Abstract
Significance: Hydrogen sulfide (H2S) metabolism leads to the formation of oxidized sulfide species, including polysulfide, persulfide, and others. Evidence is emerging that many biological effects of H2S may indeed be due to polysulfide and persulfide activation of signaling pathways and reactivity with discrete small molecules.
Recent Advances: Exogenous oxidized sulfide species, including polysulfides, are more reactive than H2S with a wide range of molecules. Importantly, endogenous polysulfide and persulfide formation has been reported to occur via transsulfuration enzymes, cystathionine γ-lyase (CSE) and cystathionine β-synthase (CBS).
Critical Issues: In light of the recent understanding of oxidized sulfide metabolite formation and reactivity, comparatively few studies have been reported comparing cellular biological and in vivo effects of H2S donors versus polysulfide and persulfide donors. Likewise, it is equally unclear when, how, and to what extent persulfide and polysulfide formation occurs in vivo under pathophysiological conditions.
Future Directions: Additional studies regarding persulfide and polysulfide formation and molecular reactions are needed in nearly all aspects of biology to better understand how sulfide metabolites contribute to key chemical biology reactions involved in cardiovascular health and immune responses. Antioxid. Redox Signal. 27, 634–653.
Keywords: : gasotransmitter, persulfide, polysulfide, sulfide, thiol
Introduction
Gasotransmitters are endogenous gaseous molecules such as hydrogen sulfide (H2S), nitric oxide (NO), and carbon monoxide (CO) that regulate biological functions at physiological relevant concentrations by targeting specific molecules. The interest in these gas molecules in mammalian cells dates back to the early 1900s when they were known as air pollutants interacting with hemoglobin. The modern idea of them as signaling molecules sparked the field when NO was identified as an endothelium-derived relaxing factor, that is, endothelial nitric oxide synthase (eNOS)-derived NO activates vascular smooth muscle soluble guanylyl cyclase (sGC) and cyclic guanosine monophosphate (cGMP) pathways to induce vasodilation.
Considering that H2S, NO, and CO can exist in the gaseous form in ambient conditions, the concept of a gasotransmitter was later created analogous to neurotransmitters (174). This classification brings attention to distinct properties of these gaseous signaling molecules, including their membrane permeability, their interaction with hemeproteins, and their ability to regulate signaling mechanisms. However, this concept is also limited by the fact that metabolic by-products (e.g., oxidation products) often mediate many biological functions rather than the parent gasotransmitter themselves. Such limitations are apparent for H2S as a signaling molecule and often misleading for studying biological effects of H2S. H2S oxidation in biological settings is inevitable and results in the production of polysulfides and persulfides that have been shown to elicit many effects also attributed to H2S (122). Moreover, the enzymatic pathway of H2S generation is embedded in the reverse transsulfuration pathway for cysteine metabolism, which may also generate oxidized sulfide species. In this review, we discuss the relationship between H2S and polysulfides–persulfides beyond the effect of H2S alone as a gasotransmitter and consider the importance of oxidized sulfide species in cardiovascular diseases and immune responses.
Hydrogen Sulfide Metabolism
Biological source of H2S and polysulfides
The ability of mammalian cells to produce H2S is based on the reverse transsulfuration pathway. The pathway is known to incorporate sulfur in methionine from diet or the folate cycle into cysteine, which is critical for the synthesis of proteins and a ubiquitous intracellular-reducing peptide glutathione (GSH). In this pathway, methionine first reacts with ATP to make S-adenosylmethionine whose methyl group is then transferred to a methyl acceptor, while the resultant S-adenosyl homocysteine loses the adenosine group to make homocysteine. From there, the conduit is limited by two pyridoxal 5′-phosphate-dependent enzymes, cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE). Initially, CBS catalyzes a β-replacement of the hydroxyl group of serine by homocysteine, yielding cystathionine. Subsequently, CSE uses cystathionine as a substrate to produce cysteine, α-ketobutyrate, and ammonia. However, like other pyridoxal 5′-phosphate-dependent enzymes, CSE and CBS are promiscuous in their use of substrates. Instead of using serine, CBS also consumes cysteine and homocysteine to generate cystathionine and H2S (157). In addition, CBS may generate H2S as well by converting cysteine to serine or lanthionine to a much lesser extent (157). Similarly, CSE uses cysteine and homocysteine to produce H2S with various by-products such as homolanthionine (31).
Another H2S-producing enzyme is 3-mercaptopyruvate sulfurtransferase (3-MST) that resides in both cytosol and mitochondria. Although 3-MST is not a part of the transsulfuration pathway, its substrate mercaptopyruvate is formed by either cysteine–aspartate aminotransferase (CAT) from l-cysteine or d-amino acid oxidase from d-cysteine (68, 154). 3-MST transfers the thiol from mercaptopyruvate to itself. In the presence of ambient reductants, 3-MST-bound persulfide is removed to release H2S. Although there is no consensus on what the endogenous reductants are, thioredoxin and dihydrolipoic acid (DHLA) appear to be particularly important (114, 124, 187).
The fate of H2S after generation is unique compared with NO and CO because it dissociates to HS- and S2- in solution, with the first and second pKa values of 6.76 and 19 at 37°C (55). It is estimated that in blood plasma where pH value is ∼7.4, 70–80% H2S exists in the HS− form. Although H2S is readily diffusible across the cell membrane, it is thought that the predominant HS- is not lipid membrane permeable and therefore requires transporters or channels. Counterintuitively, it has been shown that lipid layers do not serve as significant barriers for sulfide at the pH value of 7.4 and no facilitator is required for its passage (35, 107). This may be due to rapid diffusion of H2S and the equilibrium between H2S and HS−. In ischemic tissue where pH may drop lower than 6.8, the presence of sulfide in its full protonated state (H2S) can be more abundant than HS−, thus the permeability of H2S across membranes will be further increased (35, 170).
In aqueous solutions, auto-oxidation of H2S is inevitable with the presence of oxygen. Trace metal ions such as iron, existing both in cell culture media and blood plasma, serve as catalysts for oxidation. Protein-bound iron as in hemoglobin can also facilitate this process (171). Oxidation of H2S gives rise to highly reactive thiyl radicals, which may combine with themselves to yield hydrogen disulfide, hydrogen trisulfide, and other polysulfide compounds (129). These sulfur compounds, including persulfide and polysulfide, are named as reactive sulfur species (RSS) that arguably contribute to most of the documented biological effects of H2S (76, 118). At room temperature, up to eight sulfur atoms can bind together to form cyclic molecules and precipitate out of solution. However, longer polysulfide chains are known to occur in biology (169). Reactive oxygen species (ROS) such as superoxide and hydrogen peroxide (H2O2) have also been reported to be generated in H2S solutions (51). However, recent studies have shown that current methods for measuring ROS are equally if not more sensitive to RSS and therefore could be ambiguous in distinguishing ROS and RSS (40). In either case, the auto-oxidation of H2S seems to be inevitable even with low amounts of ambient oxygen. In cells, H2S is oxidized in the mitochondria by sulfide quinone oxidoreductase (SQR). The resultant SQR-bound persulfide can be further oxidized to sulfite, sulfate, and thiosulfate or transferred to a small molecular thiol, such as GSH or DHLA, which can be a critical source of endogenous polysulfide (67).
Recently, H2S-producing enzymes have also been shown to generate RSS. Using the monobromobimane (MBB) method, Ida et al. showed that purified CSE and CBS utilize cystine as a substrate and yield cysteine persulfide and polysulfide species (57). By overexpressing CSE and CBS in cell lines or mice, they also found that cysteine persulfide and polysulfide contents were increased using a polysulfide-specific fluorescent probe SSP2 and tag-switch assay (57). Meanwhile, Kimura et al. demonstrated the importance of 3-MST as a source of hydrogen trisulfide in the brain using the MBB method and SSP4 (78). Although these experiments addressed the contribution of CSE, CBS, and 3-MST to the bioavailability of polysulfide species, it is still not clear whether, in a biological environment, polysulfides are produced directly by these enzymes or through mitochondrial oxidation from various sources of H2S.
Oxidation of H2S is not a dead end with regard to its metabolism. Instead, this can be a critical way to store and possibly recycle H2S. The term sulfane sulfur describes sulfur bound to another sulfur. It encompasses various oxidized H2S metabolites, including persulfides, disulfides, polysulfides, and thiosulfate. We and other investigators have shown that sulfane sulfur can be a potent source of H2S and convey a wide spectrum of its biological effects (77, 84, 128, 141, 150). Among these studies, thiosulfate has emerged as an endogenous sulfane sulfur, which can be effectively converted to H2S (128). Exogenous thiosulfate may be used as an H2S donor under hypoxic conditions, suggesting that thiosulfate may be important for H2S mobilization in ischemic tissues. Thiosulfate can produce H2S through a nonenzymatic pathway or an enzymatic pathway via GSH-dependent reduction. Koj et al. reported that glutathione disulfide (GSSG), H2S, and labeled sulfite were produced when rat mitochondria were incubated with oxygen, GSH, and [S35]-thiosulfate (83). On the other hand, Curtis et al. reported that when S35 was perfused through isolated rat tissue, it was oxidized to thiosulfate and other metabolites (9, 36, 83). These findings highlight that thiosulfate can be involved in H2S production through GSH metabolism.
Regulation of enzymatic pathways
Mammalian CBS has a unique heme cofactor that enables CBS regulation by other gasotransmitters. Specifically, both NO and CO, but not H2S, can inhibit CBS activity by binding to the heme moiety (19, 167). This renders CBS oxygen sensitive in regulating cerebral circulation as it is basally inhibited under normoxia by heme oxygenase-derived CO, but released in hypoxia due to reduced CO availability (121). Additionally, CBS activity is regulated by post-translational modifications. CBS can be phosphorylated by cGMP-dependent protein kinase (PKG) at Ser227 that may regulate its enzymatic activation (121). Moreover, the C-terminal regulatory domain of CBS can also be sumoylated, although the function of this modification is unknown (69). CSE has also been shown to be phosphorylated at Ser377 by Akt/PKG to inhibit its enzymatic activity (142, 143, 196). Calcium is another important regulator for H2S production. Increased intracellular Ca2+ suppresses both CSE and 3-MST activities, while CBS is insensitive to Ca2+ regulation (27, 115, 116).
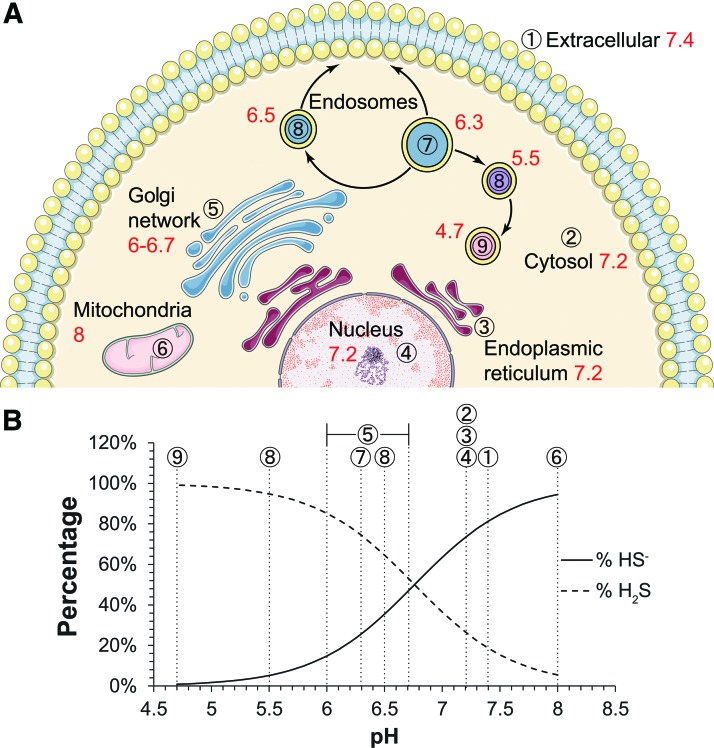
In addition to these regulatory mechanisms, it is important to note that compartmentalization is essential for cellular homeostasis and functional signaling transduction regardless of the mediator being common signaling molecules or unconventional gasotransmitters (148). For H2S or polysulfides to be signaling molecules, they should be compartmentalized as well. The first consideration of compartmentalization is the distribution of sulfide-producing enzymes and their substrates. 3-MST and CAT are ubiquitously expressed in various tissues. In comparison, CBS and CSE are somewhat differentially distributed. While CBS has greater expression in the brain, CSE is more prominent in the cardiovascular system. In the kidney and the liver, both CSE and CBS are highly expressed. Moreover, it is not only their presence that determines their ability to produce H2S but also the accessibility to the substrate and cofactors. For example, 3-MST-dependent generation of H2S relies on an endogenous reductant. As a candidate, DHLA only exists in the brain. Therefore, 3-MST is more likely to contribute to H2S generation in the brain, but it is possible that other endogenous reductants may take the place of DHLA in other tissues. However, this is a critical question that has yet to be addressed. The same question exists for cellular compartments. For example, although 3-MST can be found in both the mitochondria and the cytosol, its ability to produce H2S seems to be predominantly in the mitochondria. When cells are supplemented with 3-MP, the fluorescent signal of AzMC (H2S probe) localizes with Mitotracker, suggesting that 3-MST generates H2S predominantly in mitochondria (32). As mentioned earlier, the existing form of H2S is pH dependent and affects its membrane permeability. Although the physiological pH is often considered as ∼7, different organelles actually range from pH 4.7 to 8 (21). We calculated potential ratios of H2S to HS- in various cellular compartments based on known organelle pH (Fig. 1). It is worth noting that in the mitochondria where the normal pH value is 8, about 95% sulfide exists in the HS- form that significantly diminishes its ability to pass the mitochondrial membrane. This is important considering that mitochondria have the complete enzymatic machinery to oxidize sulfide and serve as a primary intracellular site for Fe-S cluster biosynthesis. Thus, it is not likely for mitochondrial-derived H2S to escape oxidation at least under normoxia. On the other hand, this also suggests that mitochondria could be an important source for polysulfide production regardless of the original enzymatic pathway to generate H2S. Last, but not least, for H2S and polysulfide to be signaling molecules, their production should be anchored to a microenvironment where upstream regulatory partners and downstream signaling targets are concentrated. However, little is known about such association for H2S/polysulfide biology.
FIG. 1.
Ratios of H2S to HS- in intracellular compartments. (A) Cellular organelles are denoted with a circled number and pH values are indicated in red, respectively. (B) Physiological pH values range from 4.7 to 8 where the dissociation of H2S can be considered as monoprotic (pKa1 = 6.76, pKa2 = 19). Accordingly, H2S/HS− ratios are calculated using Henderson–Hasselbalch equation with different cellular compartments represented by circled numbers. H2S, hydrogen sulfide. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Working Mechanisms of Sulfide and Reactive Sulfur Species
Atomic sulfur is very versatile in its ability to accept or donate electrons and can cycle between −2 and +6 oxidation states. This enables sulfur to play important roles in physiological and pathological processes. H2S is often considered as a reductant as its sulfur atom is −2 oxidation state, which is the same with GSH and cysteine. However, the standard reduction potential of S0/H2S, GSSG/GSH, and cystine/cysteine is −0.23, −0.24, and −0.34 V, respectively (96). Additionally, the biological level of H2S is much lower than that of cysteine or GSH. For example, in plasma samples, free H2S levels are in the pmol/mg protein range, but cysteine and GSH concentrations are in the nmol/mg protein range (16, 149). Hence, H2S is less likely to be an effective reductant under physiological conditions.
However, H2S undergoes complex oxidation, yielding thiosulfate, elemental sulfur, sulfenic acids, persulfides, polysulfides, sulfite, and sulfate. These oxidative products of H2S can be involved in protein post-translational modifications and low molecular thiol interactions. In this study, we discuss the mechanisms involved in those processes.
Protein post-translational modification
The free thiol in protein cysteine has a carbon-bound sulfur at the lowest oxidation state −2, whereas the sulfur atom in persulfide is at −1 (57). Therefore, the formation of persulfide cannot be from a direct reaction between a thiol and H2S where the sulfur valence is also −2 (181, 200). Both enzymatic and nonenzymatic-dependent mechanisms have been proposed to form persulfides.
In the mitochondria, SQR catalyzes the oxidation of H2S to sulfane sulfur. The resultant sulfane sulfur can be transferred to sulfite to form thiosulfate or GSH persulfide (62, 99). Sulfide-producing enzymes can generate cysteine persulfides. As early as 1960, Cavallini et al. have mentioned that CSE catalyzes the beta-elimination reaction of cystine, yielding cysteine persulfide (22). Recent reports showed that both CBS and CSE are also involved in the production of cysteine persulfide and polysulfide, with cysteine persulfide as their primary product (57, 130, 186). A persulfide is also known to be formed in the catalytic site of 3-MST (187). In the brain, 3-MST is found to be responsible for hydrogen trisulfide formation (78).
Sulfenic acids and S-nitrosothiols are involved in the nonenzymatic generation of persulfide. S-sulfenylation is the reversible oxidation of protein thiols to sulfenic acids (134). Sulfenic acids are known to react with thiols to form disulfides. Similarly, sulfenic acids can react with H2S to form persulfide. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and bovine serum albumin (BSA) have been proposed to be persulfidated via pre-existing S-sulfenylation (20, 200). S-nitrosothiols, another form of oxidized thiol, can also react with H2S to form persulfides (163). Some metal centers, such as copper and iron, can also oxidize H2S to form sulfhydryl radicals that can react with free thiols to generate protein persulfides (117, 200). Sulfhydryl radicals can further react with H2S to form polysulfides with varying chain lengths. Importantly, longer polysulfides display varying reactivity and are capable of completely cleaving intramolecular disulfide (178). Figure 2 illustrates several pathways whereby polysulfide and persulfide may be formed through reactions described above.
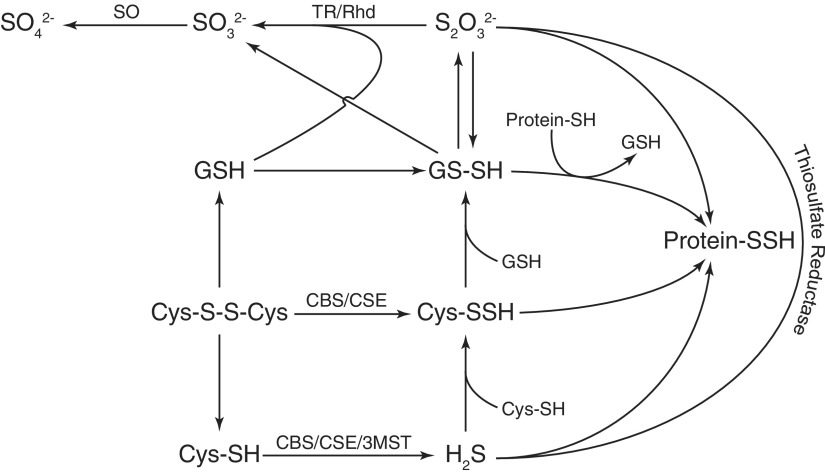
FIG. 2.
Formation of cysteine-based persulfide. Thiosulfate, glutathione persulfide, cysteine persulfide, and protein persulfide are the main forms of cysteine-based persulfide via the enzymatic/nonenzymatic pathway. Enzymes participating in this process include SQR, SO, TR, Rhd, CBS, CSE, and 3-MST. 3-MST, 3-mercaptopyruvate sulfurtransferase; CBS, cystathionine β-synthase; CSE, cystathionine γ-lyase; Rhd, rhodanese; SO, sulfite oxidase; SQR, sulfide quinone oxidoreductase; TR, thiosulfate reductase.
Interaction with small molecules
We have previously reviewed interactions between H2S and NO (85). In this study, we discuss interactions among H2S, cysteine, and GSH. The sulfane sulfur in cysteine persulfides, generated from cystine by CSE or CBS, can be transferred to GSH to form glutathione persulfide (GSSH) (57). It is worth noting that 0.5 mM substrate was used in the study, which may lack physiological relevance. Nevertheless, the study also reported the presence of GSSH and polysulfides (GSnH, GSnG) in cell culture and tissues. This can be a result of GSSH reacting with oxidized glutathione (GSSG, GSnG) and subsequent reduction by GSH reductase. Furthermore, the persulfide and polysulfides can be transferred to a protein or other small molecular thiols.
Measurement of persulfides and polysulfides
Due to the complexity of sulfur chemistry, extra caution is required for the measurement of persulfides and polysulfides. The most commonly applied approach for detecting protein-bound sulfane sulfur is a modified biotin-switch assay, adapted from the assay used for S-nitrosylation measurement (63, 122). In both the modified and the original assays, the first step is to block free thiols using an alkylating reagent such as S-methyl methanethiosulfonate (MMTS). The difference is that in the modified version, dithiothreitol (DTT) is used as a reductant for sulfane sulfurs instead of ascorbate for S-nitrosothiols. The previously modified residues can then be differentially labeled by another alkylating reagent such as N-(6-(biotinamido)hexyl)-3′-(2′-pyridyldithio)-propionamide (biotin-HPDP) and affinity-purified. A variation of this approach is to use maleimide conjugated with different fluorophores to label sulfhydryl groups of cysteines, where DTT releases the maleimide only from the persulfidated cysteine moieties, resulting in the loss of fluorescent signal (147). However, these biotin-switch methods can yield unspecific results. First, MMTS blocking can be incomplete, leaving some cysteine residues to be labeled by secondary alkylating reagent regardless of the presence of DTT or not (132). Second, DTT also reduces disulfide bonds between distinct cysteine residues and allows them to be mislabeled as persulfidated. In comparison, the tag-switch assay is more reliable for protein persulfide detection (200). This method uses methylsulfonyl benzothiazole (MSBT) as the thiol-blocking reagent, followed by selective labeling of persulfide moieties using biotinylated cyanoacetate. In both the modified biotin-switch assay and the tag-switch assay, the biotinylated products can be either verified by mass spectrometry or semiquantitatively analyzed by Western blotting.
In addition, combination of MBB and high-performance liquid chromatography (HPLC) has been applied to identify sulfane sulfurs in various biological samples. MBB reacts with sulfide in alkaline solution to yield the fluorescent sulfide dibimane, which can be measured by HPLC (151). Using the MBB method along with LC-MS/MS not only improves the assay's sensitivity but also validates the MBB/HPLC method. We developed a reliable work flow to release sulfane sulfurs using tris(2-carboxyethyl)phosphine hydrochloride (TCEP) as a reductant, re-collect volatilized H2S, and measure it with the MBB reaction and HPLC (150, 152). Furthermore, we improved the sensitivity of the MBB method to 0.25 nM with mass spectrometry (149). Other groups have also employed MBB-labeling protocols to measure hydrogen persulfide/polysulfides and sulfane sulfurs bound to small molecular thiols (57, 78).
Fluorescent probes, such as SSP2 and SSP4, have been developed for sulfane sulfurs (26, 146). These probes worked effectively under neutral to weak basic pH by releasing strong fluorescent molecules via fast intramolecular cyclization (26). Compared with other labeling methods, fluorescent probes allow spatial and temporal measurement of sulfane sulfurs in living cells.
With the emerging awareness of persulfides and polysulfides as important signaling molecules, current methods to detect these species are still limited. For example, most labeling protocols only provide resolution to a certain persulfidated protein rather than a specific cysteine residue, and it is challenging to distinguish protein persulfides and polysulfides using current techniques. Therefore, it is necessary to develop improved sulfane sulfur-labeling reagents coupled with refined mass spectrometry for unbiased investigation.
Cellular and Pathophysiological Effects of Sulfane Sulfur
The role of H2S as a gasotransmitter is first exemplified by its action on vascular tone. Today, mounting studies have shown critical roles of not only H2S but also polysulfide in maintaining cardiovascular health. In this section, we dissect the cardiovascular system into individual cell types and connect various cellular responses together in disease models to provide a glimpse into potential roles of H2S and polysulfide in cardiovascular disease and immune responses.
Endothelial solute permeability
Endothelial cells form the inner most layer of blood vessels. This monolayer of cells creates a physical barrier that separates plasma and extravascular compartments. While basal permeability of the endothelial barrier maintains normal solute exchange, vascular hyperpermeability is important for vascular remodeling and leukocyte recruitment. Initial studies reported that exogenous H2S serves to protect against particulate matter or ischemia/reperfusion-induced vascular permeability (47, 175). However, these studies focused on how H2S interferes with pathological events rather than direct and endogenous effects of H2S and its metabolites on endothelial barrier function. We have recently revealed that exogenous sulfane sulfurs from diallyl trisulfide (DATS) and inorganic sulfane sulfur compounds such as sodium disulfide, sodium trisulfide, and sodium tetrasulfide are potent inducers of solute permeability across endothelial monolayers (198). As we measured H2S levels in response to the various donor treatments using either the MBB method or a fluorescent probe (SF7-AM), we did not find significant differences in free H2S. Moreover, free H2S-releasing compounds, sodium sulfide (Na2S) or GYY4137, did not significantly alter endothelial permeability at physiological concentrations. Together, these experiments revealed that oxidized sulfide species such as polysulfides are far more potent inducers of endothelial permeability at concentrations that can be physiologically relevant (198).
We further investigated the role of endogenous H2S and polysulfides on endothelial permeability using wild-type versus CSE knockout mouse aortic endothelial cells (198). CSE knockout endothelial cells exhibited lower solute permeability and higher transendothelial electrical resistance, indicating stronger tight and adherens junctions. Compared with wild-type cells, the lack of CSE significantly reduced the sulfane sulfur pool compared with free H2S, suggesting endogenous polysulfides derived from CSE regulate basal endothelial permeability. Furthermore, we found that CSE knockout mice were more resistant to vascular endothelial growth factor (VEGF)-induced hyperpermeability in cutaneous microvasculature. These findings are in agreement with a recent report by Jiang et al. showing that CSE knockout mice have reduced postischemic sodium fluorescein extravasation in cerebral arteries (66).
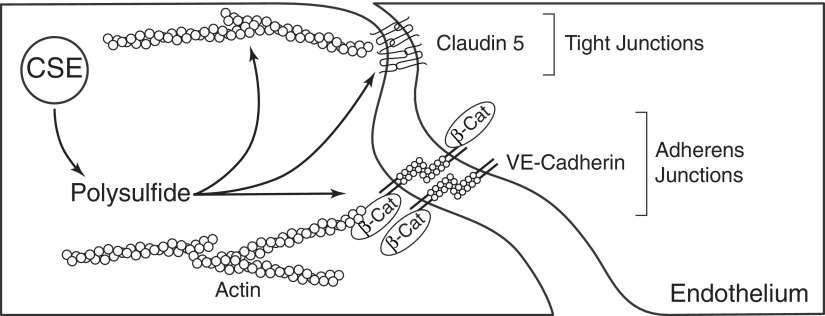
Mechanistically, we discovered that exogenous polysulfide treatment causes disruption of endothelial adherens junction proteins, VE-cadherin and β-catenin, as well as the tight junction protein claudin 5. Actin stress fiber formation and phosphorylation of myosin light chain on Thr-18/Ser-19 were also increased by polysulfides. Last, siRNA reduction of CSE expression significantly increased human umbilical vein endothelial cell (HUVEC) claudin 5 expression and enhanced its localization to the tight junction (198). Figure 3 illustrates CSE, polysulfide, and key molecular intracellular targets that modulate endothelial permeability. Intriguingly, the junction protein and permeability changes in response to polysulfides are similar to those elicited by ROS challenge; as such, it remains to be determined whether ROS and RSS share common or diverse mechanisms in regulating endothelial permeability. It is also plausible that RSS may modify reactive thiols of proteins (junction, linker, or signaling) that regulate barrier integrity. Further investigations are clearly needed to identify key molecular targets, specific polysulfide species, and potential signaling responses that may be altered by polysulfide modification.
FIG. 3.
Regulation of endothelial permeability by sulfide metabolism. CSE-derived polysulfide increases endothelial permeability through targeting junction proteins and actin cytoskeleton.
Angiogenesis
Endothelial cells in mature vascular structures are mostly quiescent, meaning they are not consistently dividing and migrating. However, the ability of endothelial cells to proliferate and migrate in response to stimuli is critical for embryonic development, angiogenesis in wound healing and ischemic tissue, and various inflammatory diseases. Others and we have examined the role of sulfide metabolites and endogenous transsulfuration enzymes on various angiogenic responses.
There is clear evidence that H2S promotes endothelial proliferation and migration. The brain endothelial cell line (bEnd3) was found to be more proliferative and migratory when treated with sodium hydrosulfide (NaHS), a salt that releases H2S, along with increased wound healing activity in an in vitro scratch assay (33). Likewise, endogenous sulfide production is important for endothelial cell growth and migration. CSE knockdown in both immortalized human umbilical endothelial cells (EA.hy 926) and primary mouse aortic endothelial cells decreased the proliferation rate, while overexpressing CSE increased it (5). Similarly, knocking down either CBS or 3-MST inhibited the rate of bEnd3 cell proliferation and reduced the number of migratory cells (32, 145). Moreover, supplement of enzymatic substrates such as l-cysteine and mercaptopyruvate also promotes proliferation and promigratory phenotypes of endothelial cells (32, 33). Although these studies provide compelling evidence on the regulatory role of sulfur metabolism in endothelial proliferation and migration, many key questions remain to be answered. First, due to the previous lack of accurate measurements of H2S and its metabolites, it is not clear whether the observed effects are mediated by free H2S or certain polysulfide derivatives. Treating cells with free H2S-releasing compounds such as NaHS does not guarantee a free H2S-induced biological effect—certainly not hours or days after the treatment (33). Others and we have previously discussed important issues surrounding free H2S-releasing reagents such as NaHS and Na2S and the popular, but erroneous, H2S measurement method using methylene blue (127, 199). It is important to remember that hydration of NaHS and Na2S immediately releases H2S whose half-life in solution may be as short as 5 min, depending on experimental conditions (127). Thus, biological effects such as cell proliferation and migration that occur hours after the initial treatment are unlikely due to free H2S. Nonetheless, H2S levels determined with the methylene blue assay indicated that sulfide continued to increase for days after the treatment (127), suggesting that the proproliferative and promigratory effects of sulfide donors may actually be due to oxidized sulfide metabolites. To further complicate this issue, it has been reported that DATS, a polysulfide donor, actually inhibits HUVEC migration and tube formation (89, 97). Therefore, it is necessary to use analytical methods to identify specific sulfide metabolites that are involved in differential regulation of endothelial cell growth and motility. Moreover, as discussed earlier, H2S-producing enzymes are closely linked to basic cell metabolism, including amino acid synthesis and redox balancing (GSH), which may also affect cell proliferation and migration that may confound the observation of sulfide serving as a gasotransmitter or polysulfide generator necessary for cell growth and survival.
These issues aside, previous studies have revealed some important pathways regulated by H2S or polysulfide that may play critical roles in endothelial cell phenotypic behavior. Chang and coworkers showed that an angiogenic factor, VEGF, increased H2S production in HUVECs using a free H2S-specific probe, SF7-AM (101). Furthermore, VEGF receptor 2 (VEGFR2), the major form in endothelial cells, has been demonstrated to possess a critical disulfide bond (Cys1045-Cys1024) that regulates its function. H2S, presumably via HS- anion, is able to reduce this disulfide bond to allow the downstream phosphorylation of phosphoinositide 3-kinase (PI3K), the activation of Akt, and the migratory effect (166). Moreover, persulfidation of the transcription factor specificity protein 1 by endogenous sulfur species derived from CBS increases VEGFR2 and its cofactor neuropilin 1 (NRP-1) (145). The PI3K/Akt pathway is known to stimulate eNOS by phosphorylation of the Ser1177 residue, and NO generated by eNOS is associated with endothelial cell proliferation and migration. It is important to consider whether these two gaseous signaling molecules crosstalk between their pathways to regulate endothelial functions. Indeed, a large collection of evidence has indicated that H2S and NO are mutually dependent on each other regarding angiogenesis (33). On the one hand, silencing CSE in bEND3 cells blunted NO-induced cell proliferation and wound healing, which was rescued by NaHS treatment. On the other hand, inhibition of eNOS by L-NAME reduced bEnd3 cell proliferation, which can be also recovered by an NO donor.
Interactions of H2S and NO remain to be fully characterized. However, the following mechanisms have been proposed on various levels of NO signaling. NaHS and Na2S have been reported to increase eNOS expression in cultured endothelial cells and a variety of endothelia, including the ear venules, the corpus cavernosum, and the capillary tuft of ischemic renal cortex (25, 56, 86, 110). However, this upregulation of eNOS seems to be limited by endothelial heterogeneity and experimental settings as no significant change of eNOS expression was observed in HUVECs or a mouse pancreatic microvascular endothelial cell line treated by either NaHS or Na2S (5, 12). Additionally, polysulfide donors, diallyl disulfide and DATS, have also been shown to increase the eNOS level in endothelial cells by suppressing proteasomal degradation under oxidized low-density lipoprotein (ox-LDL) insult (93). Furthermore, H2S metabolism also regulates eNOS activity. As mentioned above, eNOS activity can be enhanced by activation of the VEGFR2/PI3K/Akt pathway and phosphorylation of Ser1177. Interestingly, silencing any of the H2S-producing enzymes, CSE, CBS, and 3-MST, reduced eNOS phosphorylation at Ser1177 in response to shear stress, suggesting a fundamental role of H2S metabolism in shear-induced endothelial activation (52). Unfortunately, no study has investigated which sulfide metabolite is key in mediating this effect. Besides phosphorylation, eNOS is also subject to other post-translational modification. A study by Wang and coworkers demonstrated that NaHS induces eNOS persulfidation at Cys433, which favors eNOS dimer formation, thus maintaining its ability to produce NO (4). The study showed a competitive effect of NaHS and an NO donor SNP on eNOS persulfidation, suggesting that free H2S interacts with S-nitrosylated cysteine residue. The endogenous source of H2S for this regulation still requires further investigation. Moreover, eNOS is sensitive to intracellular Ca2+ availability, which is increased by NaHS. NaHS-induced Ca2+ mobilization may involve the Na+-Ca2+ exchanger, the inositol 1,4,5-trisphosphate receptor, the ryanodine receptor, and the ATP-dependent potassium channel (KATP) (72, 119, 140). H2S can open KATP channels by persulfidation of Cys43 of Kir6.1 subunit and reduction of a disulfide bridge between Cys6 and Cys26 of SUR1 subunit, where CSE plays an important role (65, 123). Moreover, intermediate conductance calcium-activated potassium channels are also persulfidated in human aortic endothelial cells by NaHS, which may contribute to vascular hyperpolarization and relaxation (123).
H2S and NO signaling pathways also converge on phosphodiesterases (PDEs) that break the phosphodiester bond in cGMP to dampen NO signaling. NaHS has been reported to inhibit PDE activity in endothelial cells in vitro (17, 33). Less is known about the mechanism of this inhibition, but it does not involve PDE persulfidation (11). Last, the major effector of NO signaling, PKG, has a redox-sensitive cysteine residue (Cys42), which can be oxidized by polysulfide and activated regardless of cGMP availability (159). However, PKG has also been shown to phosphorylate CSE at Ser377 and CBS at Ser227 that decrease H2S production, suggesting a negative feedback response, although these regulatory effects have not been investigated in endothelial cells (38, 39, 196).
Inflammatory signaling
Endothelial cells lining the vascular lumen not only form a barrier for electrolyte and soluble proteins but also regulate the process of inflammatory cell recruitment into extravascular tissues. Proinflammatory stimuli such as cytokines activate endothelial cells by upregulating expression of cell surface adhesion molecules, such as vascular cell adhesion molecule (VCAM) and intercellular adhesion molecule (ICAM) that facilitate leukocyte adhesion and recruitment. Nuclear factor kappa B (NFκB) is a transcription factor that induces and maintains the inflammatory state by coordinating the expression of proinflammatory genes, including cytokines and cell adhesion molecules. NFκB transcriptional activity depends on the nuclear translocation of the p65 subunit that is normally sequestered in the cytosol by IκB. In HUVECs, NaHS prevented tumor necrosis factor α (TNF-α)-induced IκB degradation, which attenuates p65 translocation into the nucleus and subsequent expression of VCAM and ICAM (133). On the other hand, NFκB has also been shown to be persulfidated to favor its association with ribosomal protein S3, which turns its transcription activity to prosurvival genes, although this aspect has not been investigated in endothelial cells (147).
Hypoxia and inflammatory signaling pathways are intertwined with each other. While NFκB increases the transcription of hypoxia-inducible factor (HIF), NFκB is also a target gene induced by HIF (8). NaHS has been shown to downregulate HIF-1α in EA.hy926 cells under hypoxia, which may be regulated by phosphorylation of eukaryotic initiation factor 2α (183). On the contrary, another study did not find an inhibitory effect of NaHS on hypoxia-induced HIF-1α activation in HUVECs. However, they demonstrated that NaHS only inhibits hypoxia, but not anoxia-induced HIF-1α activation and the expression of HIF-dependent genes in hepatocytes. This inhibitory effect does not involve the transcription of HIF-1α, but its proteasomal degradation is induced by von Hippel-Lindau tumor suppressor and the mitochondrial machinery (70). This discrepancy could be a result of different endothelial cell types used in the study, highlighting an important question regarding the role of endogenous H2S metabolism in different vascular cells under hypoxic conditions that require further investigation. Nuclear factor erythroid 2-related factor 2 (Nrf2) is a well-established transcription factor as a defensive mechanism against oxidative stress. The activity of Nrf2 is suppressed by Kelch-like ECH-associated protein 1 (Keap1) and targeted degradation. Xie et al. showed that in EA.hy926 cells, an H2S donor with relatively extended half-life, GYY4137, induced persulfidation of Keap1 at Cys151 determined by the tag-switch assay, which released Nrf2 from inhibition and enhanced its nuclear translocation (185). The finding is in agreement with Yang et al.’s study on mouse embryonic fibroblasts where NaHS mediated Keap1 persulfidation at the same residue measured by the modified biotin-switch assay (193). On the other hand, sodium tetrasulfide has also been reported to modify Keap1 and cause dimerization, enhancing Nrf2 nuclear translocation (82). In another study, DATS treatment induced thiol modification at only Cys288, which also increased Nrf2 expression (75). These studies support the idea that different sources of H2S and/or polysulfide influence discrete molecular targets for biological functions.
Another factor regulating endothelial inflammatory activation is endoplasmic reticulum (ER) stress. ER organelle protein processing is limited in its regulation of folding capacity under physiological conditions. This equilibrium can be disturbed by the accumulation of misfolded proteins concomitant with oxidative stress, which is commonly encountered in inflammation, resulting in ER stress. CSE is required for the persulfidation and inactivation of protein tyrosine phosphatase 1B, increasing the activity of protein kinase-like ER kinase, and restoring ER homeostasis (87).
Vascular smooth muscle cell function
Vascular smooth muscle cells (VSMCs) provide mechanical and structural support to blood vessels, which fine-tunes the compliance and resistance of arterial conduit vasculature. H2S is considered an established vasodilator with its effect being shown in different vasculatures of various animal species. H2S-induced vasodilation, usually studied by applying NaHS, is thought to be mediated mostly by hyperpolarizing the vascular wall through ion channels such as KATP, Kv7, and voltage and Ca2+-activated K+ channels (28, 50, 60, 61, 92, 98, 123). Endogenously, cysteine supplementation induces vasodilation, which is inhibited by dl-propargylglycine (PAG), a CSE inhibitor, suggesting CSE-derived H2S as an endogenous vasodilator (1, 28, 92). Furthermore, Mustafa et al. confirmed the importance of CSE in acetylcholine-induced vascular hyperpolarization using CSE knockout mice (123). Although CSE is an important source of H2S in endothelial cells, this collection of evidence does not guarantee H2S as an endothelial-derived vascular hyperpolarizing factor. Apart from being a substrate for CSE, cysteine can be also used by CBS and the CAT/3-MST pathway to generate H2S. Considering CBS, CAT, and 3-MST are present in the vasculature, it is not clear why pharmacological inhibition of CSE alone is effective to inhibit the vasodilatory effect of cysteine. Since PAG is usually used at extremely high concentrations (10–20 mM) in these experimental conditions, it is possible that some of these effects may be nonspecific. Moreover, it is not clear whether cysteine induces vasodilation in an endothelium-denuded vessel. Therefore, the endogenous source of H2S in an intact vasculature remains unclear. As a matter of fact, it has been shown that PAG also inhibited KATP current in rat aortic VSMCs, suggesting that H2S generated by VSMC CSE can be as important as endothelial CSE (164).
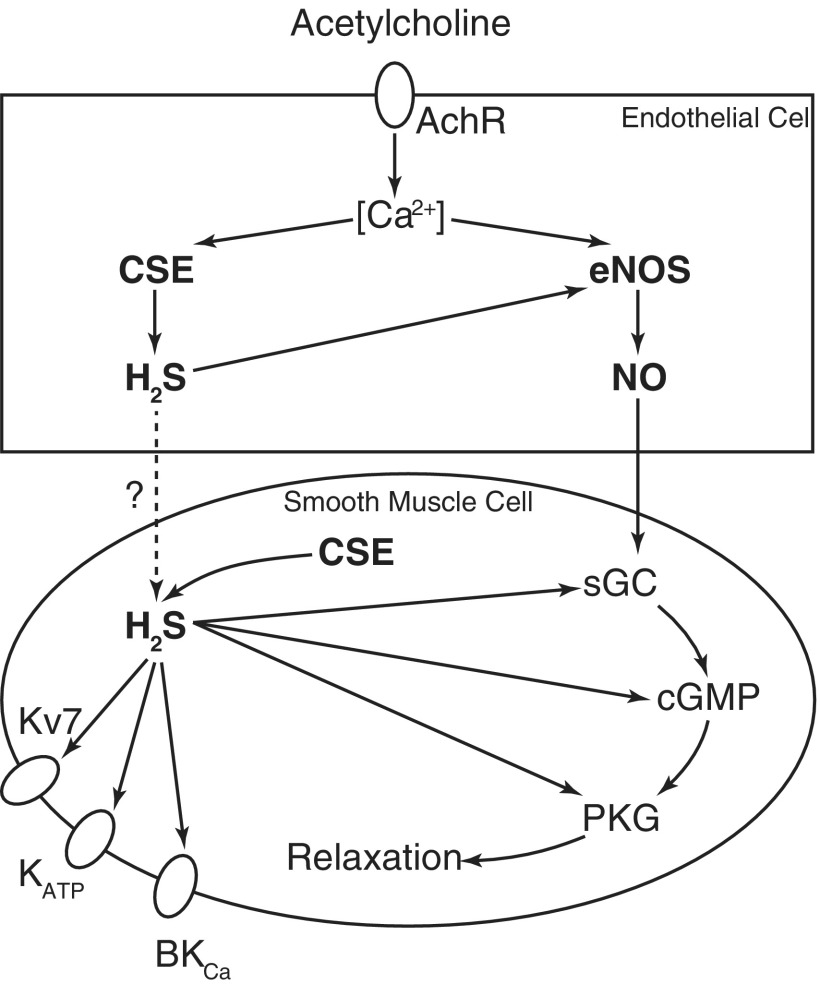
Importantly, it is possible that H2S itself may not be the direct mediator of vasodilation. As discussed earlier, H2S metabolism interacts with NO signaling in endothelial cells. Therefore, H2S-induced vasodilation can be at least partially a result of increased NO availability. Indeed, both the pharmacological inhibition and genetic deletion of eNOS reduced vascular responsiveness to NaHS (33, 165, 202). Similarly, inhibition of downstream factor PDE and PKG also attenuates NaHS-induced vasodilation (17, 33). As the target for NO signaling, sGC has a regulatory heme domain where NO interacts. H2S is known to interact with transition metals such as iron in the heme of sGC with coordination, just like NO. Surprisingly, H2S is not a direct activator for sGC (33). However, Zhou et al. recently showed that H2S potentiates the responsiveness of sGC to NO in smooth muscle cells (206). The hypothesis is that H2S reduces heme iron from the ferric state to ferrous state, which is otherwise unable to be activated by NO. The role of H2S in vasodilation is summarized in Figure 4.
FIG. 4.
Schematic illustration of H2S-induced vasodilation. CSE-derived H2S causes vasodilation mainly by activating ion channels and hyperpolarizing the vascular wall. As CSE is present in both endothelial cells and vascular smooth muscle cells, H2S from both origins can contribute to this action. H2S-mediated vasodilation also largely depends on NO signaling, which is potentiated at multiple levels by H2S. NO, nitric oxide.
Exogenous H2S has also been reported to show a biphasic effect on vascular tone. While NaHS is vasodilatory at high concentrations (>400 μM), it actually causes vascular constriction at lower concentrations, which may be due to eNOS inhibition (3, 46, 88). It is later demonstrated that this biphasic effect is a result of high oxygen tension; as with physiological levels of oxygen, NaHS leads to vasodilation at much lower concentrations (80, 81). This calls attention to the question of whether oxidative products of H2S are responsible for the constriction of vessels. Conversely, polysulfide is more effective than NaHS in dilating small mesentery arteries by oxidizing PKG Iα (159). The interaction between H2S and NO can also produce intermediates accounting for their biological effects. Nitroxyl (HNO) has been produced from H2S and NO to activate transient receptor potential channel A1 as an essential mechanism regulating vascular tone (44). Besides, the interaction of H2S and NO also gives rise to nitrosopersulfide (SSNO−) and N-nitrosohydroxylamine-N-sulfonate (SULFI/NO), involving thiyl radicals and nitrosothiols as intermediate products (34). SULFI/NO does not release NO, nor does it increase intracellular cGMP. By comparison, SSNO− is more stable in biological conditions and is also able to release NO and polysulfide. However, their presence and biological importance require further investigation in vivo.
VSMC proliferation and migration play important roles in vascular remodeling under numerous pathophysiological conditions. In this process, VSMCs make a transition from the contractile phenotype to a synthetic phenotype that allows vigorous cell proliferation and migration. Multiple studies have reported that NaHS inhibits VSMC proliferation and migration under the stimulation of serum, high levels of glucose, or homocysteine (43, 160, 177, 205). Meanwhile, the apoptotic pathway is also activated in NaHS-treated VSMCs (190, 191). Smooth muscle cells isolated from the aorta of CSE knockout mice had a higher proliferation rate with serum stimulation, but are more prone to NaHS or H2O2-induced apoptosis compared with wild-type cells (191). The mechanisms are not fully understood, but may involve ERK, calcium-sensing receptors, and mitochondrial fusion. Importantly, it was suggested that H2S acts as a reductant. However, since both H2O2 and NaHS induced SMC apoptosis, H2S or its oxidative product likely acts as an oxidant stress in this experimental setting (191).
Monocytes/macrophages
Residential macrophages and recruited circulating monocytes are important cellular components mediating inflammation and vascular/cardiac remodeling. Several lines of study have revealed the anti-inflammatory effect of exogenous H2S. Lipopolysaccharide (LPS) stimulates macrophages to produce proinflammatory cytokines such as TNF-α and interleukin-6 (IL-6) whose expression is inhibited by NaHS (53, 144). Similar results are achieved with slow H2S-releasing donors, GYY4137 and FW1256 (53, 182). In addition, these free H2S donors also inhibit LPS-induced expression of cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) and resultant prostaglandin E2 and NO production (53, 95, 126). Moreover, NaHS inhibits macrophage motility in vitro. Zhang et al. demonstrated that NaHS inhibited interferon-γ and LPS-induced expression of chemokines, CX3CR1 and chemotaxis toward CX3CL1 stimulation in RAW 264.7 cells, via regulating peroxisome proliferator-activated receptor gamma and NFκB pathways (201). Endogenously, LPS stimulation in macrophages increases the level of CSE messenger RNA (mRNA), while inhibiting CSE further increases NO production, suggesting that CSE-derived H2S is anti-inflammatory (126).
Du et al. showed that ox-LDL downregulated CBS, but not CSE or 3-MST in THP-1-derived macrophages, with elevated expression of TNFα, IL-10, monocyte chemoattractant protein-1, and macrophage migration inhibitory factor, which was inhibited by NaHS (42). In comparison, ox-LDL reduced CSE transcription in RAW 264.7 cells potentially through increased DNA methyltransferase expression and methylation of the CSE promotor region (41). GYY4137 treatment effectively inhibited ox-LDL-induced expression of lectin-like ox-LDL receptor-1, iNOS, ICAM, VCAM, and chemokines such as CXCL2, CXCR4, CXCL10, and CCL17 (103). Additionally, both endogenous and exogenous H2S alleviates ox-LDL-induced cholesterol efflux and foam cell formation in vitro (49, 204).
NFκB appears to be at the center of the H2S-mediated anti-inflammatory effect (42, 53, 91, 103, 104, 126, 176, 201). NaHS inhibits the expression of proinflammatory cytokines by persulfidation of NFκB p65 subunit at Cys38, while mutation of Cys38 to serine abolished ox-LDL-induced inflammatory effect in macrophages (42). Exogenous H2S can also inhibit NFκB activation through hemeoxygenase 1-dependent mechanism in LPS-stimulated macrophages (126). Annexin A1, a glucocorticoid-regulated inhibitor of inflammation, may also be critical for the anti-inflammatory effect of NaHS since NaHS only inhibited iNOS/COX-2 expression in bone marrow-derived macrophages from wild-type mice, but not Annexin A1 knockout mice (15). The effect of NaHS on TNF-α and IL-6 may also involve histone acetylation. However, the authors reported that both increased histone H3 acetylation by NaHS in LPS-stimulated THP-1 cells in two separate studies, without explaining the discrepancy (53, 144). Additionally, Jain et al. showed that Na2S inhibits IL-1β expression in high-glucose-treated U937 monocytes, with the upregulation of glutamate–cysteine ligase catalytic subunit and glutamate–cysteine ligase modifier subunit and increased GSH levels (64).
Although prevailing evidence indicates an anti-inflammatory role of H2S in macrophages, some studies suggest otherwise (6, 7, 74, 113). For example, Miao et al. demonstrated that NaHS increases the motility of RAW 264.7 cells through the integrin β1-Src-FAK/Pyk2-Rac pathway (113). NaHS is also shown to increase TNF-α by activating the NFκB pathway (74). The explanation for this difference may be that the role of H2S on inflammation is context dependent. However, what can be different is not only the context (the microenvironment for signaling), but also the subject (the actual signaling molecule) being studied. In addition to free H2S donors, DATS has also been found to be anti-inflammatory. Actually, DATS mimics most effects of NaHS in LPS-stimulated RAW 264.7 cells, including the reduced expression of iNOS and COX-2, a reduced release of TNF-α and IL-1β, and the suppressed activation of NFκB (91). In another interesting comparison among diallyl sulfide, diallyl disulfide, and DATS, DATS was the most effective to inhibit LPS-induced NO production in RAW 264.7 cells, while diallyl sulfide had no effect at all (102). Therefore, in activated macrophages where superoxide production is high, perhaps polysulfide is the actual signaling molecule rather than free H2S. Regardless of exogenous H2S and polysulfide for a therapeutic approach, the more important pathophysiological question is how endogenous H2S is metabolized in macrophages. Unfortunately, without properly measuring H2S in these experimental conditions, this answer remains obscure.
Neutrophils
Neutrophils are important mediators in acute inflammation caused in infectious diseases and cardiovascular diseases. Their microbiocidal activity is conveyed by myeloperoxidase (MPO). H2S has been shown to react with hypochlorous acid, the product of MPO, resulting in polysulfide species (125, 180). Palinkas et al. demonstrated that H2S is a potent, but irreversible, inhibitor of MPO for its oxidant-producing ability, while polysulfide did not show any inhibitory effect (131). The physiological role of the interaction between H2S and MPO clearly requires further investigation.
Diseases and Therapeutic Approaches
Ischemic diseases
Early studies in the isolated heart suggested a suppressed H2S production by ischemia, which was attributed to the loss of CSE activity (10, 195). However, H2S concentration and CSE activity were evaluated using the methylene blue method, which does not measure H2S in biological samples. Pharmacological inhibition of CSE by PAG on the contrary exacerbated myocardial infarction in the same model (13). PAG treatment also increased the infarction size in vivo (100). The lack of specificity and unwanted toxicity of PAG may also be an issue. Considering these limitations, the endogenous role of H2S in ischemic conditions has been revisited with improved techniques.
Using CSE knockout mice, King et al. demonstrated the cytoprotective roles of H2S metabolism in myocardial ischemia/reperfusion (I/R) injury (79). CSE deficiency increased infarction size in the ischemic heart with reduced availability of free H2S and sulfane sulfur. Conversely, cardiac-specific overexpression of CSE in mice protects against myocardial I/R injury (18). CSE transcription has been reported to be suppressed in the ischemic heart, which may be mediated by upregulation of the miR-30 family (153, 207). Meanwhile, others observed increased CSE mRNA levels (45, 153). These discrepancies could be due to different experimental procedures for ischemic injury as well as temporal regulation of CSE expression following the ischemic insult.
In patients with critical limb ischemia, the expression of CSE, CBS, and 3-MST decreased in skeletal muscle tissue, while free H2S and sulfane sulfur are also reduced (59). Conversely, patients with peripheral artery disease and/or coronary artery disease were reported to have a small but significant increase in plasma free H2S (137). These differences are likely due to different tissues studied and possible severity of ischemia. In the mouse hind limb ischemia model due to permanent femoral artery ligation, we observed a rapid increase of CSE activity and free H2S in the ischemic skeletal muscle 3 days following ligation that returned back to baseline levels by day 7 (12, 84). In CSE knockout mice, free H2S was significantly decreased in the ischemic tissue, which is associated with poor recovery of perfusion in the ischemic limb (84). This suggests that CSE-derived H2S invoked a protective mechanism against an ischemic injury and that future studies should examine regulation of CSE enzyme activity as a potential confounder of clinical ischemic tissue adaption responses. Figure 5 illustrates key CSE and sulfide-dependent responses involved in ischemia-mediated vascular remodeling involving angiogenesis and arteriogenesis.
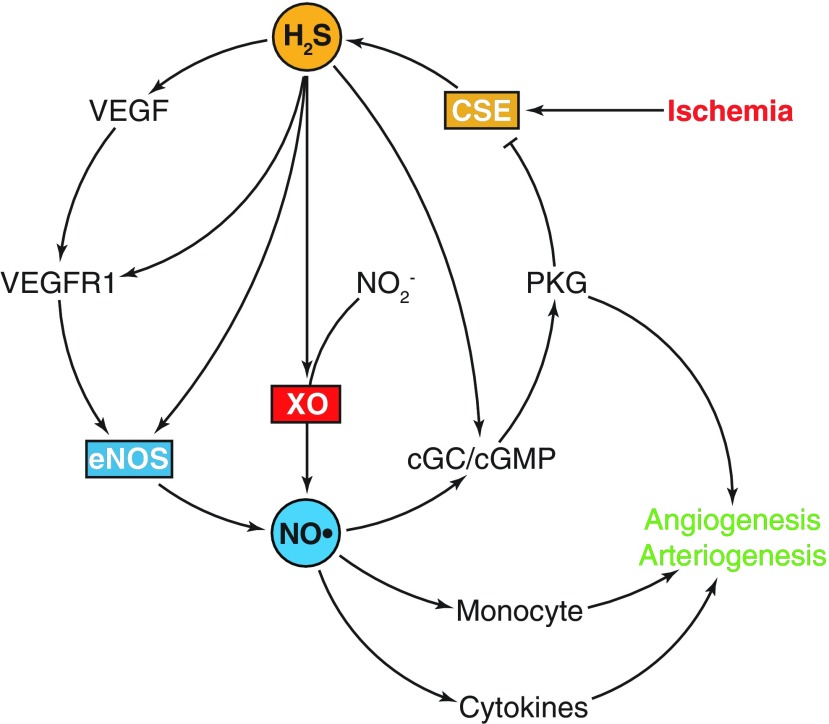
FIG. 5.
H2S and NO in ischemic vascular remodeling. Ischemia transiently enhances H2S generation by CSE. H2S increases VEGF production and post-translationally modifies VEGFR1 and eNOS, which can lead to elevated NO availability. NO in turn improves angiogenesis and arteriogenesis through cytokines and monocyte recruitment. Importantly, H2S enhances nitrite (NO2−) reduction via XO, which is a more reliable pathway of NO production under hypoxia. H2S can activate the cGC/cGMP/PKG pathway by acting on sGC and phosphodiesterase, which not only contributes to angiogenesis and arteriogenesis but may also serve as a negative feedback by PKG-dependent phosphorylation of CSE. cGMP, cyclic guanosine monophosphate; eNOS, endothelial nitric oxide synthase; PKG, cGMP-dependent protein kinase; sGC, soluble guanylyl cyclase; VEGF, vascular endothelial growth factor; VEGFR1, VEGF receptor 1; XO, xanthine oxidase. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
H2S metabolism also plays important roles in the ischemia in other tissues. In the liver, ischemic insult causes CBS translocation into mitochondria, escaping Lon protease-mediated degradation (168). The lack of CSE exacerbates I/R injury in the liver (18). In mouse retina at the neonatal stage, hyperoxygenation obliterates vessels in the center of the retina, serving as a model for ischemia-induced retinopathy. Free H2S level is increased in this model, along with the upregulation of CSE in endothelium and CBS in monocytes, while 3-MST is not altered (48). In the kidney, I/R significantly downregulated CSE, CBS, and 3-MST (14, 73, 106, 173). Similar to what we found in the femoral ligation model, ischemic kidneys also exhibit a transient increase of CSE transcription, followed by a decrease in long-term expression (14). However, the result of ischemia in the kidney is controversial as Bos et al. showed that CSE-deficient mice suffered from deteriorated kidney damage and worse kidney function, while Marko et al. demonstrated that loss of CSE is protective due to reduced inflammation (14, 106). Different strains of CSE knockout mice were used in these studies. The initial knockout mice had a mixed genetic background (C57BL/6J × 129SvEv) and exhibited age-dependent hypertension and sex-related hyperhomocysteinemia, which can affect the prognosis of kidney ischemia independent of H2S (192). In comparison, Marko used a CSE knockout strain on the C57BL/6J background that does not develop hypertension, but does have hyperhomocysteinemia and hypercysteathioninemia (58, 106).
H2S-mediated protection against ischemia involves a significant role of NO. In CSE knockout mouse, eNOS function is compromised with reduced NO bioavailability in the heart (79). Moreover, exogenous H2S failed to reduce I/R-induced myocardial infarction in mice with eNOS mutation at the active phosphorylation site (S1179A) (79). In the ischemic hind limb, we found that Na2S was able to partly recover blood flow in eNOS knockout mice to approximately half that seen in wild-type mice (12). Additional c-PTIO treatment completely abolished the remaining protective effect of exogenous H2S. Importantly, we discovered that H2S induces nitrite reduction back to NO in a xanthine oxidase (XO)-dependent manner under hypoxia, which contributes to the beneficial effect of H2S in eNOS knockout ischemic skeletal muscle (12). Thus, H2S and NO work in unison to facilitate ischemic vascular remodeling that also involves recruitment of monocytes (12, 84, 138, 197).
H2S therapy has been proved to be successful in ischemic disease models. In myocardial I/R, both preconditioning and postconditioning with free H2S-releasing compounds, including NaHS, Na2S, and GYY4137, have been shown to reduce infarction size (23, 71, 90, 109, 135). The mechanism involves the activation of antioxidant machineries and antiapoptotic and anti-inflammatory pathways as discussed in the previous section. Modis et al. recently showed that the α subunit of ATP synthase in the mitochondrial inner membrane can by persulfidated by both NaHS treatment and endogenous CSE expression, which may support mitochondrial bioenergetics (120). Moreover, autophagy is induced by NaHS treatment in vitro and in vivo (24, 156, 184). GYY4137 treatment is also effective in preventing cardiac remodeling and preserving cardiac function (100, 155). Miao et al. showed that NaHS favors M2 polarization of monocytes and macrophages at the early stage of myocardial infarction, which enhances tissue repair and function preservation (112).
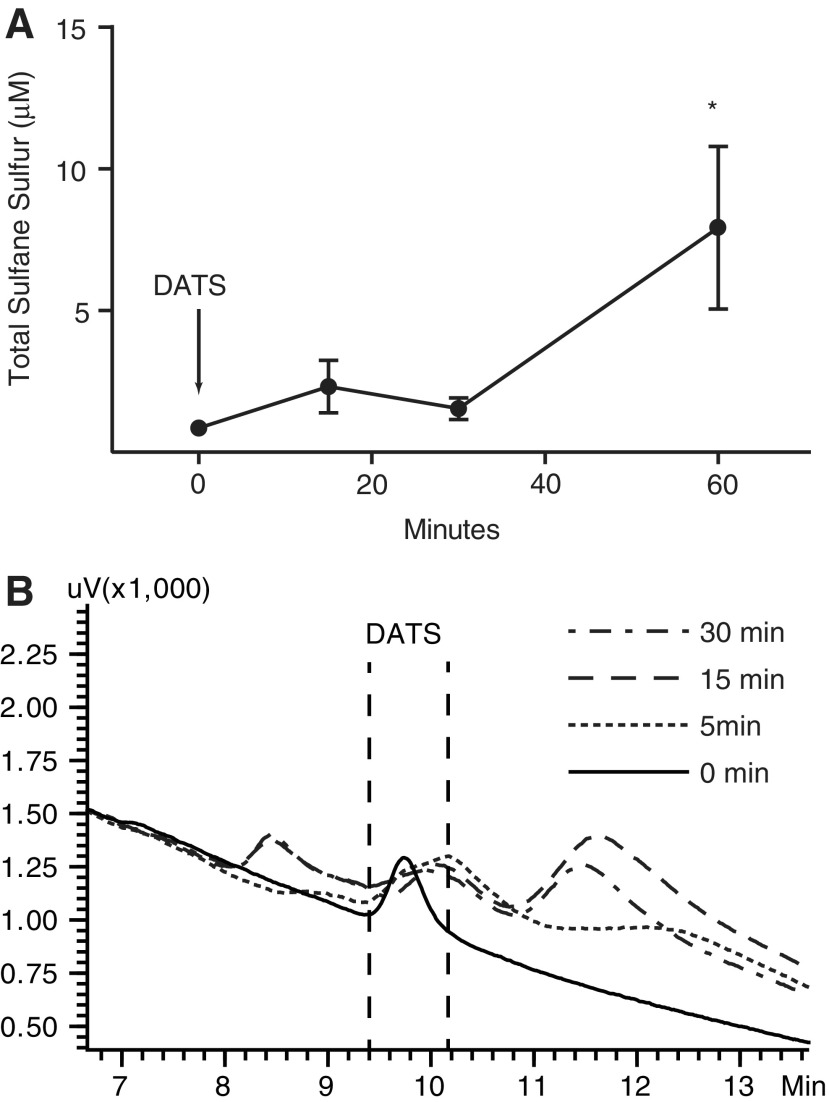
Importantly, DATS is an effective polysulfide donor in vivo and is beneficial in treating ischemic injury. Shortly after intravenous administration, DATS increases sulfane sulfur contents in the plasma and gives rise to other products (Fig. 6). Both in the ischemic heart and skeletal muscle, DATS treatment conveys protection by increasing NO availability and inducing ischemic vascular remodeling through monocyte recruitment (84, 141). Cheng et al. showed reduced CSE expression and free H2S level in bone marrow-derived macrophages (BMCs) isolated from Db/Db mice (29). The exposure of BMCs to high glucose decreased free H2S, which was rescued by DATS treatment or CSE overexpression. The beneficial effects of DATS treatment in BMCs also alleviated apoptosis and increased migration with high-glucose stimulation. More importantly, in Db/Db mice with hind limb ischemia, both the oral administration of DATS and the intramuscular transfer of CSE overexpressing BMCs successfully increased perfusion recovery in the ischemic tissue, with increased angiogenesis and arteriogenesis.
FIG. 6.
DATS as an effective polysulfide donor in vivo. (A) DATS (200 μg/kg) was given to mice by retro-orbital administration. Animals were sacrificed at indicated time and plasma was collected for sulfide measurement using the MBB method. Sulfane sulfur was significantly increased at 60-min time point (*n = 4, p < 0.05). (B) HPLC profiles of plasma samples incubated with DATS at indicated time are superimposed. Note that the peak representing DATS derivative was diminished over time, while other products appeared. DATS, diallyl trisulfide; HPLC, high-performance liquid chromatography; MBB, monobromobimane.
Hypertension
In an early clinical study, patients with severe hypertension (≥Grade 2) were reported to have lower plasma H2S concentration (108). In patients with pulmonary hypertension, both H2S concentration and CSE expression were significantly lower than the healthy population (161). Both studies used a sulfide-sensitive electrode for H2S measurement. In placenta from patients with preeclampsia, both the H2S concentration and CSE mRNA were lower compared with normotensive controls (172). Moreover, PAG treatment of pregnant mice decreased plasma H2S and increased blood pressure with abnormal placental vasculature (172). Additionally, CSE/CBS and H2S availability seem to be downregulated in animal models of hypertension. As a model of primary hypertension, the spontaneously hypertensive rat (SHR) exhibited reduced plasma H2S and CSE expression in the thoracic aorta compared with the WKY control group (188). By contrast, high salt-induced hypertension in Dahl rats showed suppressed CBS expression in the kidney and reduced H2S in the kidney and plasma, measured by a sulfide electrode (54). Increased glucocorticoid level causes hypertension, which is a clinical issue for chronic administration of glucocorticoids. Dexamethasone-induced hypertension in rats greatly reduced CSE and CBS expression in both carotid arteries and mesenteries with decreased H2S levels in these tissues (37). Hypoxia-induced pulmonary hypertension in rats also revealed reduced H2S levels in plasma and the lungs (179). However, many of these studies used the methylene blue method to measure H2S concentration, making it impossible to interpret precise roles of specific sulfide metabolites.
Supplementation with free sulfide-releasing compounds such as NaHS and GYY4137 is able to decrease blood pressure in hypertensive models (2, 139, 162, 172, 194, 203). Stubbert et al. point out that H2S is rapidly oxidized with trace oxygen and that potassium polysulfide can be a more potent vasodilator compared with NaHS. They further demonstrated that NaHS/polysulfide-induced vasodilation was compromised in the transgenic knockin mice with the redox-dead PKG (Cys42 to serine), suggesting that the vasodilatory effect of NaHS is mediated by oxidizing PKG via polysulfide formation (159). Interestingly, intraperitoneal administration of thiosulfate as a novel H2S donor not only reduced blood pressure in angiotensin II-induced hypertension but also protected against hypertensive cardiac damage (158). This further supports the hypothesis that thiosulfate can be reduced by endogenous reductant (128). Additional study is needed to identify critical endogenous reductants and more importantly the pharmacokinetics of thiosulfate with a proper method measuring H2S and polysulfide.
Atherosclerosis
In air balloon-injured carotid arteries, CSE mRNA level is increased, while NaHS treatment reduced neointimal formation (111). CSE transcription is also suppressed by vitamin D3 and nicotine-induced vascular calcification that can be mitigated by NaHS. In CSE knockout mice, neointima size induced by carotid artery ligation is greater than in wild-type mice (189). With high-fat diet, CSE knockout mice developed atherosclerotic plaques at the aortic root, with elevated plasma cholesterol and low-density lipoprotein (LDL) (105). CSE knockout mice on the apolipoprotein E (ApoE) knockout background showed further increased plaque size (105). It is worth noting that both CSE and CBS deficiency cause hyperhomocysteinemia, which is an independent risk factor for atherosclerosis and related cardiac diseases. Therefore, the atherogenic phenotype in CSE knockout mice may not be due to the deficiency of H2S synthesis, but increased homocysteine level. Nonetheless, NaHS therapy was able to reduce plaque size in the CSE/ApoE double knockout mice (105). Additionally, overexpressing CSE in ApoE knockout mice reduced plaque size (30).
H2S supplementation also alleviates atherosclerosis in conventional models. In ApoE mice with high-fat diet, NaHS treatment decreases plasma cholesterol, triglycerides, and LDL and more importantly reduces plaque size (94). Similarly, GYY4137 inhibited plaque formation in high-fat diet-fed ApoE knockout mice (103). Moreover, NaHS decreased lesion size in streptozotocin-induced LDL receptor (LDLr) knockout mice, but not in LDLr/Nrf2 double knockout mice, suggesting that the beneficial effect of H2S treatment involves Nrf2-mediated antioxidant pathways (185). Importantly, Peh et al. demonstrated that high-fat diet alone alters H2S metabolism (136). In C57BL/6 mice with 16-week high-fat diet, CSE was downregulated in the liver, the lung, and the aortic endothelium. 3-MST was also reduced in the liver. On the contrary, CBS expression was higher in the liver and the kidney. On the one hand, this suggests that H2S metabolism may be critical in metabolic disorders and atherosclerosis; on the other hand, it is currently unclear whether polysulfides are involved and how they may influence vascular pathophysiology.
Conclusions and Future Perspectives
It is now increasingly clear that H2S by itself is not solely responsible for the many different biochemical and biological responses attributed to it. As we have discussed, atomic sulfur readily reacts with numerous molecular targets via different oxidation states. It is most likely that much of the pathophysiological and pharmacological effects of exogenous H2S involve activity of oxidized metabolites. However, accounting for these chemical biology reactions and pathophysiological responses will not be easy.
Little reliable information currently exists with regard to sulfane sulfur levels (e.g., polysulfides, persulfides, thiosulfate) in various tissues under normal and disease states. Moreover, essentially no information exists regarding the levels of these metabolites in gene mutant animal models of transsulfuration enzymes, sulfide-oxidizing enzymes, and other enzymes impacting cellular redox status, not to mention animal models of aging. Correcting these deficits of knowledge is fundamental if the field is to better understand how sulfur metabolism contributes to organismal health and disease. Importantly, accurate and reliable measurement methods will be essential in answering these and many other biological questions regarding sulfane sulfur metabolites.
In conclusion, the field of polysulfides and sulfane sulfur chemistry represents an exciting and important new area of sulfide research. It is without question that significant new insights will be obtained regarding redox-dependent pathophysiological disease states, along with identification of key molecular targets. The new information gained should ultimately lead to novel and more effective therapeutic agents for a plethora of disease conditions.
Abbreviations Used
- 3-MST
3-mercaptopyruvate sulfurtransferase
- ApoE
apolipoprotein E
- BMCs
bone marrow-derived macrophages
- CAT
cysteine–aspartate aminotransferase
- CBS
cystathionine β-synthase
- cGMP
cyclic guanosine monophosphate
- CO
carbon monoxide
- COX-2
cyclooxygenase-2
- CSE
cystathionine γ-lyase
- DATS
diallyl trisulfide
- DHLA
dihydrolipoic acid
- DTT
dithiothreitol
- eNOS
endothelial nitric oxide synthase
- ER
endoplasmic reticulum
- GSH
glutathione
- GSSG
glutathione disulfide
- GSSH
glutathione persulfide
- H2O2
hydrogen peroxide
- H2S
hydrogen sulfide
- HIF
hypoxia-inducible factor
- HPLC
high-performance liquid chromatography
- HUVEC
human umbilical vein endothelial cell
- I/R
ischemia/reperfusion
- ICAM
intercellular adhesion molecule
- IL
interleukin
- iNOS
inducible nitric oxide synthase
- KATP
ATP-dependent potassium channel
- Keap1
kelch-like ECH-associated protein 1
- LDL
low-density lipoprotein
- LDLr
low-density lipoprotein receptor
- LPS
lipopolysaccharide
- MBB
monobromobimane
- MMTS
S-methyl methanethiosulfonate
- MPO
myeloperoxidase
- mRNA
messenger RNA
- Na2S
sodium sulfide
- NaHS
sodium hydrosulfide
- NFκB
nuclear factor kappa B
- NO
nitric oxide
- Nrf2
nuclear factor erythroid 2-related factor 2
- ox-LDL
oxidized low-density lipoprotein
- PAG
dl-propargylglycine
- PDE
phosphodiesterases
- PI3K
phosphoinositide 3-kinase
- PKG
cGMP-dependent protein kinase
- ROS
reactive oxygen species
- RSS
reactive sulfur species
- sGC
soluble guanylyl cyclase
- SQR
sulfide quinone oxidoreductase
- SSNO−
nitrosopersulfide
- SULFI/NO
N-nitrosohydroxylamine-N-sulfonate
- TNF-α
tumor necrosis factor alpha
- VCAM
vascular cell adhesion molecule
- VEGF
vascular endothelial growth factor
- VEGFR2
vascular endothelial growth factor receptor 2
- VSMC
vascular smooth muscle cell
Acknowledgments
This study was supported by grants HL113303 and ADA 1-15-TS-18 to C.G.K. and an LSUHSC Cardiovascular Center Malcolm Feist predoctoral fellowship to S.Y.
References
- 1.Al-Magableh MR. and Hart JL. Mechanism of vasorelaxation and role of endogenous hydrogen sulfide production in mouse aorta. Naunyn Schmiedebergs Arch Pharmacol 383: 403–413, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Al-Magableh MR, Kemp-Harper BK, and Hart JL. Hydrogen sulfide treatment reduces blood pressure and oxidative stress in angiotensin II-induced hypertensive mice. Hypertens Res 38: 13–20, 2015 [DOI] [PubMed] [Google Scholar]
- 3.Ali MY, Ping CY, Mok YY, Ling L, Whiteman M, Bhatia M, and Moore PK. Regulation of vascular nitric oxide in vitro and in vivo; a new role for endogenous hydrogen sulphide? Br J Pharmacol 149: 625–634, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altaany Z, Ju Y, Yang G, and Wang R. The coordination of S-sulfhydration, S-nitrosylation, and phosphorylation of endothelial nitric oxide synthase by hydrogen sulfide. Sci Signal 7: ra87, 2014 [DOI] [PubMed] [Google Scholar]
- 5.Altaany Z, Yang G, and Wang R. Crosstalk between hydrogen sulfide and nitric oxide in endothelial cells. J Cell Mol Med 17: 879–888, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Badiei A, Chambers ST, Gaddam RR, Fraser R, and Bhatia M. Cystathionine-gamma-lyase gene silencing with siRNA in monocytes/macrophages protects mice against acute pancreatitis. Appl Microbiol Biotechnol 100: 337–346, 2016 [DOI] [PubMed] [Google Scholar]
- 7.Badiei A, Gieseg S, Davies S, Izani Othman M, and Bhatia M. LPS up-regulates cystathionine gamma-lyase gene expression in primary human macrophages via NF-kappaB/ERK pathway. Inflamm Allergy Drug Targets 14: 99–104, 2015 [DOI] [PubMed] [Google Scholar]
- 8.Bartels K, Grenz A, and Eltzschig HK. Hypoxia and inflammation are two sides of the same coin. Proc Natl Acad Sci U S A 110: 18351–18352, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bartholomew TC, Powell GM, Dodgson KS, and Curtis CG. Oxidation of sodium sulphide by rat liver, lungs and kidney. Biochem Pharmacol 29: 2431–2437, 1980 [DOI] [PubMed] [Google Scholar]
- 10.Bian JS, Yong QC, Pan TT, Feng ZN, Ali MY, Zhou S, and Moore PK. Role of hydrogen sulfide in the cardioprotection caused by ischemic preconditioning in the rat heart and cardiac myocytes. J Pharmacol Exp Ther 316: 670–678, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Bibli SI, Yang G, Zhou Z, Wang R, Topouzis S, and Papapetropoulos A. Role of cGMP in hydrogen sulfide signaling. Nitric Oxide 46: 7–13, 2015 [DOI] [PubMed] [Google Scholar]
- 12.Bir SC, Kolluru GK, McCarthy P, Shen X, Pardue S, Pattillo CB, and Kevil CG. Hydrogen sulfide stimulates ischemic vascular remodeling through nitric oxide synthase and nitrite reduction activity regulating hypoxia-inducible factor-1alpha and vascular endothelial growth factor-dependent angiogenesis. J Am Heart Assoc 1: e004093, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bliksoen M, Kaljusto ML, Vaage J, and Stenslokken KO. Effects of hydrogen sulphide on ischaemia-reperfusion injury and ischaemic preconditioning in the isolated, perfused rat heart. Eur J Cardiothorac Surg 34: 344–349, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Bos EM, Wang R, Snijder PM, Boersema M, Damman J, Fu M, Moser J, Hillebrands JL, Ploeg RJ, Yang G, Leuvenink HG, van Goor H. Cystathionine gamma-lyase protects against renal ischemia/reperfusion by modulating oxidative stress. J Am Soc Nephrol 24: 759–770, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brancaleone V, Mitidieri E, Flower RJ, Cirino G, and Perretti M. Annexin A1 mediates hydrogen sulfide properties in the control of inflammation. J Pharmacol Exp Ther 351: 96–104, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bridgeman MM, Marsden M, MacNee W, Flenley DC, and Ryle AP. Cysteine and glutathione concentrations in plasma and bronchoalveolar lavage fluid after treatment with N-acetylcysteine. Thorax 46: 39–42, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bucci M, Papapetropoulos A, Vellecco V, Zhou Z, Pyriochou A, Roussos C, Roviezzo F, Brancaleone V, and Cirino G. Hydrogen sulfide is an endogenous inhibitor of phosphodiesterase activity. Arterioscler Thromb Vasc Biol 30: 1998–2004, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Calvert JW, Jha S, Gundewar S, Elrod JW, Ramachandran A, Pattillo CB, Kevil CG, and Lefer DJ. Hydrogen sulfide mediates cardioprotection through Nrf2 signaling. Circ Res 105: 365–374, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carballal S, Cuevasanta E, Marmisolle I, Kabil O, Gherasim C, Ballou DP, Banerjee R, and Alvarez B. Kinetics of reversible reductive carbonylation of heme in human cystathionine beta-synthase. Biochemistry 52: 4553–4562, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carballal S, Radi R, Kirk MC, Barnes S, Freeman BA, and Alvarez B. Sulfenic acid formation in human serum albumin by hydrogen peroxide and peroxynitrite. Biochemistry 42: 9906–9914, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Casey JR, Grinstein S, and Orlowski J. Sensors and regulators of intracellular pH. Nat Rev Mol Cell Biol 11: 50–61, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Cavallini D, De Marco C, Mondovi B, and Mori BG. The cleavage of cystine by cystathionase and the transulfuration of hypotaurine. Enzymologia 22: 161–173, 1960 [PubMed] [Google Scholar]
- 23.Chatzianastasiou A, Bibli SI, Andreadou I, Efentakis P, Kaludercic N, Wood ME, Whiteman M, Di Lisa F, Daiber A, Manolopoulos VG, Szabo C, and Papapetropoulos A. Cardioprotection by H2S donors: Nitric oxide-dependent and independent mechanisms. J Pharmacol Exp Ther 358: 431–440, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen J, Gao J, Sun W, Li L, Wang Y, Bai S, Li X, Wang R, Wu L, Li H, and Xu C. Involvement of exogenous H2S in recovery of cardioprotection from ischemic post-conditioning via increase of autophagy in the aged hearts. Int J Cardiol 220: 681–692, 2016 [DOI] [PubMed] [Google Scholar]
- 25.Chen PH, Fu YS, Wang YM, Yang KH, Wang DL, and Huang B. Hydrogen sulfide increases nitric oxide production and subsequent S-nitrosylation in endothelial cells. ScientificWorldJournal 2014: 480387, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen W, Liu C, Peng B, Zhao Y, Pacheco A, and Xian M. New fluorescent probes for sulfane sulfurs and the application in bioimaging. Chem Sci 4: 2892–2896, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen X, Jhee KH, and Kruger WD. Production of the neuromodulator H2S by cystathionine beta-synthase via the condensation of cysteine and homocysteine. J Biol Chem 279: 52082–52086, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Cheng Y, Ndisang JF, Tang G, Cao K, and Wang R. Hydrogen sulfide-induced relaxation of resistance mesenteric artery beds of rats. Am J Physiol Heart Circ Physiol 287: H2316–H2323, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Cheng Z, Garikipati VN, Nickoloff E, Wang C, Polhemus DJ, Zhou J, Benedict C, Khan M, Verma SK, Rabinowitz JE, Lefer D, and Kishore R. Restoration of hydrogen sulfide production in diabetic mice improves reparative function of bone marrow cells. Circulation 134: 1467–1483, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheung SH, Kwok WK, To KF, and Lau JY. Anti-atherogenic effect of hydrogen sulfide by over-expression of cystathionine gamma-lyase (CSE) gene. PLoS One 9: e113038, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chiku T, Padovani D, Zhu W, Singh S, Vitvitsky V, and Banerjee R. H2S biogenesis by human cystathionine gamma-lyase leads to the novel sulfur metabolites lanthionine and homolanthionine and is responsive to the grade of hyperhomocysteinemia. J Biol Chem 284: 11601–11612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coletta C, Modis K, Szczesny B, Brunyanszki A, Olah G, Rios EC, Yanagi K, Ahmad A, Papapetropoulos A, and Szabo C. Regulation of vascular tone, angiogenesis and cellular bioenergetics by the 3-mercaptopyruvate sulfurtransferase/H2S pathway: Functional impairment by hyperglycemia and restoration by DL-alpha-lipoic acid. Mol Med 21: 1–14, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coletta C, Papapetropoulos A, Erdelyi K, Olah G, Modis K, Panopoulos P, Asimakopoulou A, Gero D, Sharina I, Martin E, and Szabo C. Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation. Proc Natl Acad Sci U S A 109: 9161–9166, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cortese-Krott MM, Kuhnle GG, Dyson A, Fernandez BO, Grman M, DuMond JF, Barrow MP, McLeod G, Nakagawa H, Ondrias K, Nagy P, King SB, Saavedra JE, Keefer LK, Singer M, Kelm M, Butler AR, and Feelisch M. Key bioactive reaction products of the NO/H2S interaction are S/N-hybrid species, polysulfides, and nitroxyl. Proc Natl Acad Sci U S A 112: E4651–E4660, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cuevasanta E, Denicola A, Alvarez B, and Moller MN. Solubility and permeation of hydrogen sulfide in lipid membranes. PLoS One 7: e34562, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Curtis CG, Bartholomew TC, Rose FA, and Dodgson KS. Detoxication of sodium 35 S-sulphide in the rat. Biochem Pharmacol 21: 2313–2321, 1972 [DOI] [PubMed] [Google Scholar]
- 37.d'Emmanuele di Villa Bianca R, Mitidieri E, Donnarumma E, Tramontano T, Brancaleone V, Cirino G, Bucci M, and Sorrentino R. Hydrogen sulfide is involved in dexamethasone-induced hypertension in rat. Nitric Oxide 46: 80–86, 2015 [DOI] [PubMed] [Google Scholar]
- 38.d'Emmanuele di Villa Bianca R, Mitidieri E, Esposito D, Donnarumma E, Russo A, Fusco F, Ianaro A, Mirone V, Cirino G, Russo G, and Sorrentino R. Human cystathionine-beta-synthase phosphorylation on Serine 227 modulates hydrogen sulfide production in human urothelium. PLoS One 10: e0136859, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39.d'Emmanuele di Villa Bianca R, Mitidieri E, Fusco F, Russo A, Pagliara V, Tramontano T, Donnarumma E, Mirone V, Cirino G, Russo G, and Sorrentino R. Urothelium muscarinic activation phosphorylates CBS(Ser227) via cGMP/PKG pathway causing human bladder relaxation through H2S production. Sci Rep 6: 31491, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.DeLeon ER, Gao Y, Huang E, Arif M, Arora N, Divietro A, Patel S, and Olson KR. A case of mistaken identity: Are reactive oxygen species actually reactive sulfide species? Am J Physiol Regul Integr Comp Physiol 310: R549–R560, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Du HP, Li J, You SJ, Wang YL, Wang F, Cao YJ, Hu LF, and Liu CF. DNA methylation in cystathionine-gamma-lyase (CSE) gene promoter induced by ox-LDL in macrophages and in ApoE knockout mice. Biochem Biophys Res Commun 469: 776–782, 2016 [DOI] [PubMed] [Google Scholar]
- 42.Du J, Huang Y, Yan H, Zhang Q, Zhao M, Zhu M, Liu J, Chen SX, Bu D, Tang C, and Jin H. Hydrogen sulfide suppresses oxidized low-density lipoprotein (ox-LDL)-stimulated monocyte chemoattractant protein 1 generation from macrophages via the nuclear factor kappaB (NF-kappaB) pathway. J Biol Chem 289: 9741–9753, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Du J, Hui Y, Cheung Y, Bin G, Jiang H, Chen X, and Tang C. The possible role of hydrogen sulfide as a smooth muscle cell proliferation inhibitor in rat cultured cells. Heart Vessels 19: 75–80, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Eberhardt M, Dux M, Namer B, Miljkovic J, Cordasic N, Will C, Kichko TI, de la Roche J, Fischer M, Suarez SA, Bikiel D, Dorsch K, Leffler A, Babes A, Lampert A, Lennerz JK, Jacobi J, Marti MA, Doctorovich F, Hogestatt ED, Zygmunt PM, Ivanovic-Burmazovic I, Messlinger K, Reeh P, and Filipovic MR. H2S and NO cooperatively regulate vascular tone by activating a neuroendocrine HNO-TRPA1-CGRP signalling pathway. Nat Commun 5: 4381, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Geng B, Chang L, Pan C, Qi Y, Zhao J, Pang Y, Du J, and Tang C. Endogenous hydrogen sulfide regulation of myocardial injury induced by isoproterenol. Biochem Biophys Res Commun 318: 756–763, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Geng B, Cui Y, Zhao J, Yu F, Zhu Y, Xu G, Zhang Z, Tang C, and Du J. Hydrogen sulfide downregulates the aortic L-arginine/nitric oxide pathway in rats. Am J Physiol Regul Integr Comp Physiol 293: R1608–R1618, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Geng Y, Li E, Mu Q, Zhang Y, Wei X, Li H, Cheng L, and Zhang B. Hydrogen sulfide inhalation decreases early blood-brain barrier permeability and brain edema induced by cardiac arrest and resuscitation. J Cereb Blood Flow Metab 35: 494–500, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gersztenkorn D, Coletta C, Zhu S, Ha Y, Liu H, Tie H, Zhou J, Szabo C, Zhang W, and Motamedi M. Hydrogen sulfide contributes to retinal neovascularization in ischemia-induced retinopathy. Invest Ophthalmol Vis Sci 57: 3002–3009, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gong D, Cheng HP, Xie W, Zhang M, Liu D, Lan G, Huang C, Zhao ZW, Chen LY, Yao F, Tan YL, Li L, Xia XD, Zheng XL, Wang ZB, and Tang CK. Cystathionine gamma-lyase(CSE)/hydrogen sulfide system is regulated by miR-216a and influences cholesterol efflux in macrophages via the PI3K/AKT/ABCA1 pathway. Biochem Biophys Res Commun 470: 107–116, 2016 [DOI] [PubMed] [Google Scholar]
- 50.Hedegaard ER, Nielsen BD, Kun A, Hughes AD, Kroigaard C, Mogensen S, Matchkov VV, Frobert O, and Simonsen U. KV 7 channels are involved in hypoxia-induced vasodilatation of porcine coronary arteries. Br J Pharmacol 171: 69–82, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hoffman M, Rajapakse A, Shen X, and Gates KS. Generation of DNA-damaging reactive oxygen species via the autoxidation of hydrogen sulfide under physiologically relevant conditions: Chemistry relevant to both the genotoxic and cell signaling properties of H(2)S. Chem Res Toxicol 25: 1609–1615, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang B, Chen CT, Chen CS, Wang YM, Hsieh HJ, and Wang DL. Laminar shear flow increases hydrogen sulfide and activates a nitric oxide producing signaling cascade in endothelial cells. Biochem Biophys Res Commun 464: 1254–1259, 2015 [DOI] [PubMed] [Google Scholar]
- 53.Huang CW, Feng W, Peh MT, Peh K, Dymock BW, and Moore PK. A novel slow-releasing hydrogen sulfide donor, FW1256, exerts anti-inflammatory effects in mouse macrophages and in vivo. Pharmacol Res 113: 533–546, 2016 [DOI] [PubMed] [Google Scholar]
- 54.Huang P, Chen S, Wang Y, Liu J, Yao Q, Huang Y, Li H, Zhu M, Wang S, Li L, Tang C, Tao Y, Yang G, Du J, and Jin H. Down-regulated CBS/H2S pathway is involved in high-salt-induced hypertension in Dahl rats. Nitric Oxide 46: 192–203, 2015 [DOI] [PubMed] [Google Scholar]
- 55.Hughes MN, Centelles MN, and Moore KP. Making and working with hydrogen sulfide: The chemistry and generation of hydrogen sulfide in vitro and its measurement in vivo: A review. Free Radic Biol Med 47: 1346–1353, 2009 [DOI] [PubMed] [Google Scholar]
- 56.Ibrahim MY, Aziz NM, Kamel MY, and Rifaai RA. Sodium hydrosulphide against renal ischemia/reperfusion and the possible contribution of nitric oxide in adult male Albino rats. Bratisl Lek Listy 116: 681–688, 2015 [DOI] [PubMed] [Google Scholar]
- 57.Ida T, Sawa T, Ihara H, Tsuchiya Y, Watanabe Y, Kumagai Y, Suematsu M, Motohashi H, Fujii S, Matsunaga T, Yamamoto M, Ono K, Devarie-Baez NO, Xian M, Fukuto JM, and Akaike T. Reactive cysteine persulfides and S-polythiolation regulate oxidative stress and redox signaling. Proc Natl Acad Sci U S A 111: 7606–7611, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ishii I, Akahoshi N, Yamada H, Nakano S, Izumi T, and Suematsu M. Cystathionine gamma-Lyase-deficient mice require dietary cysteine to protect against acute lethal myopathy and oxidative injury. J Biol Chem 285: 26358–26368, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Islam KN, Polhemus DJ, Donnarumma E, Brewster LP, and Lefer DJ. Hydrogen sulfide levels and nuclear factor-erythroid 2-related factor 2 (NRF2) activity are attenuated in the setting of critical limb ischemia (CLI). J Am Heart Assoc 4: e001986, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jackson-Weaver O, Osmond JM, Riddle MA, Naik JS, Gonzalez Bosc LV, Walker BR, and Kanagy NL. Hydrogen sulfide dilates rat mesenteric arteries by activating endothelial large-conductance Ca(2)(+)-activated K(+) channels and smooth muscle Ca(2)(+) sparks. Am J Physiol Heart Circ Physiol 304: H1446–H1454, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jackson-Weaver O, Paredes DA, Gonzalez Bosc LV, Walker BR, and Kanagy NL. Intermittent hypoxia in rats increases myogenic tone through loss of hydrogen sulfide activation of large-conductance Ca(2+)-activated potassium channels. Circ Res 108: 1439–1447, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jackson MR, Melideo SL, and Jorns MS. Human sulfide:quinone oxidoreductase catalyzes the first step in hydrogen sulfide metabolism and produces a sulfane sulfur metabolite. Biochemistry 51: 6804–6815, 2012 [DOI] [PubMed] [Google Scholar]
- 63.Jaffrey SR, Erdjument-Bromage H, Ferris CD, Tempst P, and Snyder SH. Protein S-nitrosylation: A physiological signal for neuronal nitric oxide. Nat Cell Biol 3: 193–197, 2001 [DOI] [PubMed] [Google Scholar]
- 64.Jain SK, Huning L, and Micinski D. Hydrogen sulfide upregulates glutamate-cysteine ligase catalytic subunit, glutamate-cysteine ligase modifier subunit, and glutathione and inhibits interleukin-1beta secretion in monocytes exposed to high glucose levels. Metab Syndr Relat Disord 12: 299–302, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jiang B, Tang G, Cao K, Wu L, and Wang R. Molecular mechanism for H(2)S-induced activation of K(ATP) channels. Antioxid Redox Signal 12: 1167–1178, 2010 [DOI] [PubMed] [Google Scholar]
- 66.Jiang Z, Li C, Manuel ML, Yuan S, Kevil CG, McCarter KD, Lu W, and Sun H. Role of hydrogen sulfide in early blood-brain barrier disruption following transient focal cerebral ischemia. PLoS One 10: e0117982, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kabil O. and Banerjee R. Redox biochemistry of hydrogen sulfide. J Biol Chem 285: 21903–21907, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kabil O, Motl N, and Banerjee R. H2S and its role in redox signaling. Biochim Biophys Acta 1844: 1355–1366, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kabil O, Zhou Y, and Banerjee R. Human cystathionine beta-synthase is a target for sumoylation. Biochemistry 45: 13528–13536, 2006 [DOI] [PubMed] [Google Scholar]
- 70.Kai S, Tanaka T, Daijo H, Harada H, Kishimoto S, Suzuki K, Takabuchi S, Takenaga K, Fukuda K, and Hirota K. Hydrogen sulfide inhibits hypoxia- but not anoxia-induced hypoxia-inducible factor 1 activation in a von hippel-lindau- and mitochondria-dependent manner. Antioxid Redox Signal 16: 203–216, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Karwi QG, Whiteman M, Wood ME, Torregrossa R, and Baxter GF. Pharmacological postconditioning against myocardial infarction with a slow-releasing hydrogen sulfide donor, GYY4137. Pharmacol Res 111: 442–451, 2016 [DOI] [PubMed] [Google Scholar]
- 72.Kida M, Sugiyama T, Yoshimoto T, and Ogawa Y. Hydrogen sulfide increases nitric oxide production with calcium-dependent activation of endothelial nitric oxide synthase in endothelial cells. Eur J Pharm Sci 48: 211–215, 2013 [DOI] [PubMed] [Google Scholar]
- 73.Kim JI, Choi SH, Jung KJ, Lee E, Kim HY, and Park KM. Protective role of methionine sulfoxide reductase A against ischemia/reperfusion injury in mouse kidney and its involvement in the regulation of trans-sulfuration pathway. Antioxid Redox Signal 18: 2241–2250, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim NR, Nam SY, Ryu KJ, Kim HM, and Jeong HJ. Effects of bamboo salt and its component, hydrogen sulfide, on enhancing immunity. Mol Med Rep 14: 1673–1680, 2016 [DOI] [PubMed] [Google Scholar]
- 75.Kim S, Lee HG, Park SA, Kundu JK, Keum YS, Cha YN, Na HK, and Surh YJ. Keap1 cysteine 288 as a potential target for diallyl trisulfide-induced Nrf2 activation. PLoS One 9: e85984, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kimura H. Signaling molecules: Hydrogen sulfide and polysulfide. Antioxid Redox Signal 22: 362–376, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kimura Y, Mikami Y, Osumi K, Tsugane M, Oka J, and Kimura H. Polysulfides are possible H2S-derived signaling molecules in rat brain. FASEB J 27: 2451–2457, 2013 [DOI] [PubMed] [Google Scholar]
- 78.Kimura Y, Toyofuku Y, Koike S, Shibuya N, Nagahara N, Lefer D, Ogasawara Y, and Kimura H. Identification of H2S3 and H2S produced by 3-mercaptopyruvate sulfurtransferase in the brain. Sci Rep 5: 14774, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.King AL, Polhemus DJ, Bhushan S, Otsuka H, Kondo K, Nicholson CK, Bradley JM, Islam KN, Calvert JW, Tao YX, Dugas TR, Kelley EE, Elrod JW, Huang PL, Wang R, and Lefer DJ. Hydrogen sulfide cytoprotective signaling is endothelial nitric oxide synthase-nitric oxide dependent. Proc Natl Acad Sci U S A 111: 3182–3187, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kiss L, Deitch EA, and Szabo C. Hydrogen sulfide decreases adenosine triphosphate levels in aortic rings and leads to vasorelaxation via metabolic inhibition. Life Sci 83: 589–594, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Koenitzer JR, Isbell TS, Patel HD, Benavides GA, Dickinson DA, Patel RP, Darley-Usmar VM, Lancaster JR., Jr. Doeller JE, and Kraus DW. Hydrogen sulfide mediates vasoactivity in an O2-dependent manner. Am J Physiol Heart Circ Physiol 292: H1953–H1960, 2007 [DOI] [PubMed] [Google Scholar]
- 82.Koike S, Ogasawara Y, Shibuya N, Kimura H, and Ishii K. Polysulfide exerts a protective effect against cytotoxicity caused by t-buthylhydroperoxide through Nrf2 signaling in neuroblastoma cells. FEBS Lett 587: 3548–3555, 2013 [DOI] [PubMed] [Google Scholar]
- 83.Koj A, Frendo J, and Janik Z. [35S]thiosulphate oxidation by rat liver mitochondria in the presence of glutathione. Biochem J 103: 791–795, 1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kolluru GK, Bir SC, Yuan S, Shen X, Pardue S, Wang R, and Kevil CG. Cystathionine gamma-lyase regulates arteriogenesis through NO-dependent monocyte recruitment. Cardiovasc Res 107: 590–600, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kolluru GK, Shen X, and Kevil CG. A tale of two gases: NO and H2S, foes or friends for life? Redox Biol 1: 313–318, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kram L, Grambow E, Mueller-Graf F, Sorg H, and Vollmar B. The anti-thrombotic effect of hydrogen sulfide is partly mediated by an upregulation of nitric oxide synthases. Thromb Res 132: e112–e117, 2013 [DOI] [PubMed] [Google Scholar]
- 87.Krishnan N, Fu C, Pappin DJ, and Tonks NK. H2S-Induced sulfhydration of the phosphatase PTP1B and its role in the endoplasmic reticulum stress response. Sci Signal 4: ra86, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kubo S, Doe I, Kurokawa Y, Nishikawa H, and Kawabata A. Direct inhibition of endothelial nitric oxide synthase by hydrogen sulfide: Contribution to dual modulation of vascular tension. Toxicology 232: 138–146, 2007 [DOI] [PubMed] [Google Scholar]
- 89.Lai KC, Hsu SC, Yang JS, Yu CC, Lein JC, and Chung JG. Diallyl trisulfide inhibits migration, invasion and angiogenesis of human colon cancer HT-29 cells and umbilical vein endothelial cells, and suppresses murine xenograft tumour growth. J Cell Mol Med 19: 474–484, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lambert JP, Nicholson CK, Amin H, Amin S, and Calvert JW. Hydrogen sulfide provides cardioprotection against myocardial/ischemia reperfusion injury in the diabetic state through the activation of the RISK pathway. Med Gas Res 4: 20, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lee HH, Han MH, Hwang HJ, Kim GY, Moon SK, Hyun JW, Kim WJ, and Choi YH. Diallyl trisulfide exerts anti-inflammatory effects in lipopolysaccharide-stimulated RAW 264.7 macrophages by suppressing the Toll-like receptor 4/nuclear factor-kappaB pathway. Int J Mol Med 35: 487–495, 2015 [DOI] [PubMed] [Google Scholar]
- 92.Leffler CW, Parfenova H, Basuroy S, Jaggar JH, Umstot ES, and Fedinec AL. Hydrogen sulfide and cerebral microvascular tone in newborn pigs. Am J Physiol Heart Circ Physiol 300: H440–H447, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lei YP, Liu CT, Sheen LY, Chen HW, and Lii CK. Diallyl disulfide and diallyl trisulfide protect endothelial nitric oxide synthase against damage by oxidized low-density lipoprotein. Mol Nutr Food Res 54(Suppl. 1): S42–S52, 2010 [DOI] [PubMed] [Google Scholar]
- 94.Li D, Xiong Q, Peng J, Hu B, Li W, Zhu Y, and Shen X. Hydrogen sulfide up-regulates the expression of ATP-binding cassette transporter A1 via promoting nuclear translocation of PPARalpha. Int J Mol Sci 17: 635, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li L, Salto-Tellez M, Tan CH, Whiteman M, and Moore PK. GYY4137, a novel hydrogen sulfide-releasing molecule, protects against endotoxic shock in the rat. Free Radic Biol Med 47: 103–113, 2009 [DOI] [PubMed] [Google Scholar]
- 96.Li Q. and Lancaster JR., Jr. Chemical foundations of hydrogen sulfide biology. Nitric Oxide 35: 21–34, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li Y, Zhang J, Zhang L, Si M, Yin H, and Li J. Diallyl trisulfide inhibits proliferation, invasion and angiogenesis of osteosarcoma cells by switching on suppressor microRNAs and inactivating of Notch-1 signaling. Carcinogenesis 34: 1601–1610, 2013 [DOI] [PubMed] [Google Scholar]
- 98.Liang GH, Adebiyi A, Leo MD, McNally EM, Leffler CW, and Jaggar JH. Hydrogen sulfide dilates cerebral arterioles by activating smooth muscle cell plasma membrane KATP channels. Am J Physiol Heart Circ Physiol 300: H2088–H2095, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Libiad M, Yadav PK, Vitvitsky V, Martinov M, and Banerjee R. Organization of the human mitochondrial hydrogen sulfide oxidation pathway. J Biol Chem 289: 30901–30910, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lilyanna S, Peh MT, Liew OW, Wang P, Moore PK, Richards AM, and Martinez EC. GYY4137 attenuates remodeling, preserves cardiac function and modulates the natriuretic peptide response to ischemia. J Mol Cell Cardiol 87: 27–37, 2015 [DOI] [PubMed] [Google Scholar]
- 101.Lin VS, Lippert AR, and Chang CJ. Cell-trappable fluorescent probes for endogenous hydrogen sulfide signaling and imaging H2O2-dependent H2S production. Proc Natl Acad Sci U S A 110: 7131–7135, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu KL, Chen HW, Wang RY, Lei YP, Sheen LY, and Lii CK. DATS reduces LPS-induced iNOS expression, NO production, oxidative stress, and NF-kappaB activation in RAW 264.7 macrophages. J Agric Food Chem 54: 3472–3478, 2006 [DOI] [PubMed] [Google Scholar]
- 103.Liu Z, Han Y, Li L, Lu H, Meng G, Li X, Shirhan M, Peh MT, Xie L, Zhou S, Wang X, Chen Q, Dai W, Tan CH, Pan S, Moore PK, and Ji Y. The hydrogen sulfide donor, GYY4137, exhibits anti-atherosclerotic activity in high fat fed apolipoprotein E(-/-) mice. Br J Pharmacol 169: 1795–1809, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lohninger L, Tomasova L, Praschberger M, Hintersteininger M, Erker T, Gmeiner BM, and Laggner H. Hydrogen sulphide induces HIF-1alpha and Nrf2 in THP-1 macrophages. Biochimie 112: 187–195, 2015 [DOI] [PubMed] [Google Scholar]
- 105.Mani S, Li H, Untereiner A, Wu L, Yang G, Austin RC, Dickhout JG, Lhotak S, Meng QH, and Wang R. Decreased endogenous production of hydrogen sulfide accelerates atherosclerosis. Circulation 127: 2523–2534, 2013 [DOI] [PubMed] [Google Scholar]
- 106.Marko L, Szijarto IA, Filipovic MR, Kassmann M, Balogh A, Park JK, Przybyl L, N'Diaye G, Kramer S, Anders J, Ishii I, Muller DN, and Gollasch M. Role of cystathionine gamma-lyase in immediate renal impairment and inflammatory response in acute ischemic kidney injury. Sci Rep 6: 27517, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mathai JC, Missner A, Kugler P, Saparov SM, Zeidel ML, Lee JK, and Pohl P. No facilitator required for membrane transport of hydrogen sulfide. Proc Natl Acad Sci U S A 106: 16633–16638, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Meng G, Ma Y, Xie L, Ferro A, and Ji Y. Emerging role of hydrogen sulfide in hypertension and related cardiovascular diseases. Br J Pharmacol 172: 5501–5511, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Meng G, Wang J, Xiao Y, Bai W, Xie L, Shan L, Moore PK, and Ji Y. GYY4137 protects against myocardial ischemia and reperfusion injury by attenuating oxidative stress and apoptosis in rats. J Biomed Res 29: 203–213, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Meng J, Ganesan Adaikan P, and Srilatha B. Hydrogen sulfide promotes nitric oxide production in corpus cavernosum by enhancing expression of endothelial nitric oxide synthase. Int J Impot Res 25: 86–90, 2013 [DOI] [PubMed] [Google Scholar]
- 111.Meng QH, Yang G, Yang W, Jiang B, Wu L, and Wang R. Protective effect of hydrogen sulfide on balloon injury-induced neointima hyperplasia in rat carotid arteries. Am J Pathol 170: 1406–1414, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Miao L, Shen X, Whiteman M, Xin H, Shen Y, Xin X, Moore PK, and Zhu YZ. Hydrogen sulfide mitigates myocardial infarction via promotion of mitochondrial biogenesis-dependent M2 polarization of macrophages. Antioxid Redox Signal 25: 268–281, 2016 [DOI] [PubMed] [Google Scholar]
- 113.Miao L, Xin X, Xin H, Shen X, and Zhu YZ. Hydrogen sulfide recruits macrophage migration by integrin beta1-Src-FAK/Pyk2-Rac pathway in myocardial infarction. Sci Rep 6: 22363, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mikami Y, Shibuya N, Kimura Y, Nagahara N, Ogasawara Y, and Kimura H. Thioredoxin and dihydrolipoic acid are required for 3-mercaptopyruvate sulfurtransferase to produce hydrogen sulfide. Biochem J 439: 479–485, 2011 [DOI] [PubMed] [Google Scholar]
- 115.Mikami Y, Shibuya N, Kimura Y, Nagahara N, Yamada M, and Kimura H. Hydrogen sulfide protects the retina from light-induced degeneration by the modulation of Ca2+ influx. J Biol Chem 286: 39379–39386, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mikami Y, Shibuya N, Ogasawara Y, and Kimura H. Hydrogen sulfide is produced by cystathionine gamma-lyase at the steady-state low intracellular Ca(2+) concentrations. Biochem Biophys Res Commun 431: 131–135, 2013 [DOI] [PubMed] [Google Scholar]
- 117.Miljkovic J, Kenkel I, Ivanovic-Burmazovic I, and Filipovic MR. Generation of HNO and HSNO from nitrite by heme-iron-catalyzed metabolism with H(2)S. Angew Chem Int Ed Engl 52: 12061–12064, 2013 [DOI] [PubMed] [Google Scholar]
- 118.Mishanina TV, Libiad M, and Banerjee R. Biogenesis of reactive sulfur species for signaling by hydrogen sulfide oxidation pathways. Nat Chem Biol 11: 457–464, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Moccia F, Bertoni G, Pla AF, Dragoni S, Pupo E, Merlino A, Mancardi D, Munaron L, and Tanzi F. Hydrogen sulfide regulates intracellular Ca2+ concentration in endothelial cells from excised rat aorta. Curr Pharm Biotechnol 12: 1416–1426, 2011 [DOI] [PubMed] [Google Scholar]
- 120.Modis K, Ju Y, Ahmad A, Untereiner AA, Altaany Z, Wu L, Szabo C, and Wang R. S-Sulfhydration of ATP synthase by hydrogen sulfide stimulates mitochondrial bioenergetics. Pharmacol Res 113: 116–124, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Morikawa T, Kajimura M, Nakamura T, Hishiki T, Nakanishi T, Yukutake Y, Nagahata Y, Ishikawa M, Hattori K, Takenouchi T, Takahashi T, Ishii I, Matsubara K, Kabe Y, Uchiyama S, Nagata E, Gadalla MM, Snyder SH, and Suematsu M. Hypoxic regulation of the cerebral microcirculation is mediated by a carbon monoxide-sensitive hydrogen sulfide pathway. Proc Natl Acad Sci U S A 109: 1293–1298, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mustafa AK, Gadalla MM, Sen N, Kim S, Mu W, Gazi SK, Barrow RK, Yang G, Wang R, and Snyder SH. H2S signals through protein S-sulfhydration. Sci Signal 2: ra72, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mustafa AK, Sikka G, Gazi SK, Steppan J, Jung SM, Bhunia AK, Barodka VM, Gazi FK, Barrow RK, Wang R, Amzel LM, Berkowitz DE, and Snyder SH. Hydrogen sulfide as endothelium-derived hyperpolarizing factor sulfhydrates potassium channels. Circ Res 109: 1259–1268, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nagahara N, Yoshii T, Abe Y, and Matsumura T. Thioredoxin-dependent enzymatic activation of mercaptopyruvate sulfurtransferase. An intersubunit disulfide bond serves as a redox switch for activation. J Biol Chem 282: 1561–1569, 2007 [DOI] [PubMed] [Google Scholar]
- 125.Nagy P. and Winterbourn CC. Rapid reaction of hydrogen sulfide with the neutrophil oxidant hypochlorous acid to generate polysulfides. Chem Res Toxicol 23: 1541–1543, 2010 [DOI] [PubMed] [Google Scholar]
- 126.Oh GS, Pae HO, Lee BS, Kim BN, Kim JM, Kim HR, Jeon SB, Jeon WK, Chae HJ, and Chung HT. Hydrogen sulfide inhibits nitric oxide production and nuclear factor-kappaB via heme oxygenase-1 expression in RAW264.7 macrophages stimulated with lipopolysaccharide. Free Radic Biol Med 41: 106–119, 2006 [DOI] [PubMed] [Google Scholar]
- 127.Olson KR. A practical look at the chemistry and biology of hydrogen sulfide. Antioxid Redox Signal 17: 32–44, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Olson KR, Deleon ER, Gao Y, Hurley K, Sadauskas V, Batz C, and Stoy GF. Thiosulfate: A readily accessible source of hydrogen sulfide in oxygen sensing. Am J Physiol Regul Integr Comp Physiol 305: R592–R603, 2013 [DOI] [PubMed] [Google Scholar]
- 129.Olson KR. and Straub KD. The role of hydrogen sulfide in evolution and the evolution of hydrogen sulfide in metabolism and signaling. Physiology (Bethesda) 31: 60–72, 2016 [DOI] [PubMed] [Google Scholar]
- 130.Ono K, Akaike T, Sawa T, Kumagai Y, Wink DA, Tantillo DJ, Hobbs AJ, Nagy P, Xian M, Lin J, and Fukuto JM. Redox chemistry and chemical biology of H2S, hydropersulfides, and derived species: Implications of their possible biological activity and utility. Free Radic Biol Med 77: 82–94, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Palinkas Z, Furtmuller PG, Nagy A, Jakopitsch C, Pirker KF, Magierowski M, Jasnos K, Wallace JL, Obinger C, and Nagy P. Interactions of hydrogen sulfide with myeloperoxidase. Br J Pharmacol 172: 1516–1532, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pan J. and Carroll KS. Persulfide reactivity in the detection of protein s-sulfhydration. ACS Chem Biol 8: 1110–1116, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pan LL, Liu XH, Gong QH, Wu D, and Zhu YZ. Hydrogen sulfide attenuated tumor necrosis factor-alpha-induced inflammatory signaling and dysfunction in vascular endothelial cells. PLoS One 6: e19766, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 134.Paulsen CE. and Carroll KS. Cysteine-mediated redox signaling: Chemistry, biology, and tools for discovery. Chem Rev 113: 4633–4679, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Peake BF, Nicholson CK, Lambert JP, Hood RL, Amin H, Amin S, and Calvert JW. Hydrogen sulfide preconditions the db/db diabetic mouse heart against ischemia-reperfusion injury by activating Nrf2 signaling in an Erk-dependent manner. Am J Physiol Heart Circ Physiol 304: H1215–H1224, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Peh MT, Anwar AB, Ng DS, Atan MS, Kumar SD, and Moore PK. Effect of feeding a high fat diet on hydrogen sulfide (H2S) metabolism in the mouse. Nitric Oxide 41: 138–145, 2014 [DOI] [PubMed] [Google Scholar]
- 137.Peter EA, Shen X, Shah SH, Pardue S, Glawe JD, Zhang WW, Reddy P, Akkus NI, Varma J, and Kevil CG. Plasma free H2S levels are elevated in patients with cardiovascular disease. J Am Heart Assoc 2: e000387, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Polhemus DJ, Kondo K, Bhushan S, Bir SC, Kevil CG, Murohara T, Lefer DJ, and Calvert JW. Hydrogen sulfide attenuates cardiac dysfunction after heart failure via induction of angiogenesis. Circ Heart Fail 6: 1077–1086, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Possomato-Vieira JS, Goncalves-Rizzi VH, Graca TU, Nascimento RA, and Dias-Junior CA. Sodium hydrosulfide prevents hypertension and increases in vascular endothelial growth factor and soluble fms-like tyrosine kinase-1 in hypertensive pregnant rats. Naunyn Schmiedebergs Arch Pharmacol 389: 1325–1332, 2016 [DOI] [PubMed] [Google Scholar]
- 140.Potenza DM, Guerra G, Avanzato D, Poletto V, Pareek S, Guido D, Gallanti A, Rosti V, Munaron L, Tanzi F, and Moccia F. Hydrogen sulphide triggers VEGF-induced intracellular Ca(2)(+) signals in human endothelial cells but not in their immature progenitors. Cell Calcium 56: 225–234, 2014 [DOI] [PubMed] [Google Scholar]
- 141.Predmore BL, Kondo K, Bhushan S, Zlatopolsky MA, King AL, Aragon JP, Grinsfelder DB, Condit ME, and Lefer DJ. The polysulfide diallyl trisulfide protects the ischemic myocardium by preservation of endogenous hydrogen sulfide and increasing nitric oxide bioavailability. Am J Physiol Heart Circ Physiol 302: H2410–H2418, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Renga B, Bucci M, Cipriani S, Carino A, Monti MC, Zampella A, Gargiulo A, d'Emmanuele di Villa Bianca R, Distrutti E, and Fiorucci S. Cystathionine gamma-lyase, a H2S-generating enzyme, is a GPBAR1-regulated gene and contributes to vasodilation caused by secondary bile acids. Am J Physiol Heart Circ Physiol 309: H114–H126, 2015 [DOI] [PubMed] [Google Scholar]
- 143.Renga B, Cipriani S, Carino A, Simonetti M, Zampella A, and Fiorucci S. Reversal of endothelial dysfunction by GPBAR1 agonism in portal hypertension involves a AKT/FOXOA1 dependent regulation of H2S generation and endothelin-1. PLoS One 10: e0141082, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rios EC, Szczesny B, Soriano FG, Olah G, and Szabo C. Hydrogen sulfide attenuates cytokine production through the modulation of chromatin remodeling. Int J Mol Med 35: 1741–1746, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Saha S, Chakraborty PK, Xiong X, Dwivedi SK, Mustafi SB, Leigh NR, Ramchandran R, Mukherjee P, and Bhattacharya R. Cystathionine beta-synthase regulates endothelial function via protein S-sulfhydration. FASEB J 30: 441–456, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sakaguchi M, Marutani E, Shin HS, Chen W, Hanaoka K, Xian M, and Ichinose F. Sodium thiosulfate attenuates acute lung injury in mice. Anesthesiology 121: 1248–1257, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sen N, Paul BD, Gadalla MM, Mustafa AK, Sen T, Xu R, Kim S, and Snyder SH. Hydrogen sulfide-linked sulfhydration of NF-kappaB mediates its antiapoptotic actions. Mol Cell 45: 13–24, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Shaul PW. Regulation of endothelial nitric oxide synthase: Location, location, location. Annu Rev Physiol 64: 749–774, 2002 [DOI] [PubMed] [Google Scholar]
- 149.Shen X, Chakraborty S, Dugas TR, and Kevil CG. Hydrogen sulfide measurement using sulfide dibimane: Critical evaluation with electrospray ion trap mass spectrometry. Nitric Oxide 41: 97–104, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Shen X, Kolluru GK, Yuan S, and Kevil CG. Measurement of H2S in vivo and in vitro by the monobromobimane method. Methods Enzymol 554: 31–45, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Shen X, Pattillo CB, Pardue S, Bir SC, Wang R, and Kevil CG. Measurement of plasma hydrogen sulfide in vivo and in vitro. Free Radic Biol Med 50: 1021–1031, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Shen X, Peter EA, Bir S, Wang R, and Kevil CG. Analytical measurement of discrete hydrogen sulfide pools in biological specimens. Free Radic Biol Med 52: 2276–2283, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Shen Y, Shen Z, Miao L, Xin X, Lin S, Zhu Y, Guo W, and Zhu YZ. miRNA-30 family inhibition protects against cardiac ischemic injury by regulating cystathionine-gamma-lyase expression. Antioxid Redox Signal 22: 224–240, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Shibuya N, Koike S, Tanaka M, Ishigami-Yuasa M, Kimura Y, Ogasawara Y, Fukui K, Nagahara N, and Kimura H. A novel pathway for the production of hydrogen sulfide from D-cysteine in mammalian cells. Nat Commun 4: 1366, 2013 [DOI] [PubMed] [Google Scholar]
- 155.Shimizu Y, Nicholson CK, Lambert JP, Barr LA, Kuek N, Herszenhaut D, Tan L, Murohara T, Hansen JM, Husain A, Naqvi N, and Calvert JW. Sodium sulfide attenuates ischemic-induced heart failure by enhancing proteasomal function in an Nrf2-dependent manner. Circ Heart Fail 9: e002368, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Shui M, Liu X, Zhu Y, and Wang Y. Exogenous hydrogen sulfide attenuates cerebral ischemia-reperfusion injury by inhibiting autophagy in mice. Can J Physiol Pharmacol 94: 1187–1192, 2016 [DOI] [PubMed] [Google Scholar]
- 157.Singh S, Padovani D, Leslie RA, Chiku T, and Banerjee R. Relative contributions of cystathionine beta-synthase and gamma-cystathionase to H2S biogenesis via alternative trans-sulfuration reactions. J Biol Chem 284: 22457–22466, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Snijder PM, Frenay AR, de Boer RA, Pasch A, Hillebrands JL, Leuvenink HG, and van Goor H. Exogenous administration of thiosulfate, a donor of hydrogen sulfide, attenuates angiotensin II-induced hypertensive heart disease in rats. Br J Pharmacol 172: 1494–1504, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Stubbert D, Prysyazhna O, Rudyk O, Scotcher J, Burgoyne JR, and Eaton P. Protein kinase G Ialpha oxidation paradoxically underlies blood pressure lowering by the reductant hydrogen sulfide. Hypertension 64: 1344–1351, 2014 [DOI] [PubMed] [Google Scholar]
- 160.Sun A, Wang Y, Liu J, Yu X, Sun Y, Yang F, Dong S, Wu J, Zhao Y, Xu C, Lu F, and Zhang W. Exogenous H2S modulates mitochondrial fusion-fission to inhibit vascular smooth muscle cell proliferation in a hyperglycemic state. Cell Biosci 6: 36, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sun L, Sun S, Li Y, Pan W, Xie Y, Wang S, and Zhang Z. Potential biomarkers predicting risk of pulmonary hypertension in congenital heart disease: The role of homocysteine and hydrogen sulfide. Chin Med J (Engl) 127: 893–899, 2014 [PubMed] [Google Scholar]
- 162.Sun Y, Huang Y, Zhang R, Chen Q, Chen J, Zong Y, Liu J, Feng S, Liu AD, Holmberg L, Liu D, Tang C, Du J, and Jin H. Hydrogen sulfide upregulates KATP channel expression in vascular smooth muscle cells of spontaneously hypertensive rats. J Mol Med (Berl) 93: 439–455, 2015 [DOI] [PubMed] [Google Scholar]
- 163.Talipov MR. and Timerghazin QK. Protein control of S-nitrosothiol reactivity: Interplay of antagonistic resonance structures. J Phys Chem B 117: 1827–1837, 2013 [DOI] [PubMed] [Google Scholar]
- 164.Tang G, Wu L, Liang W, and Wang R. Direct stimulation of K(ATP) channels by exogenous and endogenous hydrogen sulfide in vascular smooth muscle cells. Mol Pharmacol 68: 1757–1764, 2005 [DOI] [PubMed] [Google Scholar]
- 165.Tang G, Yang G, Jiang B, Ju Y, Wu L, and Wang R. H(2)S is an endothelium-derived hyperpolarizing factor. Antioxid Redox Signal 19: 1634–1646, 2013 [DOI] [PubMed] [Google Scholar]
- 166.Tao BB, Liu SY, Zhang CC, Fu W, Cai WJ, Wang Y, Shen Q, Wang MJ, Chen Y, Zhang LJ, Zhu YZ, and Zhu YC. VEGFR2 functions as an H2S-targeting receptor protein kinase with its novel Cys1045-Cys1024 disulfide bond serving as a specific molecular switch for hydrogen sulfide actions in vascular endothelial cells. Antioxid Redox Signal 19: 448–464, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Taoka S. and Banerjee R. Characterization of NO binding to human cystathionine beta-synthase: Possible implications of the effects of CO and NO binding to the human enzyme. J Inorg Biochem 87: 245–251, 2001 [DOI] [PubMed] [Google Scholar]
- 168.Teng H, Wu B, Zhao K, Yang G, Wu L, and Wang R. Oxygen-sensitive mitochondrial accumulation of cystathionine beta-synthase mediated by Lon protease. Proc Natl Acad Sci U S A 110: 12679–12684, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Toohey JI. and Cooper AJ. Thiosulfoxide (sulfane) sulfur: New chemistry and new regulatory roles in biology. Molecules 19: 12789–12813, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Vandenberg JI, Metcalfe JC, and Grace AA. Mechanisms of pHi recovery after global ischemia in the perfused heart. Circ Res 72: 993–1003, 1993 [DOI] [PubMed] [Google Scholar]
- 171.Vitvitsky V, Yadav PK, Kurthen A, and Banerjee R. Sulfide oxidation by a noncanonical pathway in red blood cells generates thiosulfate and polysulfides. J Biol Chem 290: 8310–8320, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wang K, Ahmad S, Cai M, Rennie J, Fujisawa T, Crispi F, Baily J, Miller MR, Cudmore M, Hadoke PW, Wang R, Gratacos E, Buhimschi IA, Buhimschi CS, and Ahmed A. Dysregulation of hydrogen sulfide producing enzyme cystathionine gamma-lyase contributes to maternal hypertension and placental abnormalities in preeclampsia. Circulation 127: 2514–2522, 2013 [DOI] [PubMed] [Google Scholar]
- 173.Wang P, Isaak CK, and Siow YL O K. Downregulation of cystathionine beta-synthase and cystathionine gamma-lyase expression stimulates inflammation in kidney ischemia-reperfusion injury. Physiol Rep 2: e12251, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wang R. Two's company, three's a crowd: Can H2S be the third endogenous gaseous transmitter? FASEB J 16: 1792–1798, 2002 [DOI] [PubMed] [Google Scholar]
- 175.Wang T, Wang L, Zaidi SR, Sammani S, Siegler J, Moreno-Vinasco L, Mathew B, Natarajan V, and Garcia JG. Hydrogen sulfide attenuates particulate matter-induced human lung endothelial barrier disruption via combined reactive oxygen species scavenging and Akt activation. Am J Respir Cell Mol Biol 47: 491–496, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wang XH, Wang F, You SJ, Cao YJ, Cao LD, Han Q, Liu CF, and Hu LF. Dysregulation of cystathionine gamma-lyase (CSE)/hydrogen sulfide pathway contributes to ox-LDL-induced inflammation in macrophage. Cell Signal 25: 2255–2262, 2013 [DOI] [PubMed] [Google Scholar]
- 177.Wang Y, Wang X, Liang X, Wu J, Dong S, Li H, Jin M, Sun D, Zhang W, and Zhong X. Inhibition of hydrogen sulfide on the proliferation of vascular smooth muscle cells involved in the modulation of calcium sensing receptor in high homocysteine. Exp Cell Res 347: 184–191, 2016 [DOI] [PubMed] [Google Scholar]
- 178.Wedmann R, Bertlein S, Macinkovic I, Boltz S, Miljkovic J, Munoz LE, Herrmann M, and Filipovic MR. Working with “H2S”: Facts and apparent artifacts. Nitric Oxide 41: 85–96, 2014 [DOI] [PubMed] [Google Scholar]
- 179.Wei HL, Zhang CY, Jin HF, Tang CS, and Du JB. Hydrogen sulfide regulates lung tissue-oxidized glutathione and total antioxidant capacity in hypoxic pulmonary hypertensive rats. Acta Pharmacol Sin 29: 670–679, 2008 [DOI] [PubMed] [Google Scholar]
- 180.Whiteman M, Cheung NS, Zhu YZ, Chu SH, Siau JL, Wong BS, Armstrong JS, and Moore PK. Hydrogen sulphide: A novel inhibitor of hypochlorous acid-mediated oxidative damage in the brain? Biochem Biophys Res Commun 326: 794–798, 2005 [DOI] [PubMed] [Google Scholar]
- 181.Whiteman M, Li L, Kostetski I, Chu SH, Siau JL, Bhatia M, and Moore PK. Evidence for the formation of a novel nitrosothiol from the gaseous mediators nitric oxide and hydrogen sulphide. Biochem Biophys Res Commun 343: 303–310, 2006 [DOI] [PubMed] [Google Scholar]
- 182.Whiteman M, Li L, Rose P, Tan CH, Parkinson DB, and Moore PK. The effect of hydrogen sulfide donors on lipopolysaccharide-induced formation of inflammatory mediators in macrophages. Antioxid Redox Signal 12: 1147–1154, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wu B, Teng H, Yang G, Wu L, and Wang R. Hydrogen sulfide inhibits the translational expression of hypoxia-inducible factor-1alpha. Br J Pharmacol 167: 1492–1505, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Xiao J, Zhu X, Kang B, Xu J, Wu L, Hong J, Zhang Y, Ni X, and Wang Z. Hydrogen sulfide attenuates myocardial hypoxia-reoxygenation injury by inhibiting autophagy via mTOR activation. Cell Physiol Biochem 37: 2444–2453, 2015 [DOI] [PubMed] [Google Scholar]
- 185.Xie L, Gu Y, Wen M, Zhao S, Wang W, Ma Y, Meng G, Han Y, Wang Y, Liu G, Moore PK, Wang X, Wang H, Zhang Z, Yu Y, Ferro A, Huang Z, and Ji Y. Hydrogen sulfide induces Keap1 S-sulfhydration and suppresses diabetes-accelerated atherosclerosis via Nrf2 activation. Diabetes 65: 3171–3184, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Yadav PK, Martinov M, Vitvitsky V, Seravalli J, Wedmann R, Filipovic MR, and Banerjee R. Biosynthesis and reactivity of cysteine persulfides in signaling. J Am Chem Soc 138: 289–299, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Yadav PK, Yamada K, Chiku T, Koutmos M, and Banerjee R. Structure and kinetic analysis of H2S production by human mercaptopyruvate sulfurtransferase. J Biol Chem 288: 20002–20013, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yan H, Du J, and Tang C. The possible role of hydrogen sulfide on the pathogenesis of spontaneous hypertension in rats. Biochem Biophys Res Commun 313: 22–27, 2004 [DOI] [PubMed] [Google Scholar]
- 189.Yang G, Li H, Tang G, Wu L, Zhao K, Cao Q, Xu C, and Wang R. Increased neointimal formation in cystathionine gamma-lyase deficient mice: Role of hydrogen sulfide in alpha5beta1-integrin and matrix metalloproteinase-2 expression in smooth muscle cells. J Mol Cell Cardiol 52: 677–688, 2012 [DOI] [PubMed] [Google Scholar]
- 190.Yang G, Sun X, and Wang R. Hydrogen sulfide-induced apoptosis of human aorta smooth muscle cells via the activation of mitogen-activated protein kinases and caspase-3. FASEB J 18: 1782–1784, 2004 [DOI] [PubMed] [Google Scholar]
- 191.Yang G, Wu L, Bryan S, Khaper N, Mani S, and Wang R. Cystathionine gamma-lyase deficiency and overproliferation of smooth muscle cells. Cardiovasc Res 86: 487–495, 2010 [DOI] [PubMed] [Google Scholar]
- 192.Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, Meng Q, Mustafa AK, Mu W, Zhang S, Snyder SH, and Wang R. H2S as a physiologic vasorelaxant: Hypertension in mice with deletion of cystathionine gamma-lyase. Science 322: 587–590, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Yang G, Zhao K, Ju Y, Mani S, Cao Q, Puukila S, Khaper N, Wu L, and Wang R. Hydrogen sulfide protects against cellular senescence via S-sulfhydration of Keap1 and activation of Nrf2. Antioxid Redox Signal 18: 1906–1919, 2013 [DOI] [PubMed] [Google Scholar]
- 194.Yang Y, Zhang BK, Liu D, Nie W, Yuan JM, Wang Z, and Guo YM. Sodium hydrosulfide prevents hypoxia-induced pulmonary arterial hypertension in broilers. Br Poult Sci 53: 608–615, 2012 [DOI] [PubMed] [Google Scholar]
- 195.Yong QC, Lee SW, Foo CS, Neo KL, Chen X, and Bian JS. Endogenous hydrogen sulphide mediates the cardioprotection induced by ischemic postconditioning. Am J Physiol Heart Circ Physiol 295: H1330–H1340, 2008 [DOI] [PubMed] [Google Scholar]
- 196.Yuan G, Vasavda C, Peng YJ, Makarenko VV, Raghuraman G, Nanduri J, Gadalla MM, Semenza GL, Kumar GK, Snyder SH, and Prabhakar NR. Protein kinase G-regulated production of H2S governs oxygen sensing. Sci Signal 8: ra37, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Yuan S. and Kevil CG. Nitric oxide and hydrogen sulfide regulation of ischemic vascular remodeling. Microcirculation 23: 134–145, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Yuan S, Pardue S, Shen X, Alexander JS, Orr AW, and Kevil CG. Hydrogen sulfide metabolism regulates endothelial solute barrier function. Redox Biol 9: 157–166, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Yuan S, Patel RP, and Kevil CG. Working with nitric oxide and hydrogen sulfide in biological systems. Am J Physiol Lung Cell Mol Physiol 308: L403–L415, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Zhang D, Macinkovic I, Devarie-Baez NO, Pan J, Park CM, Carroll KS, Filipovic MR, and Xian M. Detection of protein S-sulfhydration by a tag-switch technique. Angew Chem Int Ed Engl 53: 575–581, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zhang H, Guo C, Wu D, Zhang A, Gu T, Wang L, and Wang C. Hydrogen sulfide inhibits the development of atherosclerosis with suppressing CX3CR1 and CX3CL1 expression. PLoS One 7: e41147, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Zhao W, Zhang J, Lu Y, and Wang R. The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. EMBO J 20: 6008–6016, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Zhao X, Zhang LK, Zhang CY, Zeng XJ, Yan H, Jin HF, Tang CS, and Du JB. Regulatory effect of hydrogen sulfide on vascular collagen content in spontaneously hypertensive rats. Hypertens Res 31: 1619–1630, 2008 [DOI] [PubMed] [Google Scholar]
- 204.Zhao ZZ, Wang Z, Li GH, Wang R, Tan JM, Cao X, Suo R, and Jiang ZS. Hydrogen sulfide inhibits macrophage-derived foam cell formation. Exp Biol Med (Maywood) 236: 169–176, 2011 [DOI] [PubMed] [Google Scholar]
- 205.Zhong X, Wang Y, Wu J, Sun A, Yang F, Zheng D, Li T, Dong S, Zhao Y, Yang G, Xu C, Sun D, Lu F, and Zhang W. Calcium sensing receptor regulating smooth muscle cells proliferation through initiating cystathionine-gamma-lyase/hydrogen sulfide pathway in diabetic rat. Cell Physiol Biochem 35: 1582–1598, 2015 [DOI] [PubMed] [Google Scholar]
- 206.Zhou Z, Martin E, Sharina I, Esposito I, Szabo C, Bucci M, Cirino G, and Papapetropoulos A. Regulation of soluble guanylyl cyclase redox state by hydrogen sulfide. Pharmacol Res 111: 556–562, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Zhu YZ, Wang ZJ, Ho P, Loke YY, Zhu YC, Huang SH, Tan CS, Whiteman M, Lu J, and Moore PK. Hydrogen sulfide and its possible roles in myocardial ischemia in experimental rats. J Appl Physiol (1985) 102: 261–268, 2007 [DOI] [PubMed] [Google Scholar]