase, PI3K = Phosphoinositide 3 kinase, PKB = Protein kinase B (also known as AKT), Stat = Signal transducer and activator of transcription, FGF21 = Fibroblast growth factor 21, GH = growth hormone, IGF-I = insulin-like growth factor-1
Under-nutrition may affect GH signaling at multiple points on the pathway and cause a state of GH resistance. In animal and cell-based models, caloric restriction is associated with a reduction in GH receptor mRNA transcription (14,15). Insulin increases hepatic GH receptor availability (16), and reduced insulin concentration during fasting may play a role in the reduction of GH receptor transcription. Calorie and protein malnutrition can also cause GH resistance through effects on post-receptor signaling. Fibroblast growth factor 21 (FGF21) is produced by adipocytes and hepatocytes, and concentrations are increased in fasting (17). FGF21 reduces STAT5b phosphorylation and increases Suppressor of Cytokine Signaling 2 (SOCS2) expression, both of which decrease IGF-I production (18). FGF21 also increases IGFBP-1 expression, which further reduces IGF-I bioavailability for signaling (19). Another potential mechanism involves Sirtuin-1, a deacetylase that mediates the metabolic response to fasting through its effects on glucose and lipid metabolism (20). Sirtuin-1 also inhibits the tyrosine phosphorylation of STAT5 (21), and represents an additional cellular mechanism of GH resistance in malnutrition. Zinc (22) magnesium (23) and vitamin B6 (24) deficiencies may also be associated with GH resistance and reduced IGF-I, although the mechanisms of each of these are unknown.
Nutrition and IGF-I from the Fetus through Adolescence
Prenatal GH deficiency (GHD) has minimal effect on birth size (25), whereas children with IGF-I receptor mutations or in utero IGF-I deficiency are born with severe intrauterine growth restriction (26). The GH-independent regulation of fetal IGF-I is poorly understood, but the pattern of changes in fetal IGF-I concentration mirrors weight gain. Fetal serum IGF-I concentrations increase steadily throughout the third trimester (27), which coincides with the period of most rapid increase in fetal weight. In addition, umbilical cord IGF-I concentrations at birth correlate with birthweight (28).
Following birth, IGF-I is closely associated with weight and nutritional intake (29,30) While adequate caloric intake is necessary for symmetrical infant growth, caloric excess will increase infant weight more than length and head circumference (31). Changes in circulating IGF-I concentrations can reflect increases in protein and caloric intake, and IGF-I measurement has been suggested as a method of monitoring feeding, particularly in preterm infants (32). In early infancy, formula fed infants have increased adiposity and higher IGF-I concentrations than breastfed infants (33–36). The effect of milk in increasing IGF-I concentrations has been studied further in 2 to 3 year-olds. In this population, circulating IGF-I is increased by 30% when milk intake increases from 200 to 600 ml/day, an effect predominantly due to the casein component of milk (37–39).
In prepubertal children, lower body mass index (BMI) is associated with lower serum IGF-I concentrations (40). Although reduced caloric intake is associated with low circulating IGF-I, nutritional factors beyond calories and protein should also be considered in children with unexpectedly low IGF-I levels. Zinc deficiency, for example, in peripubertal children is associated with lower IGF-I and IGFBP-3 concentrations. IGF-I concentrations improve with zinc replacement in these children (22,41). Iodine deficiency is also associated with low circulating IGF-I, but replacement of iodine deficiency can have the effect of reducing IGF-I concentrations further (42). This finding is most likely related to the effect of iodine replacement on thyroid function. Excess iodine can suppress thyroid function through the Wolff-Chaikoff effect, and IGF-I concentrations are reduced in hypothyroidism (43,44).
IGF-I concentrations increase during puberty, and energy requirements are also increased during this time (45). The sex steroids of puberty increase pituitary GH secretion, which in turn increases IGF-I production. The combination of increased sex steroids, GH and IGF-I cause the pubertal growth spurt. Insufficient nutrition can delay the onset of puberty (46), and leptin concentrations above a low threshold appear to be required for puberty to proceed (47,48). Low IGF-I concentrations in undernourished adolescents can appear even lower relative to reference ranges if age-based rather than pubertal stage-specific reference ranges for IGF-I are reported.
Under-Nutrition and IGF-I
Sufficient nutrition is a balance between caloric intake and energy expenditure. Caloric intake may be affected by food availability and appetite, and increased energy expenditure may put active or pubescent children at risk for undernutrition. In this section, we will review populations at particular risk.
Populations at Risk for GH/IGF Axis Changes from Insufficient Intake
Food availability can be a significant issue for many families, and may not be immediately apparent during a clinical visit. The worldwide prevalence of undernourishment is estimated at 11.3% (49), and an estimated 50 million people in the United States are uncertain of having enough food (50). This may affect the type and quantity of food available in the household (51,52). As will be discussed later in this section, even a transient 50% reduction in calorie or 33% reduction in protein availability can result in a reversible reduction in IGF-I concentrations (Figure 3) (53). This may be relevant in families where the next paycheck is required before food can be purchased. Dietary intake may be decreased despite adequate food availability due to certain feeding behaviors and/or diminished appetite. Sometimes the dietary intake is inadvertently over-restricted by the parent(s) and/or child due to fear of obesity (54–56) or hypercholesterolemia (56) to the extent that nutritional growth stunting ensues. Unstructured mealtimes, particularly those with distractions, as well as food aversions and dysfunctional parent-child interactions related to eating can all lead to failure to thrive, a topic extensively reviewed elsewhere (57). Reduced caloric intake can also result from decreased appetite, which may be endogenous (such as from delayed gastric emptying) or induced by medications. Attention deficit and hyperactivity disorder (ADHD) affects approximately 7% of children (58) and methylphenidate or dexamphetamine are commonly used to treat this disorder. These medications can be associated with appetite suppression and subsequent weight loss (59). In a small study that included healthy children treated with methylphenidate, reduced weight and BMI were seen within 4 months of treatment and an associated reduction in IGF-I concentration was observed (60).
Figure 3. Seven days of 50% caloric restriction (35 Cal/kg) resulted in a reduction in mean IGF-I amongst eight prepubertal children.

Mean IGF-I also decreased during 33% protein restriction (0.66 g/kg) in 6 other prepubertal children. IGF-I concentrations in both groups of patients before, during and after restriction are shown. Figure modified from Smith et al (53)
Populations at Risk for GH/IGF Axis Changes from Increased Demands
The recommended caloric intake in children and adolescents is dependent upon energy expenditure. The estimated energy requirements in childhood varies according to age and sex, and ranges from 520 kcal/day in the infant female to 3,152 kcal/day in the adolescent male. This is increased further in the setting of regular rigorous exercise (45). Nutritional intake should be considered in the context of an individual’s activity level, as similar dietary intakes may be sufficient for a sedentary child but insufficient for an extremely active child. This chronic nutritional insufficiency may affect IGF-I concentrations.
Failure to meet the nutritional demands of competitive sport is prevalent amongst adolescents (61–63). For example, a study of adolescent soccer players showed that the mean caloric intake was almost 500 kcal/day less than estimated requirements (64). At the extreme end of the spectrum of under-nutrition in sport, a triad that includes low energy availability, menstrual dysfunction and reduced bone mineral density has been well described in females (65). Up to 8% of athletic females have two or more features of this triad (66) and low estrogen and IGF-I levels are thought to play a role in the pathogenesis of the low bone mineral density (67). However, the adverse effects of under-nutrition in males should not be underestimated, and the International Olympic Committee’s recent guidelines has replaced the term “Female Athlete Triad” to “Relative Energy Deficiency in Sport” (68). This expands the definition to include both male athletes and other health repercussions beyond the original three, and acknowledges the variable degrees of severity of this condition (68). IGF-I concentrations in gymnasts are lower than in controls (69) and this may be a associated with the high prevalence of insufficient nutritional intake in this sport (61).
Reduced IGF-I concentrations are also seen in systemic diseases associated with increased energy requirements and/or malabsorption. These conditions include inflammatory bowel disease, cystic fibrosis, cardiac disease (particularly cyanotic conditions and congestive failure) and HIV Infection. Ensuring adequate nutrition in each of these conditions is one of the key components of management. Poor linear growth is seen in children with severe disease, and low IGF-I concentrations may reflect insufficient nutritional intake in these children. Inflammatory factors may also play a contributory role (reviewed in 70,71).
Populations at Risk for GH/IGF Axis Changes from Malabsorption
Low IGF-I concentrations may be an early sign of malabsorptive disorders, even in the absence of gastrointestinal symptoms. Celiac disease may present with a broad spectrum of symptoms and signs ranging from asymptomatic mild malabsorption (called monosymptomatic celiac disease), where the only symptom is growth failure (72) to severe malnutrition and secondary failure to thrive. Even in minimally symptomatic children, IGF-I is lower than controls at diagnosis and normalizes with a gluten- free diet in parallel to increasing BMI (73). In children with established celiac disease, gluten exposure leads to a reduction in circulating IGF-I concentration proportional to the degree of small bowel mucosal inflammation (74). The correlation of IGF-I with celiac disease activity has been replicated in many pediatric (74–76) and adult (77) studies, and IGF-I has even been suggested as an additional marker for monitoring celiac disease activity for this reason (75).
Complicating the observation of low IGF-I concentrations in children with undiagnosed celiac disease is the putative link between celiac disease and GH deficiency. Ferrante et al performed GH stimulation testing in adults with new onset celiac disease, and found that a quarter of patients were characterized has having impaired GH secretion on these tests (78). It should be noted, however, that the stimulation tests were performed at baseline and not repeated following initiation of a gluten-free diet. As mentioned previously, blunted response to GH stimulation testing similarly was found in children with protein malnutrition (79). This responded to dietary replacement, and should caution physicians against performing stimulation testing prior to dietary management. However, a potential diagnosis of comorbid GHD should be considered in children without improved linear growth despite good adherence to the gluten-free diet (80,81).
Poor growth and low IGF-I concentrations may also be a presentation of pediatric Crohn’s disease. One tenth of affected children are more than two standard deviations below the mean for height at diagnosis (82), and they may have multiple etiologies for reduced IGF-I concentrations. These include malnutrition complicated by increased metabolic rate during illness, increased inflammatory cytokines, and delayed puberty (73). IGF-I concentrations increase during disease remission (83).
The Effect of Mild Protein or Caloric Restriction on the GH/IGF-I Axis
The GH/IGF axis is sensitive to less severe and transient nutritional restrictions. Smith et al (53) measured serum IGF-I in prepubertal children prior to and after six days of 50% calorie or 33% protein restriction. IGF-I levels were reduced during the periods of decreased intake of either calories or protein (Figure 3). This reduced IGF-I concentration during restriction is due to GH resistance rather than decreased GH secretion, as has been shown in similar experiments in children with confirmed GHD. In these children, IGF-I production was reduced during fasting when a consistent dose of GH was provided (84). In normal, fasted children, IGF-I returns to baseline concentrations following resumption of a normal diet (53). Similar effects on IGF-I concentrations have also been shown in adults undergoing four days of fasting (85). In addition to reduced circulating IGF-I during caloric restriction, the growth plate is also less responsive to IGF-I and GH through reduced expression of growth plate GH and IGF-I receptors (86).
The Effect of Severe Protein or Caloric Restriction on the GH/IGF-I Axis
Kwashiorkor is defined as severe protein deficiency with adequate energy intake and is characterized by hypoproteinemia, edema, hepatomegaly and changes of the skin mucous membranes. Marasmus occurs when there is severe deficiency of energy and in its pure form does not include the aforementioned clinical features. There is occasionally overlap (marasmic kwashiorhor) where there is a deficiency of both energy and protein. The GH/IGF-I axis has been studied in children with marasmus, kwashiorkor and marasmic kwashiorkor, and provides insight into the effects of protein and calories on the axis (87). At baseline, in all of these patterns of under-nutrition, low IGF-I and increased GH concentrations are seen. When GH stimulation testing was performed in children with marasmus, kwashiorkor or marasmic kwashiorkor prior to nutrition therapy, the baseline GH concentration is higher and the increase in GH secretion upon stimulation testing is less than in controls (79).
Hintz et al (88) studied 27 Thai children under 5 years of age, and Soliman et al (79) studied 51 Egyptian children under 3 years of age with kwashiorkor or marasmus and both showed similar results. Prior to nutritional repletion, GH concentrations were increased and IGF-I concentrations were decreased relative to controls. There were no significant differences in the magnitude of these changes between the children with kwashiorkor or marasmus. Serum IGF-I levels did not correlate with albumin concentration at baseline (88), suggesting that reduced IGF-I is not merely a result of decreased amino acid availability or reduced hepatic protein synthesis. IGF-I may, however, correlate with nutritional markers such as BMI or arm circumference (79).
Nutritional replacement in children with severe protein and/ or calorie malnutrition can normalize the GH/IGF-I axis. Within two weeks of refeeding, IGF-I concentrations can double in severely malnourished children, to a level within 2 standard deviations of the mean for the population (89). After 50 days of intensive inpatient nutritional therapy (88), basal GH and IGF-I levels are indistinguishable from controls (Figure 4). Interestingly, IGF-I Z-scores are not associated with weight- for-height Z-scores in severe malnutrition, but they have a linear relationship after two weeks of refeeding (89). Similarly, nutritional interventions are associated with early increases in IGF-I levels, even before changes in anthropometric measures are observed (90). The blunted increase in GH concentrations following arginine stimulation testing also resolves with treatment (79).
Figure 4. The effect of nutritional treatment on serum IGF-I and GH in a group of children with severe protein-energy malnutrition.

Mean ± SEM IGF-I concentrations in healthy control subjects are shown in grey. Figure modified from Hintz et al (88)
Excess Nutrition and IGF-I
Approximately one fifth of children in developed countries are overweight and, even in developing countries with a high prevalence of under-nutrition, the percentage of children who are overweight is increasing (91,92) Obese children are taller than non-obese in childhood, but enter puberty earlier (93) and have comparable adult heights (94–96). Children who develop obesity earlier in childhood are more likely to have taller stature in childhood than their peers (97). As will be outlined in this section, obesity affects the GH/IGF-I axis, and this may play a role in the accelerated statural growth.
Obesity and the GH/IGF-I Axis
Increased BMI is associated with reduced spontaneous total GH secretion through a reduction in both pulse frequency and amplitude (98,99) Furthermore, the circulating half-life of GH in obese adult subjects (145 ± 8.6 kg) is reduced to 11 minutes in comparison to 15 minutes in controls (89 ± 5 kg) (99). Decreased amplitude of GH secretion is seen also during provocative testing, where peak stimulated GH concentrations in obese children (100–102) and adults (103) are lower than in non-obese controls. This can lead to children with increased BMI being misclassified as having GHD, even when BMI is within the normal range (102).
Despite the reduction in circulating GH levels, IGF-I concentrations in obese individuals are similar to, or higher than, non-obese controls (discussed later in this section) (100,104–106). Growth hormone binding protein (GHBP) is the extracellular component of the GH receptor, and serum concentrations of GHBP are increased in obesity. This may reflect increased GH sensitivity at the receptor level in obese children (107). Increased GH sensitivity in children with higher BMI is supported by a study of IGF-I concentrations in children following administration of a fixed dose of GH. Children with high normal BMI had a greater rise in serum IGF-I concentrations when compared with those with low normal BMI (108).
Numerous studies have shown an association between IGF-I and leptin concentrations (109–111). Animal studies show that administration of low doses of exogenous leptin results in a transient change in GH secretion but sustained reduction in circulating IGF-I concentrations. At higher doses, there is suppressed appetite and increased IGF-I (112). This effect of leptin on IGF-I production appears to be independent of GH. However, GH can play a role in regulating leptin, as high doses of GH can increase leptin mRNA expression (113).
Leptin also may play an independent role in linear growth, through its direct action on the growth plate. Here, leptin syngergizes with thyroid hormone in regulating chondrocyte differentiation (114,115). A phenomenon of “growth without GH” has been described in children where obesity and leptin may play a role in transiently maintaining normal growth velocity. Normal growth has been described in children with GHD following craniopharyngioma removal (116) or in obese children with severe GHD (117). In these children, GHD may be masked by normal growth and they should be followed closely. This normal rate of growth may not be maintained and intervention with GH therapy may be indicated.
IGF-I Concentrations and Bioavailability in Obesity
Reduced caloric intake and poor nutritional status consistently result in a reduction of IGF-I concentrations, but the opposite effect is not seen in obesity. Reinehr et al measured serum IGF-I concentrations in 319 obese children prior to, and after, a weight loss intervention program. They found no difference between baseline IGF-I concentrations in obese subjects or controls, and no association between reduction in BMI and change in IGF-I levels (118). The lack of association between obesity and increased IGF-I concentrations has not been shown consistently in other studies (104,119). In a population- based study of over six thousand adults, an inverse U-shaped association between IGF-I concentration and BMI was shown, with peak IGF-I concentrations at a BMI range of 22.5 to 25 kg/m2 in males and 27.5 to 30 kg/m2 in females (106).
Although total IGF-I concentrations may not be significantly increased in obesity, the amount of free IGF-I relative to total IGF-I is increased (104). This increase in IGF-I bioavailability is largely due to the effect of nutrition on the IGF binding proteins (IGFBPs). Increased BMI and high insulin levels are associated with a reduction in levels of IGFBP-1 (120,121) and IGFBP-2 (104) which can increase free IGF-I concentrations. IGFBP-3 concentrations are similar in obese and normal weight children (104).
Potential Implications of GH/IGF-I Changes from Over-Nutrition
The association between obesity and risk for several diseases of adulthood is mediated, at least in part, by the increase in GH sensitivity and IGF-I bioavailability from over-nutrition. Obesity is a risk factor for cancer (122) and increasing BMI by 5 kg/m2 will increase the relative risk of developing many different types of malignancies (123). Overweight patients who develop cancer have a worse prognosis than those with normal weight (122,124). The mechanism for this association is not fully understood, but thought to be multifactorial and include the IGF-I signaling pathway (125). There is evidence at the extreme ends of the spectrum of high and low IGF-I concentrations to support this link: the incidence of colon cancer is increased in acromegaly (126) and congenital IGF-I deficiency appears to confer protection against the development of malignancies (124,127,128). The link between the overexpression of growth factors, or their receptors, and cancer is established (reviewed in 129), and the increased concentrations of free IGF-I in obesity may be involved in this pathogenesis.
Polycystic ovarian syndrome (PCOS) is more common in obese women, and is associated with an increased risk of developing multiple comorbidities (130,131). Recent studies indicate that abnormal folliculogenesis plays a role in the anovulation seen in PCOS (132). A potential role for IGF-I in the pathogenesis of PCOS-related anovulation has been suggested (133) and is supported by the fact that PCOS is relatively common in women with acromegaly (134). IGF-I plays a role in stimulating follicle growth in normal follicle development and mRNA expression for the IGF-I receptor is increased in preantral follicles in PCOS (135). The role of IGF-I in PCOS development is currently unclear.
Conclusions and Clinical Implications
The GH/IGF-I axis is sensitive to nutritional status, and under- or over-nutrition can affect this axis at each level from regulation of secretion to intracellular signaling. Evaluating the child’s nutritional status is essential in the appropriate interpretation of IGF-I concentrations, and failure to do this can result in misdiagnosis of a disorder of the GH/IGF-I axis.
The Clinical Implications of Under-Nutrition and IGF-I Measurements
IGF-I measurement has a central role in the screening evaluation of potential disorders of the GH/IGF-I axis (2) GH stimulation testing is often used to confirm GHD in the child whose screening IGF-I concentration is low. However, these tests have poor reproducibility and specificity for this condition (136,137). Consequently many normal children will be misclassified as having GHD using these tests (138,139) and this is a risk when children with low IGF-I concentrations from under-nutrition undergo GH stimulation testing. These children have GH resistance and are unlikely to respond to GH therapy in the absence of nutritional replacement (84). Ineffective GH treatment will also have significant healthcare resource implications (140) and may expose patients to risk (141,142).
The sensitivity of the GH/IGF-I axis to transient or minor changes in nutritional substrate availability is clinically relevant in children undergoing evaluation for short stature. Brief periods of reduced caloric intake can result in reduced IGF-I concentrations, even before any effect on body composition, weight or BMI is appreciated. Thus, nutritional history should include recent, as well as chronic, dietary intake. Similarly, energy expenditure through exercise, pubertal requirements (45) or chronic disease should be considered when assessing the sufficiency of nutritional intake.
It should be remembered that the effect of nutrition on IGF-I concentration is not solely dependent on caloric intake. Inadequate protein consumption can also lower IGF-I levels. Deficiency of micro-nutrients including zinc, magnesium and iodine, as well as hormones including thyroxine (reduced in sick euthyroid syndrome during nutritional stress and/or disease) may also result in low IGF-I concentrations and should be considered as possible etiologies.
Thus, careful examination of the patient’s weight and BMI curves is an important first step after inspection of the height curve. Three-day diet recording and analysis for macro- and micronutrient sufficiency is a worthwhile diagnostic test, as is repeating the IGF-I measurement following a period of nutritional intervention, in children suspected of undernutrition prior to performing any GH stimulation testing.
The Clinical Implications of Over-Nutrition and IGF-I Measurements
Unlike under-nutrition, the results of IGF-I measurement in patients with over-nutrition are less likely to result in diagnostic confusion. Total IGF-I levels are generally normal or elevated in obesity, although free IGF-I concentrations tend to be increased. Recent dietary history may be of relevance in these children, however, short-term caloric restriction in obese children may also reduce IGF-I concentrations. The effect of obesity on the GH/IGF-I axis should be considered when interpreting the results of provocative GH stimulation testing, as a blunted response to pharmacological stimuli has been reported in these children.
The long-term implications of obesity for adverse health outcomes are of significant concern in the current obesity epidemic. Nutrition-mediated alterations in the GH/IGF-I axis in obesity may play a causative role in the pathogenesis of many of these comorbidities, and is an active area of current research.
Potential Additional Roles of IGF-I Measurement
The consistent effect of under-nutrition in reducing IGF-I concentrations may provide an additional clinical role to IGF-I measurement. Monitoring IGF-I concentrations has been suggested as a measure of nutritional sufficiency (32,90,143) Weight gain in preterm and low birth weight infants is mirrored by increases in IGF-I concentrations, and this may be an additional marker of nutritional sufficiency in this population (32). Another potential clinical role of IGF-I measurement may be the monitoring of activity of diseases associated with malabsorption, such as celiac disease (75). IGF-I concentrations are sensitive to short-term nutritional insufficiencies, and may be affected before symptoms such as weight loss are noticeable.
Conclusion
This review provides an overview of the nutritional influences on the GH/IGF axis. In many of the studies reviewed here, as in clinical practice, it is possible that various levels of multiple nutritional deficiencies coexist (e.g. calories, protein, and specific micronutrients such as zinc) and the general consequence of each of these appears to be a reversible reduction in IGF-I concentrations. IGF-1 concentration is sensitive to acute and chronic changes in nutritional status. Given the poor specificity of GH stimulation testing for GHD, we caution against proceeding to these tests prior to addressing nutritional issues in patients where nutrition may be affecting IGF-I measurements. Likewise, the diagnosis of primary IGF deficiency cannot be made until under-nutrition is excluded as a cause of the low IGF-I concentrations. GH and recombinant IGF-I treatment are neither appropriate nor effective interventions for increasing growth of children and adolescents with nutritional stunting; nutritional repletion is the therapy of choice.
Acknowledgments
Funding Sources
C.P.H is supported by a PhD grant by the National Children’s Research Centre, Dublin, Ireland and A.G. is supported by grant1R01 HD57037 from the Eunice, Kennedy Shriver National Institute of Child Health and Human Development (NICHD).
Abbreviations
- BMI
Body Mass Index
- GH
Growth Hormone
- GHD
Growth Hormone Deficiency
- IGF-I
Insulin-like Growth Factor-1
- IGFBP
Insulin-like Growth Factor Binding Protein
- JAK2
Janus Kinase 2
- MAPK
Mitogen-Activated Protein Kinase
- NPY
Neuropeptide Y
- PI3K
Phosphoinositide-3-Kinase
- STAT
Signal Transducer and Activator of Transcription
Footnotes
Disclosure
C.P.H. has no conflicts of interest to disclose. A.G. is a member of the Steering Committee for the Pfizer International Growth Study (KIGS) database.
References
- 1.Rogol AD, Hayden GF. Etiologies and early diagnosis of short stature and growth failure in children and adolescents. J Pediatr. 2014;164(Suppl 5):S1-e14–e16. doi: 10.1016/j.jpeds.2014.02.027. [DOI] [PubMed] [Google Scholar]
- 2.Juul A, Skakkebaek NE. Prediction of the outcome of growth hormone provocative testing in short children by measurement of serum levels of insulin-like growth factor I and insulin-like growth factor binding protein 3. J Pediatr. 1997;130(2):197–204. doi: 10.1016/s0022-3476(97)70343-3. [DOI] [PubMed] [Google Scholar]
- 3.Wilson TA, Rose SR, Cohen P, Rogol AD, Backeljauw P, Brown R, Hardin DS, Kemp SF, Lawson M, Radovick S, Rosenthal SM, Silverman L, Speiser P, Lawson Wilkins Pediatric Endocrinology Society, D. Therapeutics C. Update of guidelines for the use of growth hormone in children: the Lawson Wilkins Pediatric Endocrinology Society Drug and Therapeutics Committee. J Pediatr. 2003;143(4):415–421. doi: 10.1067/s0022-3476(03)00246-4. [DOI] [PubMed] [Google Scholar]
- 4.Albertsson-Wikland K, Rosberg S, Karlberg J, Groth T. Analysis of 24-hour growth hormone profiles in healthy boys and girls of normal stature: relation to puberty. J Clin Endocrinol Metab. 1994;78(5):1195–1201. doi: 10.1210/jcem.78.5.8175978. [DOI] [PubMed] [Google Scholar]
- 5.Janeckova R. The role of leptin in human physiology and pathophysiology. Physiol Res. 2001;50(5):443–459. [PubMed] [Google Scholar]
- 6.Carro E, Senaris R, Considine RV, Casanueva FF, Dieguez C. Regulation of in vivo growth hormone secretion by leptin. Endocrinology. 1997;138(5):2203–2206. doi: 10.1210/endo.138.5.5238. [DOI] [PubMed] [Google Scholar]
- 7.Okada K, Sugihara H, Minami S, Wakabayashi I. Effect of parenteral administration of selected nutrients and central injection of gamma globulin from antiserum to neuropeptide Y on growth hormone secretory pattern in food-deprived rats. Neuroendocrinology. 1993;57(4):678–686. doi: 10.1159/000126425. [DOI] [PubMed] [Google Scholar]
- 8.Rettori V, Milenkovic L, Aguila MC, McCann SM. Physiologically significant effect of neuropeptide Y to suppress growth hormone release by stimulating somatostatin discharge. Endocrinology. 1990;126(5):2296–2301. doi: 10.1210/endo-126-5-2296. [DOI] [PubMed] [Google Scholar]
- 9.Maghnie M, Valtorta A, Moretta A, Larizza D, Preti P, Palladini G, Calcante S, Severi F. Diagnosing growth hormone deficiency: the value of short-term hypocaloric diet. J Clin Endocrinol Metab. 1993;77(5):1372–1378. doi: 10.1210/jcem.77.5.8077335. [DOI] [PubMed] [Google Scholar]
- 10.Muller TD, Nogueiras R, Andermann ML, Andrews ZB, Anker SD, Argente J, Batterham RL, Benoit SC, Bowers CY, Broglio F, Casanueva FF, D’Alessio D, Depoortere I, Geliebter A, Ghigo E, Cole PA, Cowley M, Cummings DE, Dagher A, Diano S, Dickson SL, Dieguez C, Granata R, Grill HJ, Grove K, Habegger KM, Heppner K, Heiman ML, Holsen L, Holst B, Inui A, Jansson JO, Kirchner H, Korbonits M, Laferrere B, LeRoux CW, Lopez M, Morin S, Nakazato M, Nass R, Perez-Tilve D, Pfluger PT, Schwartz TW, Seeley RJ, Sleeman M, Sun Y, Sussel L, Tong J, Thorner MO, van der Lely AJ, van der Ploeg LH, Zigman JM, Kojima M, Kangawa K, Smith RG, Horvath T, Tschop MH. Ghrelin Mol Metab. 2015;4(6):437–460. doi: 10.1016/j.molmet.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miljic D, Pekic S, Djurovic M, Doknic M, Milic N, Casanueva FF, Ghatei M, Popovic V. Ghrelin has partial or no effect on appetite, growth hormone, prolactin, and cortisol release in patients with anorexia nervosa. J Clin Endocrinol Metab. 2006;91(4):1491–1495. doi: 10.1210/jc.2005-2304. [DOI] [PubMed] [Google Scholar]
- 12.Brown RJ, Adams JJ, Pelekanos RA, Wan Y, McKinstry WJ, Palethorpe K, Seeber RM, Monks TA, Eidne KA, Parker MW, Waters MJ. Model for growth hormone receptor activation based on subunit rotation within a receptor dimer. Nat Struct Mol Biol. 2005;12(9):814–821. doi: 10.1038/nsmb977. [DOI] [PubMed] [Google Scholar]
- 13.Feigerlova E, Hwa V, Derr MA, Rosenfeld RG. Current issues on molecular diagnosis of GH signaling defects. Endocr Dev. 2013;24:118–127. doi: 10.1159/000342586. [DOI] [PubMed] [Google Scholar]
- 14.Straus DS, Takemoto CD. Effect of fasting on insulin-like growth factor-I (IGF-I) and growth hormone receptor mRNA levels and IGF-I gene transcription in rat liver. Mol Endocrinol. 1990;4(1):91–100. doi: 10.1210/mend-4-1-91. [DOI] [PubMed] [Google Scholar]
- 15.Maes M, Amand Y, Underwood LE, Maiter D, Ketelslegers JM. Decreased serum insulin-like growth factor I response to growth hormone in hypophysectomized rats fed a low protein diet: evidence for a postreceptor defect. Acta Endocrinol (Copenh) 1988;117(3):320–326. doi: 10.1530/acta.0.1170320. [DOI] [PubMed] [Google Scholar]
- 16.Leung KC, Doyle N, Ballesteros M, Waters MJ, Ho KK. Insulin regulation of human hepatic growth hormone receptors: divergent effects on biosynthesis and surface translocation. J Clin Endocrinol Metab. 2000;85(12):4712–4720. doi: 10.1210/jcem.85.12.7017. [DOI] [PubMed] [Google Scholar]
- 17.Fazeli PK, Misra M, Goldstein M, Miller KK, Klibanski A. Fibroblast growth factor-21 may mediate growth hormone resistance in anorexia nervosa. J Clin Endocrinol Metab. 2010;95(1):369–374. doi: 10.1210/jc.2009-1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guasti L, Silvennoinen S, Bulstrode NW, Ferretti P, Sankilampi U, Dunkel L. Elevated FGF21 leads to attenuated postnatal linear growth in preterm infants through GH resistance in chondrocytes. J Clin Endocrinol Metab. 2014;99(11):E2198–E2206. doi: 10.1210/jc.2014-1566. [DOI] [PubMed] [Google Scholar]
- 19.Inagaki T, Lin VY, Goetz R, Mohammadi M, Mangelsdorf DJ, Kliewer SA. Inhibition of growth hormone signaling by the fasting-induced hormone FGF21. Cell Metab. 2008;8(1):77–83. doi: 10.1016/j.cmet.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gillum MP, Erion DM, Shulman GI. Sirtuin-1 regulation of mammalian metabolism. Trends Mol Med. 2011;17(1):8–13. doi: 10.1016/j.molmed.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.amamoto M, Iguchi G, Fukuoka H, Suda K, Bando H, Takahashi M, Nishizawa H, Seino S, Takahashi Y. SIRT1 regulates adaptive response of the growth hormone–insulin-like growth factor-I axis under fasting conditions in liver. Proc Natl Acad Sci U S A. 2013;110(37):14948–14953. doi: 10.1073/pnas.1220606110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cesur Y, Yordaman N, Dogan M. Serum insulin-like growth factor-I and insulin-like growth factor binding protein-3 levels in children with zinc deficiency and the effect of zinc supplementation on these parameters. J Pediatr Endocrinol Metab. 2009;22(12):1137–1143. doi: 10.1515/jpem.2009.22.12.1137. [DOI] [PubMed] [Google Scholar]
- 23.Dorup I, Flyvbjerg A, Everts ME, Clausen T. Role of insulin-like growth factor-1 and growth hormone in growth inhibition induced by magnesium and zinc deficiencies. Br J Nutr. 1991;66(3):505–521. doi: 10.1079/bjn19910051. [DOI] [PubMed] [Google Scholar]
- 24.Rao KS, Mohan PS. Plasma somatomedin activity, growth-hormone and insulin levels in vitamin B6 deficient rats. Horm Metab Res. 1982;14(11):580–582. doi: 10.1055/s-2007-1019086. [DOI] [PubMed] [Google Scholar]
- 25.Mehta A, Hindmarsh PC, Stanhope RG, Turton JP, Cole TJ, Preece MA, Dattani MT. The role of growth hormone in determining birth size and early postnatal growth, using congenital growth hormone deficiency (GHD) as a model. Clin Endocrinol (Oxf) 2005;63(2):223–231. doi: 10.1111/j.1365-2265.2005.02330.x. [DOI] [PubMed] [Google Scholar]
- 26.Woods KA, Camacho-Hubner C, Savage MO, Clark AJ. Intrauterine growth retardation and postnatal growth failure associated with deletion of the insulin-like growth factor I gene. N Engl J Med. 1996;335(18):1363–1367. doi: 10.1056/NEJM199610313351805. [DOI] [PubMed] [Google Scholar]
- 27.Lassarre C, Hardouin S, Daffos F, Forestier F, Frankenne F, Binoux M. Serum insulin-like growth factors and insulin-like growth factor binding proteins in the human fetus. Relationships with growth in normal subjects and in subjects with intrauterine growth retardation. Pediatr Res. 1991;29(3):219–225. doi: 10.1203/00006450-199103000-00001. [DOI] [PubMed] [Google Scholar]
- 28.Bennett A, Wilson DM, Liu F, Nagashima R, Rosenfeld RG, Hintz RL. Levels of insulin-like growth factors I and II in human cord blood. J Clin Endocrinol Metab. 1983;57(3):609–612. doi: 10.1210/jcem-57-3-609. [DOI] [PubMed] [Google Scholar]
- 29.Hansen-Pupp I, Lofqvist C, Polberger S, Niklasson A, Fellman V, Hellstrom A, Ley D. Influence of insulin-like growth factor I and nutrition during phases of postnatal growth in very preterm infants. Pediatr Res. 2011;69(5 Pt 1):448–453. doi: 10.1203/PDR.0b013e3182115000. [DOI] [PubMed] [Google Scholar]
- 30.Engstrom E, Niklasson A, Wikland KA, Ewald U, Hellstrom A. The role of maternal factors, postnatal nutrition, weight gain, and gender in regulation of serum IGF-I among preterm infants. Pediatr Res. 2005;57(4):605–610. doi: 10.1203/01.PDR.0000155950.67503.BC. [DOI] [PubMed] [Google Scholar]
- 31.Kashyap S, Forsyth M, Zucker C, Ramakrishnan R, Dell RB, Heird WC. Effects of varying protein and energy intakes on growth and metabolic response in low birth weight infants. J Pediatr. 1986;108(6):955–963. doi: 10.1016/s0022-3476(86)80940-4. [DOI] [PubMed] [Google Scholar]
- 32.Smith WJ, Underwood LE, Keyes L, Clemmons DR. Use of insulin-like growth facto I (IGF-I) and IGF-binding protein measurements to monitor feeding of premature infants. J Clin Endocrinol Metab. 1997;82(12):3982–3988. doi: 10.1210/jcem.82.12.4452. [DOI] [PubMed] [Google Scholar]
- 33.de Zegher F, Sebastiani G, Diaz M, Gomez-Roig MD, Lopez-Bermejo A, Ibanez L. Breast-feeding vs formula-feeding for infants born small-for-gestational-age: divergent effects on fat mass and on circulating IGF-I and high-molecular-weight adiponectin in late infancy. J Clin Endocrinol Metab. 2013;98(3):1242–1247. doi: 10.1210/jc.2012-3480. [DOI] [PubMed] [Google Scholar]
- 34.de Zegher F, Sebastiani G, Diaz M, Sanchez-Infantes D, Lopez-Bermejo A, Ibanez L. Body composition and circulating high-molecular-weight adiponectin and IGF-I in infants born small for gestational age: breast-versus formula-feeding. Diabetes. 2012;61(8):1969–1973. doi: 10.2337/db11-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Madsen AL, Larnkjaer A, Molgaard C, Michaelsen KF. IGF-I and IGFBP-3 in healthy 9 month old infants from the SKOT cohort: breastfeeding, diet, and later obesity. Growth Horm IGF Res. 2011;21(4):199–204. doi: 10.1016/j.ghir.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 36.Hawkes CP, Grimberg A. Measuring growth hormone and insulin-like growth factor-I in infants: what is normal? Pediatr Endocrinol Rev. 2013;11(2):126–146. [PMC free article] [PubMed] [Google Scholar]
- 37.Hoppe C, Molgaard C, Dalum C, Vaag A, Michaelsen KF. Differential effects of casein versus whey on fasting plasma levels of insulin, IGF-1 and IGF-1/IGFBP-3: results from a randomized 7-day supplementation study in prepubertal boys. Eur J Clin Nutr. 2009;63(9):1076–1083. doi: 10.1038/ejcn.2009.34. [DOI] [PubMed] [Google Scholar]
- 38.Hoppe C, Molgaard C, Juul A, Michaelsen KF. High intakes of skimmed milk, but not meat, increase serum IGF-I and IGFBP-3 in eight-year-old boys. Eur J Clin Nutr. 2004;58(9):1211–1216. doi: 10.1038/sj.ejcn.1601948. [DOI] [PubMed] [Google Scholar]
- 39.Hoppe C, Molgaard C, Vaag A, Barkholt V, Michaelsen KF. High intakes of milk, but not meat, increase s-insulin and insulin resistance in 8-year-old boys. Eur J Clin Nutr. 2005;59(3):393–398. doi: 10.1038/sj.ejcn.1602086. [DOI] [PubMed] [Google Scholar]
- 40.Sen TA, Aycicek A. Do children with adenotonsillar hypertrophy have lower IGF-1 and ghrelin levels than the normal children? Int J Pediatr Otorhinolaryngol. 2010;74(6):665–668. doi: 10.1016/j.ijporl.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 41.Imamoglu S, Bereket A, Turan S, Taga Y, Haklar G. Effect of zinc supplementation on growth hormone secretion, IGF-I, IGFBP-3, somatomedin generation, alkaline phosphatase, osteocalcin and growth in prepubertal children with idiopathic short stature. J Pediatr Endocrinol Metab. 2005;18(1):69–74. doi: 10.1515/jpem.2005.18.1.69. [DOI] [PubMed] [Google Scholar]
- 42.Ozon A, Alikasifoglu A, Yordam N. Influence of iodine supplementation on serum insulin-like growth factor-I (IGF-I) and IGF-binding protein-3 (IGFBP-3) levels in severe iodine deficiency. Turk J Pediatr. 2004;46(4):303–308. [PubMed] [Google Scholar]
- 43.Angervo M, Toivonen J, Leinonen P, Valimaki M, Seppala M. Thyroxine withdrawal is accompanied by decreased circulating levels of insulin-like growth factor-binding protein-1 in thyroidectomized patients. J Clin Endocrinol Metab. 1993;76(5):1199–1201. doi: 10.1210/jcem.76.5.7684392. [DOI] [PubMed] [Google Scholar]
- 44.Miell JP, Zini M, Quin JD, Jones J, Portioli I, Valcavi R. Reversible effects of cessation and recommencement of thyroxine treatment on insulin-like growth factors (IGFs) and IGF-binding proteins in patients with total thyroidectomy. J Clin Endocrinol Metab. 1994;79(5):1507–1512. doi: 10.1210/jcem.79.5.7525638. [DOI] [PubMed] [Google Scholar]
- 45.Institute of Medicine. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients) Washington, DC: The National Academies Press; 2005. [Google Scholar]
- 46.Soliman A, De Sanctis V, Elalaily R. Nutrition and pubertal development. Indian J Endocrinol Metab. 2014;18(Suppl 1):S39–S47. doi: 10.4103/2230-8210.145073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Elias CF, Purohit D. Leptin signaling and circuits in puberty and fertility. Cellular and molecular life sciences. CMLS. 2013;70(5):841–862. doi: 10.1007/s00018-012-1095-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mann DR, Plant TM. Leptin and pubertal development. Semin Reprod Med. 2002;20(2):93–102. doi: 10.1055/s-2002-32500. [DOI] [PubMed] [Google Scholar]
- 49.Food and Agriculture Organization of the United Nations, International Fund for Agriculture Development, World Food Programme. The state of food insecurity in the world Rome. 2014 [Google Scholar]
- 50.Gundersen C. Food insecurity is an ongoing national concern. Adv Nutr. 2013;4(1):36–41. doi: 10.3945/an.112.003244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stephens LD, McNaughton SA, Crawford D, MacFarlane A, Ball K. Correlates of dietary resilience among socioeconomically disadvantaged adolescents. Eur J Clin Nutr. 2011;65(11):1219–1232. doi: 10.1038/ejcn.2011.107. [DOI] [PubMed] [Google Scholar]
- 52.Cook JT, Black M, Chilton M, Cutts D, Ettinger de Cuba S, Heeren TC, Rose-Jacobs R, Sandel M, Casey PH, Coleman S, Weiss I, Frank DA. Are food insecurity’s health impacts underestimated in the US population? Marginal food security also predicts adverse health outcomes in young US children and mothers. Adv Nutr. 2013;4(1):51–61. doi: 10.3945/an.112.003228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith WJ, Underwood LE, Clemmons DR. Effects of caloric or protein restriction on insulin-like growth factor-I (IGF-I) and IGF-binding proteins in children and adults. J Clin Endocrinol Metab. 1995;80(2):443–449. doi: 10.1210/jcem.80.2.7531712. [DOI] [PubMed] [Google Scholar]
- 54.Moses N, Banilivy MM, Lifshitz F. Fear of obesity among adolescent girls. Pediatrics. 1989;83(3):393–398. [PubMed] [Google Scholar]
- 55.Pugliese MT, Lifshitz F, Grad G, Fort P, Marks-Katz M. Fear of obesity. A cause of short stature and delayed puberty. N Engl J Med. 1983;309(9):513–518. doi: 10.1056/NEJM198309013090901. [DOI] [PubMed] [Google Scholar]
- 56.Lifshitz F, Moses N. Nutritional dwarfing: growth, dieting, and fear of obesity. J Am Coll Nutr. 1988;7(5):367–376. doi: 10.1080/07315724.1988.10720254. [DOI] [PubMed] [Google Scholar]
- 57.Romano C, Hartman C, Privitera C, Cardile S, Shamir R. Current topics in the diagnosis and management of the pediatric non organic feeding disorders (NOFEDs) Clin Nutr. 2015;34(2):195–200. doi: 10.1016/j.clnu.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 58.Thomas R, Sanders S, Doust J, Beller E, Glasziou P. Prevalence of attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Pediatrics. 2015;135(4):e994–e1001. doi: 10.1542/peds.2014-3482. [DOI] [PubMed] [Google Scholar]
- 59.Davis C, Fattore L, Kaplan AS, Carter JC, Levitan RD, Kennedy JL. The suppression of appetite and food consumption by methylphenidate: the moderating effects of gender and weight status in healthy adults. Int J Neuropsychopharmacol. 2012;15(2):181–187. doi: 10.1017/S1461145711001039. [DOI] [PubMed] [Google Scholar]
- 60.Bereket A, Turan S, Karaman MG, Haklar G, Ozbay F, Yazgan MY. Height, weight, IGF-I, IGFBP-3 and thyroid functions in prepubertal children with attention deficit hyperactivity disorder: effect of methylphenidate treatment. Horm Res. 2005;63(4):159–164. doi: 10.1159/000084683. [DOI] [PubMed] [Google Scholar]
- 61.Lopez-Varela S, Montero A, Chandra RK, Marcos A. Nutritional status of young female elite gymnasts. Int J Vitam Nutr Res. 2000;70(4):185–190. doi: 10.1024/0300-9831.70.4.185. [DOI] [PubMed] [Google Scholar]
- 62.Iglesias-Gutierrez E, Garcia-Roves PM, Rodriguez C, Braga S, Garcia-Zapico P, Patterson AM. Food habits and nutritional status assessment of adolescent soccer players. A necessary and accurate approach. Can J Appl Physiol. 2005;30(1):18–32. doi: 10.1139/h05-102. [DOI] [PubMed] [Google Scholar]
- 63.Weimann E, Witzel C, Schwidergall S, Bohles HJ. Peripubertal perturbations in elite gymnasts caused by sport specific training regimes and inadequate nutritional intake. Int J Sports Med. 2000;21(3):210–215. doi: 10.1055/s-2000-8875. [DOI] [PubMed] [Google Scholar]
- 64.Gibson JC, Stuart-Hill L, Martin S, Gaul C. Nutrition status of junior elite Canadian female soccer athletes. Int J Sport Nutr Exerc Metab. 2011;21(6):507–514. doi: 10.1123/ijsnem.21.6.507. [DOI] [PubMed] [Google Scholar]
- 65.Deimel JF, Dunlap BJ. The female athlete triad. Clin Sports Med. 2012;31(2):247–254. doi: 10.1016/j.csm.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 66.Nichols JF, Rauh MJ, Lawson MJ, Ji M, Barkai HS. Prevalence of the female athlete triad syndrome among high school athletes. Arch Pediatr Adolesc Med. 2006;160(2):137–142. doi: 10.1001/archpedi.160.2.137. [DOI] [PubMed] [Google Scholar]
- 67.De Souza MJ, Williams NI. Beyond hypoestrogenism in amenorrheic athletes: energy deficiency as a contributing factor for bone loss. Curr Sports Med Rep. 2005;4(1):38–44. doi: 10.1007/s11932-005-0029-1. [DOI] [PubMed] [Google Scholar]
- 68.Mountjoy M, Sundgot-Borgen J, Burke L, Carter S, Constantini N, Lebrun C, Meyer N, Sherman R, Steffen K, Budgett R, Ljungqvist A. The IOC consensus statement: beyond the Female Athlete Triad-Relative Energy Deficiency in Sport (RED-S) Br J Sports Med. 2014;48(7):491–497. doi: 10.1136/bjsports-2014-093502. [DOI] [PubMed] [Google Scholar]
- 69.Adiyaman P, Ocal G, Berberoglu M, Evliyaoglu O, Aycan Z, Cetinkaya E, Bulca Y, Ersoz G, Akar N. Alterations in serum growth hormone (GH)/GH dependent ternary complex components (IGF-I, IGFBP-3, ALS, IGF-I/IGFBP-3 molar ratio) and the influence of these alterations on growth pattern in female rhythmic gymnasts. J Pediatr Endocrinol Metab. 2004;17(6):895–903. doi: 10.1515/jpem.2004.17.6.895. [DOI] [PubMed] [Google Scholar]
- 70.Ahmed SF, Farquharson C. The effect of GH and IGF1 on linear growth and skeletal development and their modulation by SOCS proteins. J Endocrinol. 2010;206(3):249–259. doi: 10.1677/JOE-10-0045. [DOI] [PubMed] [Google Scholar]
- 71.MacRae VE, Wong SC, Farquharson C, -Ahmed SF. Cytokine actions in growth disorders associated with pediatric chronic inflammatory diseases (review) Int J Mol Med. 2006;18(6):1011–1018. doi: 10.3892/ijmm.18.6.1011. [DOI] [PubMed] [Google Scholar]
- 72.Bonamico M, Scire G, Mariani P, Pasquino AM, Triglione P, Scaccia S, Ballati G, Boscherini B. Short stature as the primary manifestation of monosymptomatic celiac disease. J Pediatr Gastroenterol Nutr. 1992;14(1):12–16. doi: 10.1097/00005176-199201000-00003. [DOI] [PubMed] [Google Scholar]
- 73.Gasparetto M, Guariso G. Crohn’s disease and growth deficiency in children and adolescents. World J Gastroenterol. 2014;20(37):13219–13233. doi: 10.3748/wjg.v20.i37.13219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jansson UH, Kristiansson B, Magnusson P, Larsson L, Albertsson-Wikland K, Bjarnason R. The decrease of IGF-I, IGF-binding protein-3 and bone alkaline phosphatase isoforms during gluten challenge correlates with small intestinal inflammation in children with coeliac disease. EurJ Endocrinol. 2001;144(4):417–423. doi: 10.1530/eje.0.1440417. [DOI] [PubMed] [Google Scholar]
- 75.Locuratolo N, Pugliese G, Pried F, Romeo G, Mariani P, Diaz-Horta O, Calvani L, Montuori M, Cipolletta E, Di Mario U, Bonamico M. The circulating insulin-like growth factor system in children with coeliac disease: an additional marker for disease activity. Diabetes Metab Res Rev. 1999;15(4):254–260. doi: 10.1002/(sici)1520-7560(199907/08)15:4<254::aid-dmrr47>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 76.Street ME, Volta C, Ziveri MA, Zanacca C, Banchini G, Viani I, Rossi M, Virdis R, Bernasconi S. Changes and relationships of IGFS and IGFBPS and cytokines in coeliac disease at diagnosis and on gluten-free diet. Clin Endocrinol (Oxf) 2008;68(1):22–28. doi: 10.1111/j.1365-2265.2007.02992.x. [DOI] [PubMed] [Google Scholar]
- 77.Valdimarsson T, Arnqvist HJ, Toss G, Jarnerot G, Nystrom F, Strom M. Low circulating insulin-like growth factor I in coeliac disease and its relation to bone mineral density. Scand J Gastroenterol. 1999;34(9):904–908. doi: 10.1080/003655299750025381. [DOI] [PubMed] [Google Scholar]
- 78.Ferrante E, Giavoli C, Elli L, Redaelli A, Novati E, De Bellis A, Ronchi CL, Bergamaschi S, Lania A, Bardella MT, Bellastella G, Beck-Peccoz P. Evaluation of GH-IGF-I axis in adult patients with coeliac disease. Horm Metab Res. 2010;42(1):45–49. doi: 10.1055/s-0029-1241169. [DOI] [PubMed] [Google Scholar]
- 79.Soliman AT, Hassan AE, Aref MK, Hintz RL, Rosenfeld RG, Rogol AD. Serum insulin-like growth factors I and II concentrations and growth hormone and insulin responses to arginine infusion in children with protein-energy malnutrition before and after nutritional rehabilitation. Pediatr Res. 1986;20(11):1122–1130. doi: 10.1203/00006450-198611000-00012. [DOI] [PubMed] [Google Scholar]
- 80.Giovenale D, Meazza C, Cardinale GM, Sposito M, Mastrangelo C, Messini B, Citro G, Delvecchio M, Di Maio S, Bozzola M. The prevalence of growth hormone deficiency and celiac disease in short children. Clin Med Res. 2006;4(3):180–183. doi: 10.3121/cmr.4.3.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Giovenale D, Meazza C, Cardinale GM, Farinelli E, Mastrangelo C, Messini B, Citro G, Del Vecchio M, Di Maio S, Possenti I, Bozzola M. Growth hormone treatment in prepubertal children with celiac disease and growth hormone deficiency. J Pediatr Gastroenterol Nutr. 2007;45(4):433–437. doi: 10.1097/MPG.0b013e3180de5e0a. [DOI] [PubMed] [Google Scholar]
- 82.Vasseur F, Gower-Rousseau C, Vernier-Massouille G, Dupas JL, Merle V, Merlin B, Lerebours E, Savoye G, Salomez JL, Cortot A, Colombel JF, Turck D. Nutritional status and growth in pediatric Crohn’s disease: a population-based study. Am J Gastroenterol. 2010;105(8):1893–1900. doi: 10.1038/ajg.2010.20. [DOI] [PubMed] [Google Scholar]
- 83.Corkins MR, Gohil AD, Fitzgerald JF. The insulin-like growth factor axis in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2003;36(2):228–234. doi: 10.1097/00005176-200302000-00014. [DOI] [PubMed] [Google Scholar]
- 84.Merimee TJ, Zapf J, Froesch ER. Insulin-like growth factors in the fed and fasted states. J Clin Endocrinol Metab. 1982;55(5):999–1002. doi: 10.1210/jcem-55-5-999. [DOI] [PubMed] [Google Scholar]
- 85.Grinspoon SK, Baum HB, Peterson S, Klibanski A. Effects of rhIGF-I administration on bone turnover during short-term fasting. J Clin Invest. 1995;96(2):900–906. doi: 10.1172/JCI118137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gat-Yablonski G, Shtaif B, Abraham E, Phillip M. Nutrition-induced catch-up growth at the growth plate. J Pediatr Endocrinol Metab. 2008;21(9):879–893. doi: 10.1515/jpem.2008.21.9.879. [DOI] [PubMed] [Google Scholar]
- 87.McLaren DS, Pellett PL, Read WW. A simple scoring system for classifying the severe forms of protein-calorie malnutrition of early childhood. Lancet. 1967;1(7489):533–535. doi: 10.1016/s0140-6736(67)92113-7. [DOI] [PubMed] [Google Scholar]
- 88.Hintz RL, Suskind R, Amatayakul K, Thanangkul O, Olson R. Plasma somatomedin and growth hormone values in children with protein-calorie malnutrition. J Pediatr. 1978;92(1):153–156. doi: 10.1016/s0022-3476(78)80099-7. [DOI] [PubMed] [Google Scholar]
- 89.Kouanda S, Doulougou B, De Coninck V, Habimana L, Sondo B, Tonglet R, Ketelslegers JM, Robert A. Insulin Growth Factor-I in Protein-Energy Malnutrition during Rehabilitation in Two Nutritional Rehabilitation Centres in Burkina Faso. J Trop Med. 2009;2009:832589. doi: 10.1155/2009/832589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Caregaro L, Favaro A, Santonastaso P, Alberino F, Di Pascoli L, Nardi M, Favaro S, Gatta A. Insulin-like growth factor 1 (IGF-1), a nutritional marker in patients with eating disorders. Clin Nutr. 2001;20(3):251–257. doi: 10.1054/clnu.2001.0397. [DOI] [PubMed] [Google Scholar]
- 91.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, Mullany EC, Biryukov S, Abbafati C, Abera SF, Abraham JP, Abu-Rmeileh NM, Achoki T, AlBuhairan FS, Alemu ZA, Alfonso R, Ali MK, Ali R, Guzman NA, Ammar W, Anwari P, Banerjee A, Barquera S, Basu S, Bennett DA, Bhutta Z, Blore J, Cabral N, Nonato IC, Chang JC, Chowdhury R, Courville KJ, Criqui MH, Cundiff DK, Dabhadkar KC, Dandona L, Davis A, Dayama A, Dharmaratne SD, Ding EL, Durrani AM, Esteghamati A, Farzadfar F, Fay DF, Feigin VL, Flaxman A, Forouzanfar MH, Goto A, Green MA, Gupta R, Hafezi-Nejad N, Hankey GJ, Harewood HC, Havmoeller R, Hay S, Hernandez L, Husseini A, Idrisov BT, Ikeda N, Islami F, Jahangir E, Jassal SK, Jee SH, Jeffreys M, Jonas JB, Kabagambe EK, Khalifa SE, Kengne AP, Khader YS, Khang YH, Kim D, Kimokoti RW, Kinge JM, Kokubo Y, Kosen S, Kwan G, Lai T, Leinsalu M, Li Y, Liang X, Liu S, Logroscino G, Lotufo PA, Lu Y, Ma J, Mainoo NK, Mensah GA, Merriman TR, Mokdad AH, Moschandreas J, Naghavi M, Naheed A, Nand D, Narayan KM, Nelson EL, Neuhouser ML, Nisar MI, Ohkubo T, Oti SO, Pedroza A, Prabhakaran D, Roy N, Sampson U, Seo H, Sepanlou SG, Shibuya K, Shiri R, Shiue I, Singh GM, Singh JA, Skirbekk V, Stapelberg NJ, Sturua L, Sykes BL, Tobias M, Tran BX, Trasande L, Toyoshima H, van de Vijver S, Vasankari TJ, Veerman JL, Velasquez-Melendez G, Vlassov VV, Vollset SE, Vos T, Wang C, Wang X, Weiderpass E, Werdecker A, Wright JL, Yang YC, Yatsuya H, Yoon J, Yoon SJ, Zhao Y, Zhou M, Zhu S, Lopez AD, Murray CJ, Gakidou E. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Singh GK, Kogan MD, van Dyck PC. Changes in state-specific childhood obesity and overweight prevalence in the United States from 2003 to 2007. Arch Pediatr Adolesc Med. 2010;164(7):598–607. doi: 10.1001/archpediatrics.2010.84. [DOI] [PubMed] [Google Scholar]
- 93.Aksglaede L, Juul A, Olsen LW, Sorensen TI. Age at puberty and the emerging obesity epidemic. PLoS One. 2009;4(12):e8450. doi: 10.1371/journal.pone.0008450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vignolo M, Naselli A, Di Battista E, Mostert M, Aicardi G. Growth and development in simple obesity. Eur J Pediatr. 1988;147(3):242–244. doi: 10.1007/BF00442687. [DOI] [PubMed] [Google Scholar]
- 95.He Q, Karlberg J. Bmi in childhood and its association with height gain, timing of puberty, and final height. Pediatr Res. 2001;49(2):244–251. doi: 10.1203/00006450-200102000-00019. [DOI] [PubMed] [Google Scholar]
- 96.Denzer C, Weibel A, Muche R, Karges B, Sorgo W, Wabitsch M. Pubertal development in obese children and adolescents. Int J Obes (Lond) 2007;31(10):1509–1519. doi: 10.1038/sj.ijo.0803691. [DOI] [PubMed] [Google Scholar]
- 97.Papadimitriou A, Gousi T, Giannouli O, Nicolaidou P. The growth of children in relation to the timing of obesity development. Obesity. 2006;14(12):2173–2176. doi: 10.1038/oby.2006.254. [DOI] [PubMed] [Google Scholar]
- 98.Iranmanesh A, Lizarralde G, Veldhuis JD. Age and relative adiposity are specific negative determinants of the frequency and amplitude of growth hormone (GH) secretory bursts and the halflife of endogenous GH in healthy men. J Clin Endocrinol Metab. 1991;73(5):1081–1088. doi: 10.1210/jcem-73-5-1081. [DOI] [PubMed] [Google Scholar]
- 99.Veldhuis JD, Iranmanesh A, Ho KK, Waters MJ, Johnson ML, Lizarralde G. Dual defects in pulsatile growth hormone secretion and clearance subserve the hyposomatotropism of obesity in man. J Clin Endocrinol Metab. 1991;72(1):51–59. doi: 10.1210/jcem-72-1-51. [DOI] [PubMed] [Google Scholar]
- 100.Loche S, Cappa M, Borrelli P, Faedda A, Crino A, Cella SG, Corda R, Muller EE, Pintor C. Reduced growth hormone response to growth hormone-releasing hormone in children with simple obesity: evidence for somatomedin-C mediated inhibition. Clin Endocrinol (Oxf) 1987;27(2):145–153. doi: 10.1111/j.1365-2265.1987.tb01139.x. [DOI] [PubMed] [Google Scholar]
- 101.Patel L, Skinner AM, Price DA, Clayton PE. The influence of body mass index on growth hormone secretion in normal and short statured children. Growth Regul. 1994;4(1):29–34. [PubMed] [Google Scholar]
- 102.Stanley TL, Levitsky LL, Grinspoon SK, Misra M. Effect of body mass index on peak growth hormone response to provocative testing in children with short stature. J Clin Endocrinol Metab. 2009;94(12):4875–4881. doi: 10.1210/jc.2009-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Williams T, Berelowitz M, Joffe SN, Thorner MO, Rivier J, Vale W, Frohman LA. Impaired growth hormone responses to growth hormone-releasing factor in obesity. A pituitary defect reversed with weight reduction. N Engl J Med. 1984;311(22):1403–1407. doi: 10.1056/NEJM198411293112203. [DOI] [PubMed] [Google Scholar]
- 104.Nam SV, Lee EJ, Kim KR, Cha BS, Song YD, Lim SK, Lee HC, Huh KB. Effect of obesity on total and free insulin-like growth factor (IGF)- 1, and their relationship to IGF-binding protein (BP)-1, IGFBP-2, IGFBP-3, insulin, and growth hormone Int J Obes Relat Metab Disord. 1997;21(5):355–359. doi: 10.1038/sj.ijo.0800412. [DOI] [PubMed] [Google Scholar]
- 105.Radetti G, Bozzola M, Pasquino B, Paganini C, Aglialoro A, Livieri C, Barreca A. Growth hormone bioactivity, insulin-like growth factors (IGFs), and IGF binding proteins in obese children. Metabolism. 1998;47(12):1490–1493. doi: 10.1016/s0026-0495(98)90075-0. [DOI] [PubMed] [Google Scholar]
- 106.Schneider HJ, Saller B, Klotsche J, Marz W, Erwa W, Wittchen HU, Stalla GK. Opposite associations of age-dependent insulin-like growth factor-l standard deviation scores with nutritional state in normal weight and obese subjects. Eur J Endocrinol. 2006;154(5):699–706. doi: 10.1530/eje.1.02131. [DOI] [PubMed] [Google Scholar]
- 107.Argente J, Caballo N, Barrios V, Pozo J, Munoz MT, Chowen JA, Hernandez M. Multiple endocrine abnormalities of the growth hormone and insulin-like growth factor axis in prepubertal children with exogenous obesity: effect of short- and long-term weight reduction. J Clin Endocrinol Metab. 1997;82(7):2076–2083. doi: 10.1210/jcem.82.7.4089. [DOI] [PubMed] [Google Scholar]
- 108.Roman R, Iniguez G, Lammoglia JJ, Avila A, Salazar T, Cassorla F. The IGF-I response to growth hormone is related to body mass index in short children with normal weight. Horm Re. 2009;72(1):10–14. doi: 10.1159/000224335. [DOI] [PubMed] [Google Scholar]
- 109.Mohammadzadeh G, Zarghami N. Serum leptin level is reduced in non-obese subjects with type 2 diabetes. Int J Endocrinol Metab. 2013;11(1):3–10. doi: 10.5812/ijem.6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ostadrahimi A, Moradi T, Zarghami N, Shoja MM. Correlates of serum leptin and insulin-like growth factor-l concentrations in normal weight and overweight/obese Iranian women. J Womens Health (Larchmt) 2008;17(8):1389–1397. doi: 10.1089/jwh.2007.0736. [DOI] [PubMed] [Google Scholar]
- 111.Haspolat K, Ece A, Gurkan F, Atamer Y, Tutanc M, Yolbas I. Relationships between leptin, insulin, IGF-1 and IGFBP-3 in children with energy malnutrition. Clin Biochem. 2007;40(3–4):201–205. doi: 10.1016/j.clinbiochem.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 112.Ajuwon KM, Kuske JL, Ragland D, Adeola O, Hancock DL, Anderson DB, Spurlock ME. The regulation of IGF-1 by leptin in the pig is tissue specific and independent of changes in growth hormone. J Nutr Biochem. 2003;14(9):522–530. doi: 10.1016/s0955-2863(03)00103-7. [DOI] [PubMed] [Google Scholar]
- 113.Houseknecht KL, Portocarrero CP, Ji S, Lemenager R, Spurlock ME. Growth hormone regulates leptin gene expression in bovine adipose tissue: correlation with adipose IGF-1 expression. J Endocrinol. 2000;164(1):51–57. doi: 10.1677/joe.0.1640051. [DOI] [PubMed] [Google Scholar]
- 114.Kishida Y, Hirao M, Tamai N, Nampei A, Fujimoto T, Nakase T, Shimizu N, Yoshikawa H, Myoui A. Leptin regulates chondrocyte differentiation and matrix maturation during endochondral ossification. Bone. 2005;37(5):607–621. doi: 10.1016/j.bone.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 115.Wang L, Shao YY, Bailock RT. Leptin synergizes with thyroid hormone signaling in promoting growth plate chondrocyte proliferation and terminal differentiation in vitro. Bone. 2011;48(5):1022–1027. doi: 10.1016/j.bone.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 116.Blethen SL, Weldon VV. Outcome in children with normal growth following removal of a craniopharyngioma. Am J Med Sci. 1986;292(1):21–24. doi: 10.1097/00000441-198607000-00004. [DOI] [PubMed] [Google Scholar]
- 117.Geffner ME, Lippe BM, Bersch N, Van Herle A, Kaplan SA, Elders MJ, Golde DW. Growth without growth hormone: evidence for a potent circulating human growth factor. Lancet. 1986;1(8477):343–347. doi: 10.1016/s0140-6736(86)92316-0. [DOI] [PubMed] [Google Scholar]
- 118.Reinehr T, Panteliadou A, de Sousa G, Andler W. Insulin-like growth factor-l, insulin-like growth factor binding protein-3 and growth in obese children before and after reduction of overweight. J Pediatr Endocrinol Metab. 2009;22(3):225–233. doi: 10.1515/jpem.2009.22.3.225. [DOI] [PubMed] [Google Scholar]
- 119.Hosick PA, McMurray RG, Hackney AC, Battaglini CL, Combs TP, Harrell JS. Differences in the GH-IGF-I axis in children of different weight and fitness status. Growth Horm IGF Res. 2012;22(2):87–91. doi: 10.1016/j.ghir.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Frystyk J, Vestbo E, Skjaerbaek C, Mogensen CE, Orskov H. Free insulin-like growth factors in human obesity. Metabolism. 1995;44(10 Suppl 4):37–44. doi: 10.1016/0026-0495(95)90219-8. [DOI] [PubMed] [Google Scholar]
- 121.Katz LE, DeLeon DD, Zhao H, Jawad AF. Free and total insulin-like growth factor (IGF)-I levels decline during fasting: relationships with insulin and IGF-binding protein-1. J Clin Endocrinol Metab. 2002;87(6):2978–2983. doi: 10.1210/jcem.87.6.8601. [DOI] [PubMed] [Google Scholar]
- 122.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348(17):1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 123.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 124.Demark-Wahnefried W, Platz EA, Ligibel JA, Blair CK, Courneya KS, Meyerhardt JA, Ganz PA, Rock CL, Schmitz KH, Wadden T, Philip EJ, Wolfe B, Gapstur SM, Ballard-Barbash R, McTiernan A, Minasian L, Nebeling L, Goodwin PJ. The role of obesity in cancer survival and recurrence. Cancer Epidemiol Biomarkers Prev. 2012;21(8):1244–1259. doi: 10.1158/1055-9965.EPI-12-0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Aleman JO, Eusebi LH, Ricciardiello L, Patidar K, Sanyal AJ, Holt PR. Mechanisms of obesity-induced gastrointestinal neoplasia. Gastroenterology. 2014;146(2):357–373. doi: 10.1053/j.gastro.2013.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Orme SM, McNally RJ, Cartwright RA, Belchetz PE. Mortality and cancer incidence in acromegaly: a retrospective cohort study. United Kingdom Acromegaly Study Group. J Clin Endocrinol Metab. 1998;83(8):2730–2734. doi: 10.1210/jcem.83.8.5007. [DOI] [PubMed] [Google Scholar]
- 127.Steuerman R, Shevah O, Laron Z. Congenital IGF1 deficiency tends to confer protection against post-natal development of malignancies. Eur J Endocrinol. 2011;164(4):485–489. doi: 10.1530/EJE-10-0859. [DOI] [PubMed] [Google Scholar]
- 128.Shevah O, Laron Z. Patients with congenital deficiency of IGF-I seem protected from the development of malignancies: a preliminary report. Growth Horm IGF Res. 2007;17(1):54–57. doi: 10.1016/j.ghir.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 129.Samani AA, Yakar S, LeRoith D, Brodt P. The role of the IGF system in cancer growth and metastasis: overview and recent insights. Endocr Rev. 2007;28(1):20–47. doi: 10.1210/er.2006-0001. [DOI] [PubMed] [Google Scholar]
- 130.Hart R, Doherty DA. The potential implications of a PCOS diagnosis on a woman’s long-term health using data linkage. J Clin Endocrinol Metab. 2015;100(3):911–919. doi: 10.1210/jc.2014-3886. [DOI] [PubMed] [Google Scholar]
- 131.Sirmans SM, Parish RC, Blake S, Wang X. Epidemiology and comorbidities of polycystic ovary syndrome in an indigent population. J Investig Med. 2014;62(6):868–874. doi: 10.1097/01.JIM.0000446834.90599.5d. [DOI] [PubMed] [Google Scholar]
- 132.Webber LJ, Stubbs S, Stark J, Trew GH, Margara R, Hardy K, Franks S. Formation and early development of follicles in the polycystic ovary. Lancet. 2003;362(9389):1017–1021. doi: 10.1016/s0140-6736(03)14410-8. [DOI] [PubMed] [Google Scholar]
- 133.Franks S, Stark J, Hardy K. Follicle dynamics and anovulation in polycystic ovary syndrome. Hum Reprod Update. 2008;14(4):367–378. doi: 10.1093/humupd/dmn015. [DOI] [PubMed] [Google Scholar]
- 134.Kaltsas GA, Androulakis II, Tziveriotis K, Papadogias D, Tsikini A, Makras P, Dimitriou K, Stathopoulou A, Piaditis G. Polycystic ovaries and the polycystic ovary syndrome phenotype in women with active acromegaly. Clin Endocrinol (Oxf) 2007;67(6):917–922. doi: 10.1111/j.1365-2265.2007.02987.x. [DOI] [PubMed] [Google Scholar]
- 135.Stubbs SA, Webber LJ, Stark J, Rice S, Margara R, Lavery S, Trew GH, Hardy K, Franks S. Role of Insulin-like growth factors in initiation of follicle growth in normal and polycystic human ovaries. J Clin Endocrinol Metab. 2013;98(8):3298–3305. doi: 10.1210/jc.2013-1378. [DOI] [PubMed] [Google Scholar]
- 136.E, Tassoni P, Cicognani A, Pirazzoli P, Salardi S, Balsamo A, Cassio A, Zucchini S, Colli C, Tassinari D. Value and limits of pharmacological and physiological tests to diagnose growth hormone (GH) deficiency and predict therapy response: first and second retesting during replacement therapy of patients defined as GH deficient. J Clin Endocrinol Metab. 1994;79(6):1663–1669. doi: 10.1210/jcem.79.6.7989472. [DOI] [PubMed] [Google Scholar]
- 137.Tassoni P, Cacciari E, Cau M, Colli C, Tosi M, Zucchini S, Cicognani A, Pirazzoli P, Salardi S, Balsamo A, Frejaville E, Cassio A, Zappulla F. Variability of growth hormone response to pharmacological and sleep tests performed twice in short children. J Clin Endocrinol Metab. 1990;71(1):230–234. doi: 10.1210/jcem-71-1-230. [DOI] [PubMed] [Google Scholar]
- 138.Ghigo E, Bellone J, Aimaretti G, Bellone S, Loche S, Cappa M, Bartolotta E, Dammacco F, Camanni F. Reliability of provocative tests to assess growth hormone secretory status. Study in 472 normally growing children. J Clin Endocrinol Metab. 1996;81(9):3323–3327. doi: 10.1210/jcem.81.9.8784091. [DOI] [PubMed] [Google Scholar]
- 139.Marin G, Domene HM, Barnes KM, Blackwell BJ, Cassorla FG, Cutler GB., Jr The effects of estrogen priming and puberty on the growth hormone response to standardized treadmill exercise and arginine-insulin in normal girls and boys. J Clin Endocrinol Metab. 1994;79(2):537–541. doi: 10.1210/jcem.79.2.8045974. [DOI] [PubMed] [Google Scholar]
- 140.Shalet SM. Extensive expertise in endocrinology: UK stance on adult GH replacement: the economist vs the endocrinologist. Eur J Endocrinol. 2013;169(4):R81–R87. doi: 10.1530/EJE-13-0418. [DOI] [PubMed] [Google Scholar]
- 141.Carel JC, Ecosse E, Landier F, Meguellati-Hakkas D, Kaguelidou F, Rey G, Coste J. Long-term mortality after recombinant growth hormone treatment for isolated growth hormone deficiency or childhood short stature: preliminary report of the French SAGhE study. J Clin Endocrinol Metab. 2012;97(2):416–425. doi: 10.1210/jc.2011-1995. [DOI] [PubMed] [Google Scholar]
- 142.Poidvin A, Touze E, Ecosse E, Landier F, Bejot Y, Giroud M, Rothwell PM, Carel JC, Coste J. Growth hormone treatment for childhood short stature and risk of stroke in early adulthood. Neurology. 2014;83(9):780–786. doi: 10.1212/WNL.0000000000000737. [DOI] [PubMed] [Google Scholar]
- 143.Livingstone C. The insulin-like growth factor system and nutritional assessment. Scientifica (Cairo) 2012;2012:768731. doi: 10.6064/2012/768731. [DOI] [PMC free article] [PubMed] [Google Scholar]


