Abstract
Endothelial cells (ECs) play a major role in blood vessel formation and function. While there is longstanding evidence for the potential of chemical exposures to adversely affect EC function and vascular development, the hazard potential of chemicals with respect to vascular effects is not routinely evaluated in safety assessments. Induced pluripotent stem cell (iPSC)-derived ECs promise to provide a physiologically relevant, organotypic culture model that is amenable for high-throughput (HT) EC toxicant screening and may represent a viable alternative to traditional in vitro models, including human umbilical vein endothelial cells (HUVECs). To evaluate the utility of iPSC-ECs for multidimensional HT toxicity profiling of chemicals, both iPSC-ECs and HUVECs were exposed to selected positive (angiogenesis inhibitors, cytotoxic agents) and negative compounds in concentration response for either 16 or 24 h in a 384-well plate format. Furthermore, chemical effects on vascularization were quantified using EC angiogenesis on biological (Geltrex™) and synthetic (SP-105 angiogenesis hydrogel) extracellular matrices. Cellular toxicity was assessed using high-content live cell imaging and the CellTiter-Glo® assay. Assay performance indicated good to excellent assay sensitivity and reproducibility for both cell types investigated. Both iPSC-derived ECs and HUVECs formed tube-like structures on Geltrex™ and hydrogel, an effect that was inhibited by angiogenesis inhibitors and cytotoxic agents in a concentration-dependent manner. The quality of HT assays in HUVECs was generally higher than that in iPSC-ECs. Altogether, this study demonstrates the capability of ECs for comprehensive assessment of the biological effects of chemicals on vasculature in a HT compatible format.
Keywords: : endothelial cells, high-throughput, angiogenesis, iPSC-derived cells
Introduction
Adverse chemical effects on the vasculature remain a major concern from a public health perspective. According to WHO estimates, up to 23% of all cardiovascular disease cases are environmental exposure related, resulting in ∼2.5 million deaths annually.1 Endothelial cells (ECs) play a major role in the vascular system, providing the structural foundation of blood vessels and contributing to vascular function. While there is longstanding evidence for the potential of chemical exposures to adversely affect EC function and vascular development, the identities and associated hazard potentials of vascular toxicants remain largely unknown.2 Considering the large number of environmentally relevant chemicals that have yet to be evaluated for their potential to exert adverse vascular effects, there is increasing demand for physiologically relevant, high-throughput (HT) applicable in vitro alternatives to traditional toxicity testing strategies for rapid identification and hazard characterization of EC toxicants.
To date, primary human umbilical vein endothelial cells (HUVECs) remain the most widely used cell culture model for in vitro assessment of vascular hazards.3 However, primary human cells have a number of limitations that impede their routine application in HT screening (HTS) approaches.4,5 Most importantly, batch-to-batch variations in pooled HUVEC preparations, associated with underlying genetic and other variability among the donors, introduce an inherent biological variability component that is difficult to control and which may negatively impact assay reproducibility.6 Moreover, a potential limited supply of primary human cell preparations can also be a limitation for large-scale screening studies.7
Significant advances in stem cell engineering have now resulted in the availability of human induced pluripotent stem cell (iPSC)-derived ECs, a physiologically relevant, organotypic in vitro model that promises to overcome the key limitations associated with traditional cell culture systems. iPSC-ECs can be generated from a genetically defined iPSC, that is, derived from a single individual, in virtually unlimited supplies, thereby alleviating concerns associated with HUVECs.8,9 This also creates an opportunity to use organotypic cells from a large number of genetically defined donors and evaluate them for in vitro population variability testing. Thus, iPSC-ECs potentially represent a useful in vitro alternative that is capable of informing mechanism-based hazard identification using multidimensional phenotypic characterization in a HT applicable format.
ECs are known to self-assemble into cellular networks when plated on certain extracellular matrices or when cocultured in the presence of other cell types.3,10,11 This characteristic EC tube formation has been proven a useful phenotype to investigate mechanisms of angiogenesis and to estimate and quantify antiangiogenic properties of chemicals, especially in preclinical drug screening for cancer therapeutics.12–14 Traditional matrices that have been used include Matrigel or collagen,10,15–17 both of which consist of extracellular proteins or protein mixtures that are susceptible to lot-to-lot variations that may also jeopardize standardization in HTS efforts. In addition, recent reports demonstrate the propensity of direct chemical matrix effects that can result in false positive findings, that is, unspecific, matrix-dependent inhibition of EC tube formation as was the case with suramin.18 More recently, synthetic polyethylene glycol hydrogels have emerged as synthetic, but fully functional alternatives to traditional matrices as an extracellular matrix for EC tube formation, allowing for more accurately defined chemical composition and thus better assay reproducibility. However, these initial studies did not address the HT applicability of hydrogels in iPSC-EC-based screenings and also included direct exposure of cells to ultraviolet light during hydrogel polymerization.19–21 To avoid physical interference with cellular angiogenesis, a more refined and less intrusive assay is needed for the assessment of vascular growth or angiogenesis.
In this article, we describe a multidimensional HTS approach for comprehensive chemical characterization of functional vascularization and cellular toxicity evaluation in iPSC-ECs and HUVECs. The overall objective was to determine if iPSC-ECs provide a better cellular model for chemical screening compared with HUVECs for both of these endpoints.
Materials and Methods
Chemicals and Biologicals
iCell Endothelial Cells (Catalog No. ECC-100-010-001; Lot No. 1825866) and their media supplement were purchased from Cellular Dynamics International, Inc. (Madison, WI). The VascuLife® VEGF Medium Complete Kits were purchased from Lifeline Cell Technology (Frederick, MD). Pooled HUVECs in EGM-2 media (Catalog No. CC2519A; Lot No. 0000409274) and the EGM™-2 BulletKits™ were obtained from Lonza (Walkersville, MD). Chloroquine phosphate, colchicine, concanamycin A, nocodazole, suramin, and tetraoctylammonium bromide (TAB) were all purchased from Cayman Chemical (Ann Arbor, MI). SU5402 and formaldehyde solution was purchased from Sigma-Aldrich (St. Louis, MO). Dimethyl sulfoxide (DMSO) was from Santa Cruz Biotechnology (Dallas, TX). Calcein AM, CellMask Green, fibronectin, Geltrex™ LDEV-Free Reduced Growth Factor Basement Membrane, Hoechst 33342, and TrypLE Express™ were purchased from Life Technologies (Grand Island, NY). Fetal bovine serum (FBS), Histamine, FluoroBrite DMEM, and Medium 199 were purchased from Fisher Scientific (Waltham, MA). Recombinant human interferon-gamma (IFN-γ), interleukin-1 beta (IL-1β), and tumor necrosis factor-alpha (TNF-α) were obtained from R&D Systems (Minneapolis, MN). SP-105 angiogenesis hydrogels were provided by StemPharm, Inc. (Madison, WI).
iPSC-ECs Culture
iCell Endothelial Cells (iPSC-EC) were plated and expanded on T-75 tissue culture flasks according to instructions provided by Cellular Dynamics International. iPSC-ECs are quality controlled by the manufacturer for positive expression of the EC-specific markers, CD31 and CD105, and a typical EC response to vascular endothelial growth factor (VEGF) and TNF-α. Briefly, T-75 flasks were coated with fibronectin solution at 3 μg/cm2 and incubated for 1–2 h. Cells were removed from vapor-phase liquid nitrogen storage and thawed for 3 min in a water bath at 37°C. The thawed cells were added to maintenance medium containing the VascuLife VEGF Medium Complete Kit, without FBS, and iCell Endothelial Cells Medium Supplement. The final formulation of maintenance medium was VascuLife© Basal Medium with fibroblast growth factor (FGF) (5 ng/mL), ascorbic acid (50 μg/mL), hydrocortisone hemisuccinate (1 μg/mL), l-glutamine (4 mM), insulin-like growth factor 1 (IGF-1) (15 ng/mL), epidermal growth factor (EGF) (5 ng/mL), VEGF (5 ng/mL), heparin sulfate (0.75 U/mL), and, finally, 10% iCell Endothelial Cells Medium Supplement. Cell density was determined using Trypan Blue exclusion test and a cell suspension was prepared that results in 1.0 × 104 cells/cm2. The fibronectin solution was aspirated and cells were seeded in a T-75 flask. Cells were incubated at 37°C and 5% CO2 with media changes every 2 days and passaged every 3–4 days by TrypLE Express. Experiments were conducted with cells between passages 1 and 5.
HUVECs Culture
HUVECs were seeded and grown on T-75 tissue culture flasks in Medium199 with the EGM-2 BulletKits. The EGM-2 BulletKits consisted of hEGF, hydrocortisone, GA-1000 (Gentamicin, Amphotericin-B), FBS, VEGF, hFGF-B, R3-IGF-1, ascorbic acid, and heparin. HUVECs were incubated at 37°C and 5% CO2 and passaged every 2–3 days using TrypLE Express. Cell density was determined by cell counting with Trypan Blue. Experiments were performed with cells between passages 1 and 5.
Chemical Preparations
Chemicals were prepared as 200 × concentration stocks in cell culture grade DMSO. Stocks were diluted serially with DMSO in glass-coated 96-well plates. These chemical masterplates were stored at −20°C. Chemical stock solutions were diluted in medium to prepare 2 × and 4 × working solutions for the angiogenic and cytotoxicity assays, respectively. The final DMSO concentration was 0.5% for all assays under investigation. Plates were equilibrated at 37°C and 5% CO2 before use.
Angiogenesis Assay Using Geltrex
A portion of the angiogenic assays were performed on Geltrex LDEV-Free Reduced Growth Factor Basement Membrane for both iCell ECs and HUVECs in 384-well format and assessed by live cell high-content imaging using the following instructions (Table 1). iPSC-ECs were incubated with VascuLife® Basal Medium containing 4 mM l-glutamine LifeFactor and 0.1% iCell Endothelial Cells Medium Supplement. HUVECs were incubated with Medium 199 containing the EGM-2 BulletKits at 2 × concentration, also the VEGF component was replaced with 12.5 ng/mL VEGF, and this was referred to as “2 × Assay Medium.” Geltrex was thawed at 4°C and dispensed to coat the plates (10 μL/well) on the ice. The plates were incubated for 1 h at 37°C. Following the incubation, a 2 × chemical working solution (25 μL/well), prepared in basal medium, was added to the plate and cells resuspended in 2 × assay medium (25 μL/well) were seeded at the density of 7,500 (iPSC-ECs) or 3,500 (HUVECs) cells/well. Cells were exposed to chemicals overnight at 37°C at 5% CO2 and stained with 3 × concentration Calcein AM (25 μL/well, 6 μmol/L) for 15 min.
Table 1.
Angiogenesis Assay Using Geltrex in Induced Pluripotent Stem Cell–Endothelial Cells and Human Umbilical Vein Endothelial Cells
| Step | Parameter | Value | Description |
|---|---|---|---|
| 1 | Dispense Geltrex™ | 10 μL | 4°C |
| 2 | Incubation time | 1 h | 37°C |
| 3 | Library compounds | 25 μL | 2 × concentration stock |
| 4 | Plate cells | 25 μL | 7.5 × 103 |
| iCell endothelial cells | |||
| 3.5 × 103 | |||
| HUVECs | |||
| 5 | Incubation time | 16 h | 37°C, 5% CO2 |
| 6 | Dispense 3 × staining solution | 25 μL | Calcein AM (6 μmol/L) in medium |
| 7 | Incubation time | 15 min | 37°C, 5% CO2 |
| 8 | Acquire images | 4 × Objective | With FITC filter |
Step Notes
1. Plate: Black clear bottom 384-well plate. Dispense Geltrex on ice.
3, 4, 6. For the assay in iCell endothelial cells, each solution and cell suspension were prepared by VascuLife® Basal Medium containing 4 mM l-glutamine LifeFactor and 0.1% iCell Endothelial Cells Medium Supplement.
For the assay in HUVECs, cell suspension was prepared by “2 × Assay Medium” and each solution was prepared by Medium 199. “2 × Assay Medium” consisted of Medium199 containing the EGM™-2 BulletKits™ at 2 × concentration, also the VEGF component was replaced with 12.5 ng/mL VEGF from R&D Systems.
4. Final DMSO concentration on assay plate: 0.5%.
8. ImageXpress was used for image acquisition.
DMSO, dimethyl sulfoxide; EC, endothelial cell; HUVEC, human umbilical vein endothelial cell; VEGF, vascular endothelial growth factor.
Angiogenesis Assay Using SP-105 Angiogenesis Hydrogel
The angiogenic assays using SP-105 angiogenesis hydrogel for iPSC-ECs and HUVECs were performed as follows (Table 2), and conducted in 384-well Small Volume™ LoBase Microplates (Greiner Bio-One, Monroe, NC). SP-105 angiogenesis hydrogel was added to each well (3 μL/well) and the plates were centrifuged at 200 g for 30 s. The plates were irradiated with UV at 302 nm for 8 min and incubated overnight at 37°C and 5% CO2 with 20 μL/well phosphate-buffered saline (PBS). Following overnight incubation, for iPSC-ECs, PBS on hydrogels was replaced with 20 μL VascuLife Basal Medium with 0.5% FBS and 25 ng/mL VEGF (Starvation Medium) and incubated at 37°C for at least 30 min. For HUVECs, the hydrogel was washed twice with PBS before addition of chemicals/cells. For both iPSC-ECs and HUVECs, cells were detached from T-75 flask with TrypLE Express and counted on disposable hemocytometer with Trypan Blue exclusion. The total number of cells needed for the entire plate was spun at 200 g for 5 min (Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/adt). The cell pellet was resuspended in 2 mL of FluoroBrite DMEM with 0.1% CellMask Green and incubated at 37°C for 10 min. Twelve milliliters of FluoroBrite DMEM was added to the cell suspension and the resultant solution was spun at 200 g for 5 min. The final cell pellet was resuspended in 2 × assay media, similarly prepared as for the Geltrex angiogenesis assay. Starvation Medium/PBS was removed and 2 × concentration test chemical working solution (10 μL/well; Basal Medium containing test chemicals) was added. Subsequently, iPSC-ECs/HUVECs were seeded at a cell density of 3,500 and 6,125 cells/well, respectively, and incubated with chemicals overnight at 37°C and 5% CO2. After incubation, cells were fixed by adding 6 μL of 16% formaldehyde followed by imaging.
Table 2.
Angiogenesis Assay Using Hydrogel in Induced Pluripotent Stem Cell–Endothelial Cells and Human Umbilical Vein Endothelial Cells
| Step | Parameter | Value | Description |
|---|---|---|---|
| 1 | Dispense hydrogel | 3 μL | 4°C |
| 2 | Centrifugation | 30 s | 200 g for 30 s |
| 3 | UV polymerization | 8 min | 302 nm; 2 × 15 W bulbs; 9 cm |
| 4 | Dispense PBS | 20 μL | Sterile |
| 5 | Incubation time | 24 h | 37°C, 5% CO2 |
| 6 | Serum starve cells | 24 h | Basal media w/0.5% FBS |
| 7 | Wash hydrogel | 20 μL | Sterile PBS |
| 8 | Equilibrate hydrogel | 20 μL | Starvation media |
| 9 | Incubate hydrogel | 30 min | 37°C, 5% CO2 |
| 10 | Wash hydrogel | 20 μL | Sterile PBS |
| 11 | Cells stained | 2 mL | CellMask Green |
| 12 | Library compounds | 10 μL | 2 × concentration stock |
| 13 | Plate cells | 10 μL | 3.5 × 103 |
| iCell endothelial cells | |||
| 6.125 × 103 | |||
| HUVECs | |||
| 14 | Incubation time | 24 h | 37°C, 5% CO2 |
| 15 | Dispense fixation solution | 6 μL | 16% formaldehyde in water |
| 16 | Acquire images | 4 × Objective | With FITC filter |
Step Notes
1. Plate: 384-well Small Volume™, LoBase, PS, μclear, black, TC plates. Dispense SP-105 hydrogel on ice.
6. Serum starvation is only needed with the iCell endothelial cells.
9. Treatment of hydrogel is only needed with the iCell endothelial cells.
7, 10. For washing of the hydrogel, dispense 15 μL of sterile PBS to each well resulting in a meniscus above the well. Flip the plate over onto a sponge cloth and add weight to apply pressure then incubate for 1 min. Additional tapping may be required for removal of liquid in the well. Add 20 μL of sterile PBS and repeat washing.
8. Starvation media: Basal media with 0.5% FBS and 25 ng/mL VEGF.
11. For staining of the cells, cells were counted and desired number of cells transferred to a centrifuge tube. The cell suspension was centrifuged at 200 g for 5 min. Supernatant was discarded. The cell pellet was resuspended in 2 mL of FluoroBrite DMEM with 1:1,000 CellMask Green dye. Cells were incubated for 10 min at 37°C, 5% CO2. Following incubation, 12 mL of FluoroBrite DMEM was added to cell suspension and spun at 200 g for 5 min. The resulting cell pellet was resuspended in 2 × assay media.
For iCell endothelial cell 2 × assay media: VascuLife Basal Medium with 10 ng/mL FGF, 100 μg/mL ascorbic acid, 2 μg/mL hydrocortisone hemisuccinate, 8 mM l-glutamine, 30 ng/mL IGF-1, 10 ng/mL EGF, 10 ng/mL VEGF, 1.5 U/mL heparin sulfate, and 20% iCell endothelial cells media supplement.
For HUVEC 2 × assay media: Medium 199 containing the EGM™-2 BulletKits™ at 2 × concentration, also the VEGF component was replaced with 12.5 ng/mL VEGF from R&D Systems.
13. Final DMSO concentration on assay plate: 0.5%.
16. ImageXpress was used for image acquisition.
FBS, fetal bovine serum; PBS, phosphate-buffered saline.
Cytotoxicity Assay
The evaluation of the cytotoxicity was assessed using the following instructions (Table 3). Tissue culture 384-well plates for iPSC-ECs were coated by adding 10 μL/well of a 30 μg/mL fibronectin solution and incubated for 2 h. Subsequently, iPSC-ECs were dissociated from the flasks with TrypLE Express. The fibronectin solution was removed and cells were plated at the cell density of 750 cells/well. HUVECs were dissociated from the flasks with TrypLE Express and plated to the tissue culture 384-well plates at the cell density of 750 cells/well. The plates were incubated at 37°C and 5% CO2 until the monolayer was formed. Before the assay, the medium was exchanged with 25 μL/well fresh medium. 4 × concentration test chemical solutions (12.5 μL/well) were added to cells 1 h before the incubation with or without a 12.5 μL/well cytokine cocktail (IL-1β, 1 ng/mL; TNF-α, 5 ng/mL; and IFN-γ, 100 ng/mL) for 24 h at 37°C at 5% CO2. After 24 h incubation with chemicals and cytokines, cells were stained with 4 × concentration Hoechst 33342 (1 μg/mL) and Calcein AM (0.1 μmol/L) for 20 min.
Table 3.
High-Content Imaging Analysis of Cytotoxicity in Induced Pluripotent Stem Cell–Endothelial Cells and Human Umbilical Vein Endothelial Cells
| Step | Parameter | Value | Description |
|---|---|---|---|
| 1 | Dispense fibronectin solution | 10 μL | 30 μg/mL fibronectin in water |
| 2 | Incubation time | 2 h | Room temperature |
| 3 | Plate cells | 25 μL | 7.5 × 102 |
| 4 | Incubation time | 5–6 days | 37°C, 5% CO2 |
| 5 | Change media | 25 μL | Every other day |
| 6 | Library compounds | 12.5 μL | 4 × concentration stock |
| 7 | Incubation time | 1 h | 37°C, 5% CO2 |
| 8 | Cytokine stimulation (optional) | 12.5 μL | A cocktail of cytokines (IL-1β, 1 ng/mL; TNF-α, 5 ng/mL; and IFN-γ, 100 ng/mL) in maintenance medium. |
| 9 | Incubation time | 24 h | 37°C, 5% CO2 |
| 10 | Dispense 4 × staining solution | 16.7 μL | With HBSS |
| 11 | Incubation time | 20 min | Room temperature |
| 12 | Wash | 2 Times | With HBSS |
| 13 | Dispense HBSS | 25 μL | |
| 14 | Acquire images | 10 × Objective | With DAPI, FITC filter |
| 15 | Dispense CellTiter-Glo® solution | 25 μL | CellTiter-Glo Luminescent Cell Viability Assay |
| 16 | Incubation time | 10 min | Room temperature |
| 17 | Reading | Luminescence | FLIPR Tetra® |
Step Notes
1, 2. These steps are for iCell endothelial cells.
3. Plate for iCell endothelial cells and HUVECs: black clear-bottom 384-well plate. iCell endothelial cells: remove fibronectin solution before plating cells.
5. For cell maintenance, 25 μL of medium were exchanged every other day. Cell maintenance continued until the monolayer was formed. On the evening before the experiment, old medium was replaced with 25 μL fresh medium.
8. For nonstimulated cells, 12.5 μL/well normal maintenance medium was dispended.
14. ImageXpress was used for image acquisition.
HBSS, Hank's balanced buffer solution.
High-Content Imaging
Images of cell culture plates were acquired using The ImageXpress Micro Confocal High-Content Imaging System (Molecular Devices LLC, Sunnyvale, CA). The angiogenic images were captured at 4 × and 10 × magnification with the FITC (Ex. 409 nm, Em. 447/60 nm) filter (Calcein AM/CellMask Green). Images were analyzed by the angiogenesis module or a custom module in MetaXpress (Molecular Devices) software. The custom module was designed to quantify the protrusions away from the node area (Supplementary Fig. S1) and is provided along with the directions of use in the Supplementary Data. Only a selection of outputs is presented here to illustrate the type of output available from such image processing, and details are included in the Supplementary Data. Images for cytotoxicity assay were acquired at 10 × magnification with DAPI (Ex. 409 nm, Em. 447/60 nm) and FITC (Ex. 506 nm, Em. 536/40 nm) filter. Acquired images were analyzed by the multiwavelength cell scoring applications module in MetaXpress.
Data Processing and Assay Quality Controls
Each experiment, for both the angiogenesis assays and cytotoxicity assays, was conducted on three occasions with at least one of those occasions occurring on a separate day. The HUVECs and iPSC-ECs were from a single lot of cells and the potential for lot-to-lot differences was not investigated here. Data for each treatment were normalized to vehicle (0.5% DMSO)-treated controls and fitted to a curve with a quantitative logistics function to determine point-of-departure (POD) values, defined as one standard deviation of vehicle controls, using R software-based script as previously described.22 The interday and interplate replicability were tested using the normalized data. Coefficients of variation (%CV) were determined from the standard deviation of the mean of vehicle-treated controls. Z′ values were calculated from the normalized value of vehicle control wells and chemical-treated wells with the following formula Z′-factor = 1 − [3(σp + σn)/(|μp − μn|)], where μn and σn represent the mean and standard deviation of the negative controls and μp and σp represent the mean and standard deviation of the positive controls.23 Additional statistics used for determining significance of positive controls selected for Z′-factor calculation include one-way ANOVA with Dunnett's test (angiogenesis assay – 100 nM nocodazole, 50 μM suramin) and a two-tailed unpaired Student's t-test (cytotoxicity – 50 μM TAB).
Results
Experimental Approach
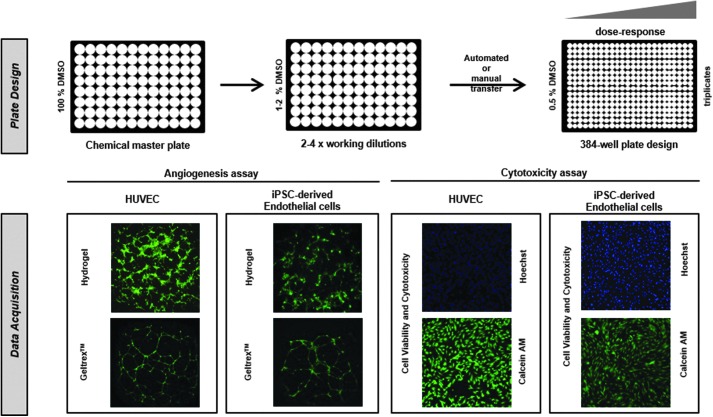
The goal of this study was the development and evaluation of HTS assays for assessing chemical effects on the angiogenesis potential and general cytotoxicity of ECs (Fig. 1). For functional angiogenesis measurements, a classical 2 × 2 approach was employed incorporating two cell types, HUVECs and iPSC-derived ECs, tested on two different extracellular matrices, Geltrex Basement Membrane Matrix and SP-105 angiogenesis hydrogel. Cytotoxicity was then evaluated in both EC types using high-content cellular imaging upon staining with the nuclear dye Hoechst 33342 and the functional cytoplasmic stain Calcein AM. Total adenosine triphosphate (ATP) content was subsequently quantified using the CellTiter-Glo® assay.
Fig. 1.
Assay breakdown of toxicity screening in iPSC-derived ECs and HUVECs. In this study, we present a multidimensional comparison of high-throughput in vitro assays for assessing the alterations in vascularization (angiogenesis assays in both Geltrex™ and SP-105 hydrogel) and cell viability (Hoechst Nuclei Content, Viable Cell Staining with Calcein AM and ATP content with CellTiter-Glo®) caused by chemicals. EC, endothelial cell; HUVEC, human umbilical vein endothelial cell; iPSC, induced pluripotent stem cell.
Assessment of Angiogenesis Assay for ECs
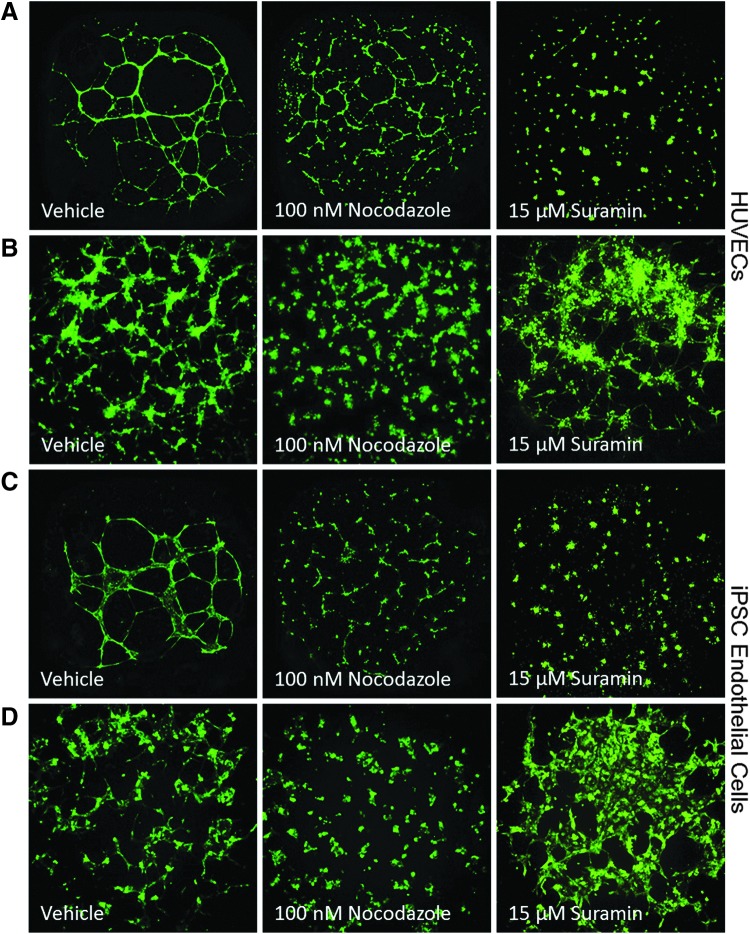
ECs exhibited significantly different morphologies when plated on semisynthetic hydrogels as compared with the traditional matrix, Geltrex (Fig. 2). Seeding cells on the Geltrex resulted in the formation of a well-defined tube-like network within 16 h for both the HUVECs and iPSC-ECs (Fig. 2A, C). The hydrogel matrix resulted in a thicker network of cellular nodes with small protrusions invading into areas of the matrix (Fig. 2B, D). Similar to the observations with the Geltrex, morphological appearance on the hydrogel was similar for both HUVECs and iPSC-ECs. Exposure to a known chemical inhibitor of angiogenesis, nocodazole, resulted in a concentration-dependent decrease or disruption of angiogenic growth in both cell types and both extracellular matrices (Fig. 2) with cytotoxicity only occurring at higher concentrations (Supplementary Table S2).
Fig. 2.
Representative images for HUVECs (A, B) and iPSC-derived ECs (C, D) grown on different extracellular matrices [Geltrex™ (A, C) and SP-105 hydrogel (B, D)] with treatment of vehicle (0.5% DMSO), 100 nM nocodazole, and 15 μM suramin from 16 to 24 h. DMSO, dimethyl sulfoxide.
The %CV of the vehicle-treated controls, was lower for HUVECs (2.7–14) as compared with iPSC-ECs (4.3–31). Reproducibility was also evaluated using interplate and interday replicates for three different morphological features of the EC growth: total tube length, mean tube length, and total tube area. This consisted of three experiments being conducted with at least one experiment occurring on a separate day. Pearson and Spearman correlation coefficients for these morphological features were determined for both cell types. For HUVECs, Pearson's r and Spearman's ρ values ranged from 0.54 to 0.86 and 0.56 to 0.83 on the Geltrex and 0.75–0.88 and 0.57–0.81 on the hydrogel (Table 4). In iPSC-ECs, Pearson's r values ranged from 0.63 to 0.84 on Geltrex and 0.55 to 0.76 on hydrogel, whereas Spearman's ρ values ranged from 0.53 to 0.75 and 0.35 to 0.65, respectively (Table 4). The HUVECs exhibited consistently higher reproducibility across both extracellular matrices with the higher correlations and the lowest %CVs seen on the hydrogel.
Table 4.
Assay Quality Control—Angiogenesis Assays
| Interplate reproducibilitya | Interday reproducibilitya | %CV of negative controlsb | Z′-factorb | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Metric | Cells | Gelc | Hydrod | Gel | Hydro | Gel | Hydro | Chemicals | Gel | Hydro |
| Total tube length | HUVEC | S: 0.70 (<0.0001) | S: 0.81 (<0.0001) | S: 0.72 (<0.0001) | S: 0.75 (<0.0001) | 12 | 6.6 | Nocoe | 0.35 | 0.67 |
| P: 0.66 (<0.0001) | P: 0.88 (<0.0001) | P: 0.86 (<0.0001) | P: 0.86 (<0.0001) | Suraminf | −0.46 | −2.8 | ||||
| iPSC-Endog | S: 0.61 (<0.0001) | S: 0.35 (0.0024) | S: 0.55 (<0.0001) | S: 0.37 (0.0016) | 15 | 28 | Noco | −1.5 | −0.31 | |
| P: 0.70 (<0.0001) | P: 0.56 (<0.0001) | P: 0.63 (<0.0001) | P: 0.59 (<0.0001) | Suramin | 0.49 | −5.7 | ||||
| Mean tube length | HUVEC | S: 0.82 (<0.0001) | S: 0.64 (<0.0001) | S: 0.80 (<0.0001) | S: 0.57 (<0.0001) | 6.1 | 2.7 | Noco | −0.011 | −0.66 |
| P: 0.80 (<0.0001) | P: 0.80 (<0.0001) | P: 0.84 (<0.0001) | P: 0.75 (<0.0001) | Suramin | −1.5 | −2.0 | ||||
| iPSC-Endog | S: 0.72 (<0.0001) | S: 0.40 (0.0004) | S: 0.75 (<0.0001) | S: 0.65 (<0.0001) | 14 | 4.3 | Noco | −7.0 | −0.58 | |
| P: 0.84 (<0.0001) | P: 0.61 (<0.0001) | P: 0.83 (<0.0001) | P: 0.76 (<0.0001) | Suramin | −3.4 | −77 | ||||
| Total tube area | HUVEC | S: 0.56 (<0.0001) | S: 0.81 (<0.0001) | S: 0.70 (<0.0001) | S: 0.71 (<0.0001) | 14 | 7.1 | Noco | 0.44 | 0.65 |
| P: 0.54 (<0.0001) | P: 0.86 (<0.0001) | P: 0.83 (<0.0001) | P: 0.80 (<0.0001) | Suramin | −0.35 | −3.9 | ||||
| iPSC-Endog | S: 0.61 (<0.0001) | S: 0.38 (0.001) | S: 0.53 (<0.0001) | S: 0.56 (<0.0001) | 15 | 31 | Noco | −1.9 | −0.42 | |
| P: 0.71 (<0.0001) | P: 0.55 (<0.0001) | P: 0.63 (<0.0001) | P: 0.71 (<0.0001) | Suramin | 0.51 | −7.0 | ||||
aS: Spearman's ρ, P: Pearson's r. Correlation analysis sample size ranged from n = 60–72. P values provided below correlation values.
bSample size ranges from n = 18–36.
cGel: Geltrex™.
dHydro: SP-105 hydrogel.
eNoco: 100 nM nocodazole.
fSuramin: 50 μM suramin.
giPSC-Endo: iCell endothelial cells from Cellular Dynamics International.
Plots of the data used to calculate Z′-factors shown in this table are included as Supplementary Figure S2.
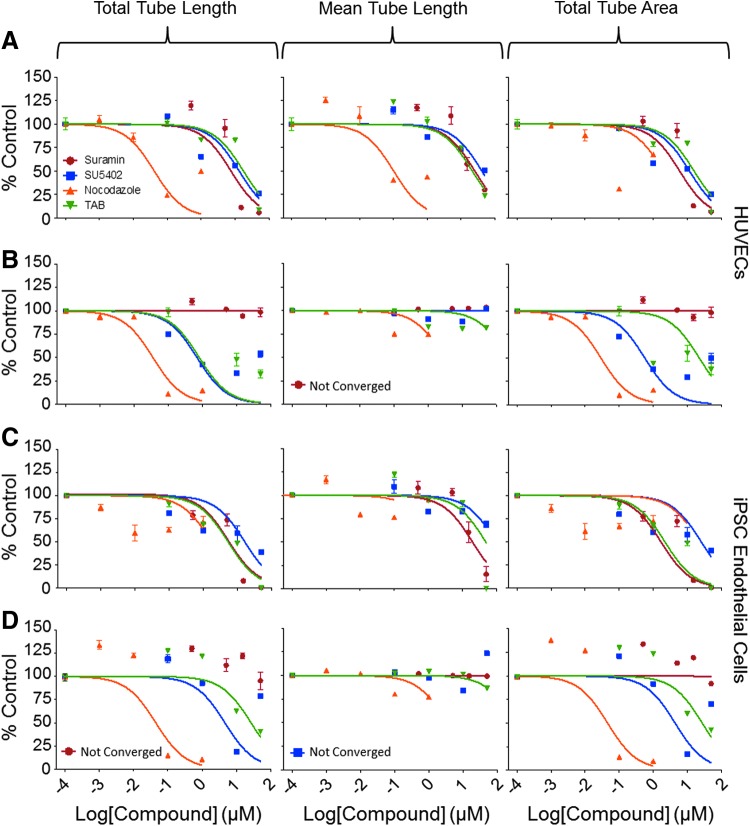
Treatment with different known inhibitors of angiogenesis had concentration-dependent effects on the EC angiogenic potential as evident by tube formation phenotypes (Fig. 3 and Table 5). The effect of chemical treatments on the various phenotypes was consistent for both the HUVECs and the iPSC-ECs, as reflected by comparable derived POD values for both cell types. This was true for all chemicals, except for suramin. Suramin showed no effect on the hydrogel for both cell types, but exhibited a robust inhibition of angiogenesis on the Geltrex (Figs. 2 and 3), reflected by a shift in the calculated POD values from 50 μM ( = highest tested concentration, i.e., nontoxic) on hydrogel to ∼10 μM on the Geltrex matrix, consistent with previous findings of chemical matrix effects.10
Fig. 3.
Assessment of angiogenesis using HUVECs (A, B) and iPSC-derived ECs (C, D) on both Geltrex™ (A, C) and SP-105 hydrogel (B, D) extracellular matrices treated with angiogenic inhibitors. Concentration–response plots for ECs treated with suramin (red), SU5402 (blue), nocodazole (orange), and TAB (green). Concentration–response graphs shown were prepared in GraphPad using a normalized nonlinear fit. Data points in each concentration–response plot represent the average ± SEM of three separate experiments. SEM, standard error of the mean; TAB, tetraoctylammonium bromide.
Table 5.
Concentration Response Analysis—Angiogenesis Assay
| Average POD values ± SDa | ||||
|---|---|---|---|---|
| Metric | Cells | Chemical | Geltrex™ | Hydrogel |
| Total tube length | HUVECs | Nocodazole | 0.028 ± 0.029 | 0.010 ± 0.00042 |
| Suramin | 8.0 ± 2.6 | >50 | ||
| SU5402 | 2.9 ± 3.9 | 0.063 ± 0.0046 | ||
| TAB | 14 ± 17 | 0.38 ± 0.34 | ||
| iPSC-Endob | Nocodazole | 0.0018 ± 0.0024 | 0.030 ± 0.029 | |
| Suramin | 2.8 ± 2.2 | >50 | ||
| SU5402 | 0.057 ± 0.061 | 0.64 ± 0.57 | ||
| TAB | 1.0 ± 0.94 | 8.1 ± 9.5 | ||
| Mean tube length | HUVECs | Nocodazole | 0.036 ± 0.0048 | 0.017 ± 0.014 |
| Suramin | 12 ± 3.2 | >50 | ||
| SU5402 | 9.4 ± 10 | >50 | ||
| TAB | 10 ± 2.7 | 0.17 ± 0.045 | ||
| iPSC-Endob | Nocodazole | 0.064 ± 0.10 | 0.032 ± 0.036 | |
| Suramin | 9.0 ± 4.7 | >50 | ||
| SU5402 | 2.5 ± 2.7 | 42 ± 11 | ||
| TAB | 10 ± 1.7 | 20 ± 20 | ||
| Total tube area | HUVECs | Nocodazole | 0.33 ± 0.53 | 0.010 ± 0.0002 |
| Suramin | 7.6 ± 3.7 | >50 | ||
| SU5402 | 0.96 ± 0.96 | 0.064 ± 0.0052 | ||
| TAB | 13 ± 15 | 0.42 ± 0.35 | ||
| iPSC-Endob | Nocodazole | 0.0012 ± 0.0019 | 0.030 ± 0.027 | |
| Suramin | 3.2 ± 2.5 | >50 | ||
| SU5402 | 0.13 ± 0.13 | 0.65 ± 0.57 | ||
| TAB | 7.9 ± 13 | 9.4 ± 11.0 | ||
aValue (μM) at which the concentration–response fit curve crosses beyond 1 SD of the mean control value. Values shown represent the mean and SD of the single curve fit for three separate experiments each containing three replicates per concentration.
biPSC-Endo: iCell endothelial cells from Cellular Dynamics International.
POD, point-of-departure; SD, standard deviation; TAB, tetraoctyl ammonium bromide.
While a number of quantitative phenotypes could be deduced from the angiogenesis assay, their potential utility is not uniform. In particular, mean tube length on the hydrogel showed no concentration–response effect with chemical exposure, resulting in Z′ factors well below the 0.5 mark of a good screening assay in both cell types and matrices. Although 100 nM nocodazole did have a significant effect on mean tube length, the magnitude of the effect was only modest (Supplementary Fig. S2). In addition, 50 μM suramin did not alter the mean tube length. Total tube length and total tube area exhibited higher Z′ factors with nocodazole-treated HUVECs on the hydrogel resulting in 0.67 and 0.65, respectively. The magnitude and statistical significance of positive controls, 100 nM nocodazole and 50 μM suramin, indicate that total tube length and total tube area are better metrics for evaluating the angiogenic potential of chemicals (Supplementary Fig. S2). Similar to the reproducibility, HUVECs performed better for the angiogenesis assays on both matrices.
Evaluation of Cytotoxic Endpoints in ECs
Three cellular characteristics were investigated to determine the robustness and utility of the various cytotoxicity endpoints. Nuclear content was evaluated with Hoechst 33342, functional intracellular esterase activity with Calcein AM, and total ATP content with CellTiter-Glo assay. Reproducibility was determined similarly to the angiogenesis assays with interplate and interday replicates. In addition, HUVECs and iPSC-ECs were either stimulated with a cytokine cocktail, or left unstimulated to determine if activation alters the response of ECs to chemicals. Correlation coefficients indicated good reproducibility across the different endpoints for both cell types, ranging from 0.51 to 0.97 with the majority above 0.8 (Table 6). %CVs showed acceptable variability with the iPSC-ECs having a higher degree of variability with values from 7.0 to 15.4 compared with 6.4 to 6.8 for the HUVECs. Assay quality control metrics between stimulated and unstimulated conditions were similar with the exception of nuclear content and calcein AM for the iPSC-ECs. For the latter, the stimulated cells had a higher %CV; nuclear content, 14.4 for stimulated compared with 7.4 for unstimulated; calcein AM, 15.4 for stimulated compared with 7.4 for unstimulated.
Table 6.
Assay Quality Control—Cytotoxicity Assay
| Interplate reproducibilitya | Interday reproducibilitya | %CV of negative controls | Z′-factorb (N = 12–36) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Metric | Cells | (+)c | (−)d | (+) | (−) | (+) | (−) | (+) | (−) |
| Nuclei content | HUVEC | S: 0.81 (<0.0001) | S: 0.72 (<0.0001) | S: 0.79 (<0.0001) | S: 0.71 (<0.0001) | 6.4 | 6.6 | 0.73 | 0.55 |
| P: 0.84 (<0.0001) | P: 0.89 (<0.0001) | P: 0.88 (<0.0001) | P: 0.90 (<0.0001) | ||||||
| iPSC-Endoe | S: 0.81 (<0.0001) | S: 0.78 (<0.0001) | S: 0.71 (<0.0001) | S: 0.58 (<0.0001) | 14.4 | 7.4 | −1.2 | −0.19 | |
| P: 0.83 (<0.0001) | P: 0.89 (<0.0001) | P: 0.77 (<0.0001) | P: 0.80 (<0.0001) | ||||||
| Viable cell staining | HUVEC | S: 0.81 (<0.0001) | S: 0.72 (<0.0001) | S: 0.81 (<0.0001) | S: 0.71 (<0.0001) | 6.4 | 6.6 | 0.78 | 0.80 |
| P: 0.90 (<0.0001) | P: 0.90 (<0.0001) | P: 0.90 (<0.0001) | P: 0.91 (<0.0001) | ||||||
| iPSC-Endoe | S: 0.87 (<0.0001) | S: 0.77 (<0.0001) | S: 0.82 (<0.0001) | S: 0.51 (<0.0001) | 15.4 | 7.4 | 0.53 | 0.64 | |
| P: 0.97 (<0.0001) | P: 0.95 (<0.0001) | P: 0.89 (<0.0001) | P: 0.78 (<0.0001) | ||||||
| ATP content | HUVEC | S: 0.87 (<0.0001) | S: 0.78 (<0.0001) | S: 0.80 (<0.0001) | S: 0.62 (<0.0001) | 6.5 | 6.8 | 0.79 | 0.72 |
| P: 0.91 (<0.0001) | P: 0.89 (<0.0001) | P: 0.86 (<0.0001) | P: 0.91 (<0.0001) | ||||||
| iPSC-Endoe | S: 0.87 (<0.0001) | S: 0.80 (<0.0001) | S: 0.80 (<0.0001) | S: 0.66 (<0.0001) | 8.3 | 7.0 | 0.74 | 0.77 | |
| P: 0.91 (<0.0001) | P: 0.97 (<0.0001) | P: 0.86 (<0.0001) | P: 0.93 (<0.0001) | ||||||
aS: Spearman's ρ, P: Pearson's r. Correlation analysis sample size ranged from n = 96. P values provided below correlation values.
bTreatment with TAB at 50 μM.
c(+): Cells stimulated with cytokines.
d(−): Unstimulated cells.
eiPSC-Endo: iCell endothelial cells from Cellular Dynamics International.
Plots of the data used to calculate Z′-factors shown in this table are included as Supplementary Figure S3.
ATP, adenosine triphosphate.
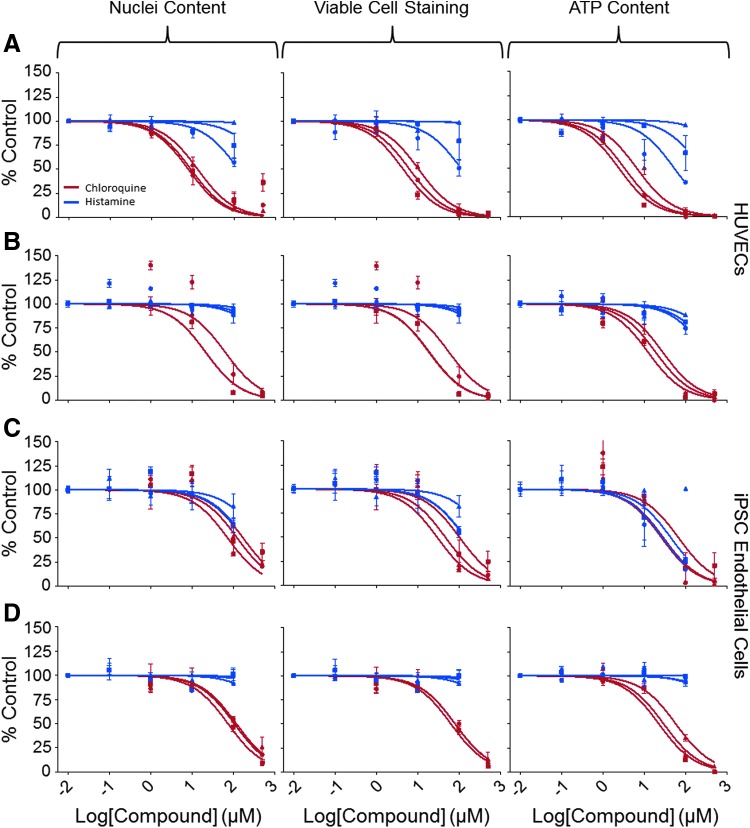
Several chemicals that are known to have effects on ECs were used for studies of concentration-dependent cytotoxicity. Satisfactory reproducibility of concentration-responses was seen across plates and across days for chloroquine phosphate and histamine (Fig. 4) with similar response seen between HUVECs and iPSC-ECs (Table 7). Cytokine stimulation did have an effect on the chemical response for certain chemicals. This was especially the case with histamine, where stimulated POD values were about 50% lower as compared with unstimulated POD values. Similar effects were seen in HUVECs and iPSC-ECs. Chloroquine phosphate also demonstrated this effect, but only in HUVECs (Table 7).
Fig. 4.
Assessment of cytotoxic effects of select agents on HUVECs (A, B) and iPSC-derived ECs (C, D) after 24-h chemical treatment with (A, C) and without (B, D) cytokine exposure. Reproducibility of concentration–response plots for histamine (blue) and chloroquine (red) for the three cytotoxic endpoints each represented by the different shapes. Concentration–response graphs shown were prepared in GraphPad using a normalized nonlinear fit. Each line represents the mean ± SEM for a single experiment with three replicates.
Table 7.
Concentration Response Analysis—Cytotoxicity Assay
| Average POD values ± SDa | ||||
|---|---|---|---|---|
| Metric | Cells | Chemical | (+)b | (−)c |
| Nuclei content | HUVECs | Colchicine | 0.038 ± 0.0062 | 0.04 ± 0.001 |
| Histamine | 35 ± 45 | 75 ± 20 | ||
| Concanamycin A | 0.00073 ± 0.0009 | 0.0056 ± 0.0055 | ||
| Chloroquine phosphate | 1.0 ± 0.42 | 30 ± 24 | ||
| iPSC-Endod | Colchicine | 0.050 ± 0.014 | 0.048 ± 0.012 | |
| Histamine | 44 ± 22 | >100 | ||
| Concanamycin A | 0.0033 ± 0.0033 | 0.020 ± 0.024 | ||
| Chloroquine phosphate | 58 ± 5.0 | 17 ± 18 | ||
| Viable cell staining | HUVECs | Colchicine | 0.053 ± 0.030 | 0.039 ± 0.0005 |
| Histamine | 35 ± 46 | 76 ± 20 | ||
| Concanamycin A | 0.0009 ± 0.0001 | 0.0056 ± 0.0055 | ||
| Chloroquine phosphate | 1.2 ± 0.45 | 28 ± 23 | ||
| iPSC-Endod | Colchicine | 0.040 ± 0.0040 | 0.050 ± 0.016 | |
| Histamine | 33 ± 28 | >100 | ||
| Concanamycin A | 0.00016 ± 0.00006 | 0.017 ± 0.017 | ||
| Chloroquine phosphate | 32 ± 11 | 18 ± 19 | ||
| ATP content | HUVECs | Colchicine | 0.023 ± 0.016 | 0.044 ± 0.004 |
| Histamine | 39 ± 43 | 62 ± 28 | ||
| Concanamycin A | 0.00005 ± 0.00001 | 0.0075 ± 0.0075 | ||
| Chloroquine phosphate | 0.6 ± 0.22 | 5.3 ± 4.7 | ||
| iPSC-Endod | Colchicine | 0.035 ± 0.0038 | 0.039 ± 0.0069 | |
| Histamine | 36 ± 46 | >100 | ||
| Concanamycin A | 0.00019 ± 0.00009 | 0.0004 ± 0.00014 | ||
| Chloroquine phosphate | 9.3 ± 2.6 | 17 ± 18 | ||
aValue (μM) at which the concentration–response fit curve crosses beyond one SD of the mean control value. Values shown represent the mean and SD of the single curve fit for three separate experiments each containing three replicates per concentration.
b(+): Cells stimulated with cytokines.
c(−): Unstimulated cells.
diPSC-Endo: iCell endothelial cells from Cellular Dynamics International.
The different cytotoxic endpoints had consistent results for both the HUVECs and the iPSC-ECs. This was seen in the reproducibility, the POD values, and also the Z′ factor values. All endpoints for both types of cells had Z′ values greater than 0.5 upon treatment with 50 μM TAB with the exception of the nuclear content for the iPSC-ECs (Table 6).
Discussion
This study set out to examine endpoints specific to ECs (angiogenesis assays) and general endpoints of cellular dysfunction (cytotoxicity assays) and conduct a side-by-side performance comparison of HUVECs and iPSC-derived ECs with the hypothesis that the iPSC-derived ECs would be similar or better in performance. Several of these assay endpoints provided Z′-factors greater than 0.5 suggesting potential utility for HTS.
The angiogenic assessment of ECs showed the HUVECs performing relatively more consistently on both extracellular matrices. The iPSC-derived ECs used in this study were more likely to form sheets if seeding density was too high (data not shown), so a fine balance is needed for the amount of cells plated on the extracellular matrix. The hydrogel, in the experiments with the HUVECs, had higher Z′-factors and lower %CV for the negative controls suggesting a more robust and reproducible extracellular matrix assay compared with the traditional Geltrex. The difference in performance is largely due to the higher %CV of negative controls, although this may be a result of the different image processing/quantification used here. We also note that there is a prominent difference in angiogenic appearance of both cell types depending on the matrix. In Geltrex, cells formed thicker tubes and smaller nodes, whereas the opposite was true in hydrogel. Consequently, positive control compounds used in these experiments yielded more consistent concentration–response effects in the experiments with hydrogel. This was true for both cell types, as exemplified with the results with nocodazole, which failed to block angiogenesis by iPSC-ECs in Geltrex, but was effective in hydrogel.
In addition, a chemical matrix effect was observed on Geltrex for suramin. Previous publications have indicated that suramin exerts its effects by disrupting the matrix, thus not allowing the ECs to self-assemble.10 This phenomenon was observed in this study, as suramin had profound effects on ECs in the Geltrex, yet had little to no effect on cells in the hydrogel. Due to the photopolymerization of the hydrogel, it is less likely to be completely disrupted by chemical matrix effects as was observed with the Geltrex. Thus, chemical matrix effects should be an important consideration for screening assays, especially for chemicals that have been poorly characterized like a large number of environmental chemicals. Hydrogel may be a preferred matrix for future studies with ECs as it provides greater consistency in the readouts and is not prone to chemical matrix effects.
Multiple cytotoxic endpoints were also evaluated in a companion assay, and these included nuclei content, functional intracellular esterases (viable cell staining), and total ATP content. Good concordance was seen across these different endpoints; however, nuclei content was a parameter with the lowest Z′-factor values, especially for the iPSC-ECs. This phenomenon was driven by a wider range of values in the TAB-treated cells, although the effect was still statistically significant (Supplementary Fig. S3). One reason for the lower quality in this readout is that cellular debris was counted as nuclei content, an issue that was difficult to resolve by adjusting the parameters of the image analysis. Therefore, we conclude that nuclei content is not a reliable endpoint for inclusion into multiplex assays; additional probes such as phalloidin for cytoskeletal integrity may be included, if needed, to increase assay output. In addition, a difference was observed between cytokine stimulated ECs and nonstimulated for some chemicals, specifically histamine and chloroquine phosphate. Thus, attention should be given to the type of chemicals being investigated as to whether cytokine stimulation is warranted or not, thus probing the anti-inflammatory effects of chemicals. Strong reproducibility and Z′-factors above 0.5 suggest any of these endpoints would work well in a screening assay, with or without stimulation.
It is important to note several divergences between the performance of the HUVECs and the iPSC-ECs; in particular, the difference seen with responses to concanamycin A and chloroquine phosphate with cytokine treatment for the cytotoxicity evaluation, as well as the performance difference on the two extracellular matrices. It has been demonstrated for other types of iPSC-derived cells that they can exhibit fetal characteristics.8,24–26 This could be playing a role here as chemical responses may be dictated by the expression or activity of proteins variably expressed between fully matured and immature cells. In addition, ECs are present in two fully differentiated forms in vivo, macro and microvascular.3 HUVECs are of the macrovascular type, whereas the iPSC-ECs under investigation in this study have not been validated for either type. Currently, this is quite common in the iPSC-EC literature as few researchers consider the finally differentiated form of their ECs given that techniques for validating the different vascular types have not fully been elucidated, although differences have been described.27,28 This presents an important additional consideration that may need to be addressed to ensure that the screening approach is fit for purpose.
Given the vast number of environmental chemicals that have little to no vascular-related toxicity data and the increasing prevalence of vascular diseases, a HT-relevant assay is needed to ascertain base level data regarding the vascular effects of these chemicals. Described in this study is a vascular-specific and a general cytotoxic assay for ECs as well as a comparison of the generally used cell model, HUVECs, and an iPSC-derived EC. Our data show that HUVECs performed more consistently than the iPSC-ECs for the various endpoints investigated, in particular, the angiogenic endpoints. This is not surprising as this is a primary cell type that has been propagated through a limited number of passages and the culture media and other experimental conditions have been refined using this model over the past years. iPSC-ECs are still in development and one key advantage they offer is the opportunity to create a virtually unlimited supply of cells from the same individuals and a possibility of population-wide testing. Once a standardized protocol for derivation of iPSC-EC are developed by the larger scientific community, a fine tuning of assay protocols and matrix materials should lead to optimization in iPSC-EC performance. We also find that the use of hydrogels may be preferred with respect to the stability of the matrix and robustness of the readouts when screening a diverse array of compounds. The information presented here can be used as a screening approach itself, with additional validation, or provide valuable data for future HT assay development either with material optimization or the cellular system being used. In addition, the assays presented here could be further enhanced by incorporating other nonimaging techniques, such as transcriptomic or metabolomic endpoints, to increase data generated and further refine the toxicity profile of the chemicals being screened.
Supplementary Material
Abbreviations Used
- %CV
coefficient of variation
- DMSO
dimethyl sulfoxide
- EC
endothelial cell
- FBS
fetal bovine serum
- HCS
high-content screening
- HT
high throughput
- HTS
high-throughput screening
- HUVEC
human umbilical vein endothelial cell
- iPSC
induced pluripotent stem cell
- POD
point-of-departure
- TAB
tetraoctyl ammonium bromide
- VEGF
vascular endothelial growth factor
Acknowledgments
The authors appreciate useful discussions and technical support from Oksana Sirenko and particularly appreciate Paula Gedraitis for assistance with the imaging module (Molecular Devices; LLC, Sunnyvale, CA). This work was supported by grants from Concawe (“New Technologies to Underpin Category Approaches and Read-Across in Regulatory Programs —CAT-APP”) and the United States Environmental Protection Agency (STAR RD83516602). F. Grimm is the recipient of the 2017 Society of Toxicology Syngenta Fellowship Award in Human Health Application of New Technologies. William Klaren is a T32 training fellow supported by the Regulatory Science in Environmental Health and Toxicology Training Grant (T32 ES026568).
Disclosure Statement
C.S.L. was employed by StemPharm, Incorporated, the manufacturer of the hydrogel used in this study.
References
- 1.Pruss-Ustun A, Corvalan C: Preventing Disease Through Healthy Environments: Towards an Estimate of the Environmental Burden of Disease. World Health Organization, Geneva, Switzerland, 2006 [Google Scholar]
- 2.Eckers A, Haendeler J: Endothelial cells in health and disease. Antioxid Redox Signal 2015;22:1209–1211 [DOI] [PubMed] [Google Scholar]
- 3.Smith EJ, Staton CA: Tubule Formation Assays. John Wiley & Sons, Ltd., Hoboken, NJ, 2006 [Google Scholar]
- 4.Sarkanen JR, Mannerstrom M, Vuorenpaa H, Uotila J, Ylikomi T, Heinonen T: Intra-laboratory pre-validation of a human cell based in vitro angiogenesis assay for testing angiogenesis modulators. Front Pharmacol 2010;1:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jennings P: The future of in vitro toxicology. Toxicol In Vitro 2015;29:1217–1221 [DOI] [PubMed] [Google Scholar]
- 6.Auerbach R, Lewis R, Shinners B, Kubai L, Akhtar N: Angiogenesis assays: A critical overview. Clin Chem 2003;49:32–40 [DOI] [PubMed] [Google Scholar]
- 7.Robitaille K, Rourke JL, McBane JE, et al. : High-throughput functional genomics identifies regulators of primary human beta cell proliferation. J Biol Chem 2016;291:4614–4625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh VK, Kalsan M, Kumar N, Saini A, Chandra R: Induced pluripotent stem cells: Applications in regenerative medicine, disease modeling, and drug discovery. Front Cell Dev Biol 2015;3:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen G, Gulbranson DR, Hou Z, et al. : Chemically defined conditions for human iPSC derivation and culture. Nat Methods 2011;8:424–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arnaoutova I, Kleinman HK: In vitro angiogenesis: Endothelial cell tube formation on gelled basement membrane extract. Nat Protoc 2010;5:628–635 [DOI] [PubMed] [Google Scholar]
- 11.Falcon BL, Swearingen M, Gough WH, et al. : An in vitro cord formation assay identifies unique vascular phenotypes associated with angiogenic growth factors. PLoS One 2014;9:e106901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evensen L, Micklem DR, Link W, Lorens JB: A novel imaging-based high-throughput screening approach to anti-angiogenic drug discovery. Cytometry A 2010;77:41–51 [DOI] [PubMed] [Google Scholar]
- 13.Cote MC, Lavoie JR, Houle F, Poirier A, Rousseau S, Huot J: Regulation of vascular endothelial growth factor-induced endothelial cell migration by LIM kinase 1-mediated phosphorylation of annexin 1. J Biol Chem 2010;285:8013–8021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Folkman J, Haudenschild C: Angiogenesis in vitro. Nature 1980;288:551–556 [DOI] [PubMed] [Google Scholar]
- 15.Grant DS, Kinsella JL, Fridman R, et al. : Interaction of endothelial cells with a laminin A chain peptide (SIKVAV) in vitro and induction of angiogenic behavior in vivo. J Cell Physiol 1992;153:614–625 [DOI] [PubMed] [Google Scholar]
- 16.Kleinman HK, Martin GR: Matrigel: Basement membrane matrix with biological activity. Semin Cancer Biol 2005;15:378–386 [DOI] [PubMed] [Google Scholar]
- 17.Orkin RW, Gehron P, McGoodwin EB, Martin GR, Valentine T, Swarm R: A murine tumor producing a matrix of basement membrane. J Exp Med 1977;145:204–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prigozhina NL, Heisel AJ, Seldeen JR, Cosford ND, Price JH: Amphiphilic suramin dissolves Matrigel, causing an ‘inhibition’ artefact within in vitro angiogenesis assays. Int J Exp Pathol 2013;94:412–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nguyen EH, Zanotelli MR, Schwartz MP, Murphy WL: Differential effects of cell adhesion, modulus and VEGFR-2 inhibition on capillary network formation in synthetic hydrogel arrays. Biomaterials 2014;35:2149–2161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Belair DG, Schwartz MP, Knudsen T, Murphy WL: Human iPSC-derived endothelial cell sprouting assay in synthetic hydrogel arrays. Acta Biomater 2016;39:12–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zanotelli MR, Ardalani H, Zhang J, et al. : Stable engineered vascular networks from human induced pluripotent stem cell-derived endothelial cells cultured in synthetic hydrogels. Acta Biomater 2016;35:32–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sirenko O, Cromwell EF, Crittenden C, Wignall JA, Wright FA, Rusyn I: Assessment of beating parameters in human induced pluripotent stem cells enables quantitative in vitro screening for cardiotoxicity. Toxicol Appl Pharmacol 2013;273:500–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang JH, Chung TD, Oldenburg KR: A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J Biomol Screen 1999;4:67–73 [DOI] [PubMed] [Google Scholar]
- 24.Robertson C, Tran DD, George SC: Concise review: Maturation phases of human pluripotent stem cell-derived cardiomyocytes. Stem Cells 2013;31:829–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sauer V, Roy-Chowdhury N, Guha C, Roy-Chowdhury J: Induced pluripotent stem cells as a source of hepatocytes. Curr Pathobiol Rep 2014;2:11–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Odawara A, Katoh H, Matsuda N, Suzuki I: Physiological maturation and drug responses of human induced pluripotent stem cell-derived cortical neuronal networks in long-term culture. Sci Rep 2016;6:26181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shlyonsky V, Soussia IB, Cheriaa N, Naeije R, Mies F: Functional response to endothelin in macro- and microvascular endothelial cells. FASEB J 2010;24:976–978 [Google Scholar]
- 28.Jackson CJ, Nguyen M: Human microvascular endothelial cells differ from macrovascular endothelial cells in their expression of matrix metalloproteinases. Int J Biochem Cell Biol 1997;29:1167–1177 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.