Abstract
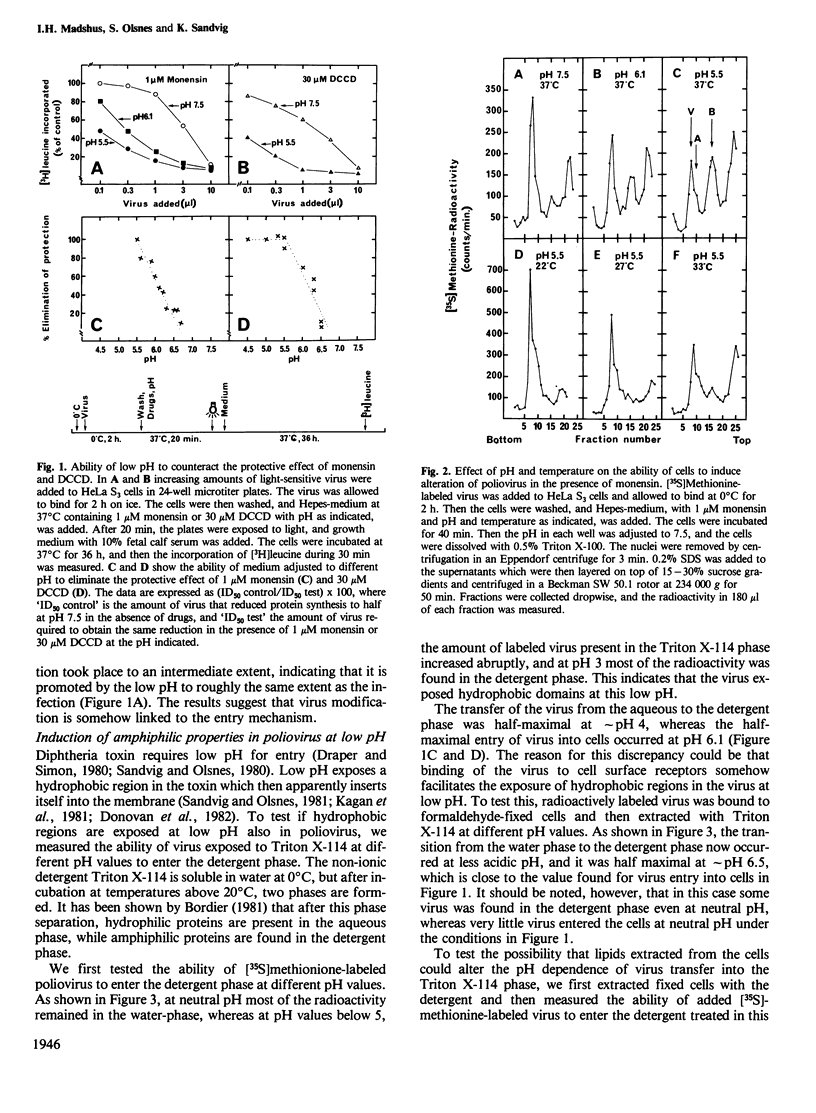
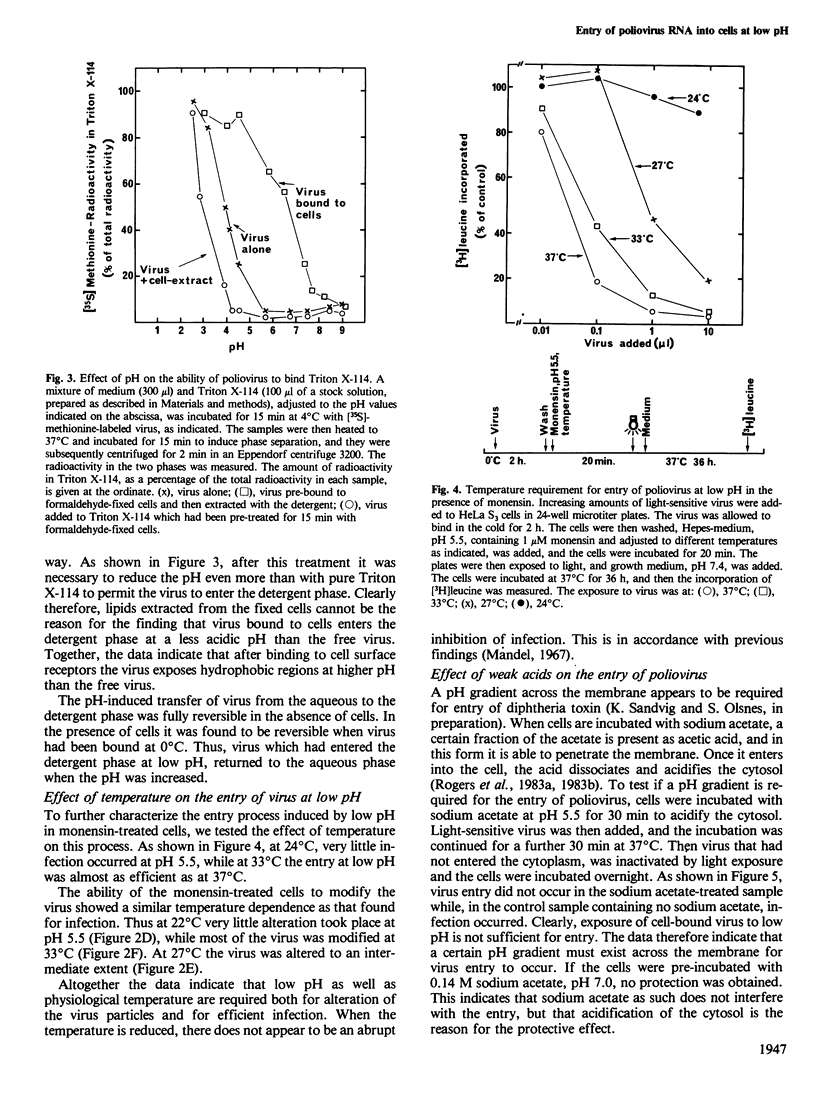
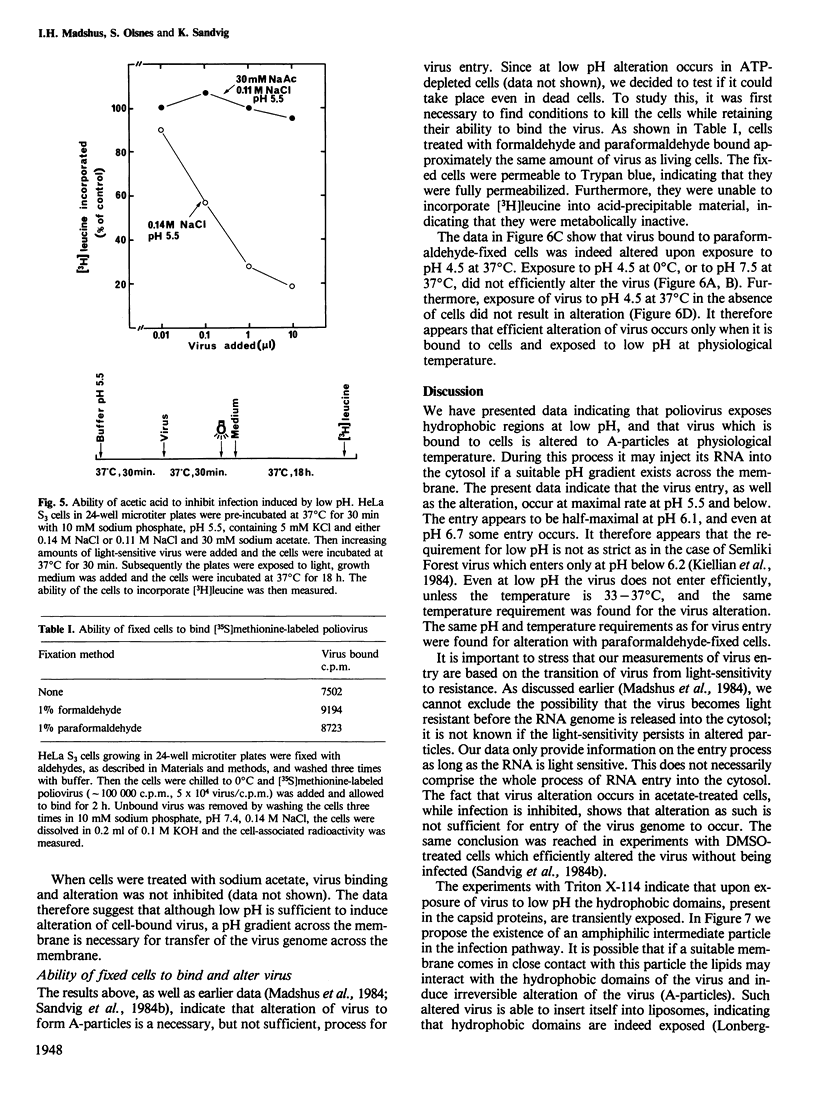
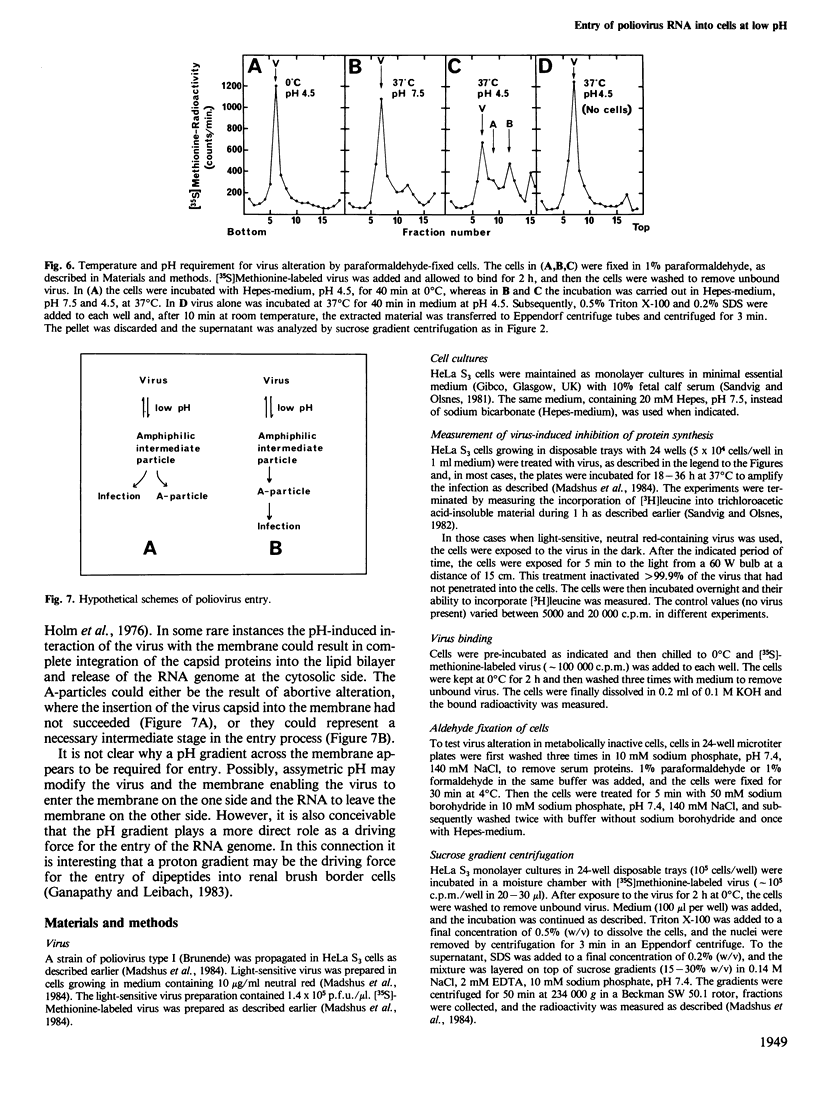
HeLa S3 cells were protected against infection by poliovirus type I by the presence of monensin and N,N'-dicyclohexylcarbodiimide (DCCD), compounds elevating the pH of acidic intracellular compartments. The protection was fully overcome by exposing the cells to pH 5.5 and lower, and at approximately pH 6.1 it was reduced by half. Measurements of the ability of the virus to enter the detergent phase under conditions where Triton X-114 was separated from water indicated that the virus is hydrophilic at neutral pH, and that it exposes hydrophobic regions at low pH. When the cells were pretreated with acetic acid, which reduces the intracellular pH, virus entry was inhibited, indicating that a pH gradient across the membrane is necessary for infection. Under all conditions which induced infection, the virus particles were altered to more slowly sedimenting material. Also, virus bound to aldehyde-fixed cells was altered when exposed to low pH at 37 degrees C. The data indicate that poliovirus bound to receptors on cells exposes hydrophobic regions at low pH, and that at physiological temperature it undergoes alteration. This alteration may be a necessary, but not sufficient requirement for infection.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Crowell R. L., Philipson L. Specific alterations of coxsackievirus B3 eluted from HeLa cells. J Virol. 1971 Oct;8(4):509–515. doi: 10.1128/jvi.8.4.509-515.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan J. J., Simon M. I., Montal M. Insertion of diphtheria toxin into and across membranes: role of phosphoinositide asymmetry. Nature. 1982 Aug 12;298(5875):669–672. doi: 10.1038/298669a0. [DOI] [PubMed] [Google Scholar]
- Draper R. K., Simon M. I. The entry of diphtheria toxin into the mammalian cell cytoplasm: evidence for lysosomal involvement. J Cell Biol. 1980 Dec;87(3 Pt 1):849–854. doi: 10.1083/jcb.87.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FitzGerald D., Morris R. E., Saelinger C. B. Receptor-mediated internalization of Pseudomonas toxin by mouse fibroblasts. Cell. 1980 Oct;21(3):867–873. doi: 10.1016/0092-8674(80)90450-x. [DOI] [PubMed] [Google Scholar]
- Ganapathy V., Leibach F. H. Role of pH gradient and membrane potential in dipeptide transport in intestinal and renal brush-border membrane vesicles from the rabbit. Studies with L-carnosine and glycyl-L-proline. J Biol Chem. 1983 Dec 10;258(23):14189–14192. [PubMed] [Google Scholar]
- Helenius A., Kartenbeck J., Simons K., Fries E. On the entry of Semliki forest virus into BHK-21 cells. J Cell Biol. 1980 Feb;84(2):404–420. doi: 10.1083/jcb.84.2.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan B. L., Finkelstein A., Colombini M. Diphtheria toxin fragment forms large pores in phospholipid bilayer membranes. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4950–4954. doi: 10.1073/pnas.78.8.4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielian M. C., Keränen S., Käriäinen L., Helenius A. Membrane fusion mutants of Semliki Forest virus. J Cell Biol. 1984 Jan;98(1):139–145. doi: 10.1083/jcb.98.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonberg-Holm K., Gosser L. B., Kauer J. C. Early alteration of poliovirus in infected cells and its specific inhibition. J Gen Virol. 1975 Jun;27(3):329–342. doi: 10.1099/0022-1317-27-3-329. [DOI] [PubMed] [Google Scholar]
- Lonberg-Holm K., Gosser L. B., Shimshick E. J. Interaction of liposomes with subviral particles of poliovirus type 2 and rhinovirus type 2. J Virol. 1976 Aug;19(2):746–749. doi: 10.1128/jvi.19.2.746-749.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonberg-Holm K., Korant B. D. Early interaction of rhinoviruses with host cells. J Virol. 1972 Jan;9(1):29–40. doi: 10.1128/jvi.9.1.29-40.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madshus I. H., Olsnes S., Sandvig K. Mechanism of entry into the cytosol of poliovirus type 1: requirement for low pH. J Cell Biol. 1984 Apr;98(4):1194–1200. doi: 10.1083/jcb.98.4.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel B. The relationship between penetration and uncoating of poliovirus in HeLa cells. Virology. 1967 Apr;31(4):702–712. doi: 10.1016/0042-6822(67)90198-5. [DOI] [PubMed] [Google Scholar]
- Matlin K. S., Reggio H., Helenius A., Simons K. Pathway of vesicular stomatitis virus entry leading to infection. J Mol Biol. 1982 Apr 15;156(3):609–631. doi: 10.1016/0022-2836(82)90269-8. [DOI] [PubMed] [Google Scholar]
- Rogers J., Hesketh T. R., Smith G. A., Beaven M. A., Metcalfe J. C., Johnson P., Garland P. B. Intracellular pH and free calcium changes in single cells using quene 1 and quin 2 probes and fluorescence microscopy. FEBS Lett. 1983 Sep 5;161(1):21–27. doi: 10.1016/0014-5793(83)80722-4. [DOI] [PubMed] [Google Scholar]
- Rogers J., Hesketh T. R., Smith G. A., Metcalfe J. C. Intracellular pH of stimulated thymocytes measured with a new fluorescent indicator. J Biol Chem. 1983 May 25;258(10):5994–5997. [PubMed] [Google Scholar]
- Sandvig K., Madshus I. H., Olsnes S. Dimethyl sulphoxide protects cells against polypeptide toxins and poliovirus. Biochem J. 1984 May 1;219(3):935–940. doi: 10.1042/bj2190935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvig K., Olsnes S. Diphtheria toxin entry into cells is facilitated by low pH. J Cell Biol. 1980 Dec;87(3 Pt 1):828–832. doi: 10.1083/jcb.87.3.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvig K., Olsnes S. Entry of the toxic proteins abrin, modeccin, ricin, and diphtheria toxin into cells. II. Effect of pH, metabolic inhibitors, and ionophores and evidence for toxin penetration from endocytotic vesicles. J Biol Chem. 1982 Jul 10;257(13):7504–7513. [PubMed] [Google Scholar]
- Sandvig K., Olsnes S. Rapid entry of nicked diphtheria toxin into cells at low pH. Characterization of the entry process and effects of low pH on the toxin molecule. J Biol Chem. 1981 Sep 10;256(17):9068–9076. [PubMed] [Google Scholar]
- Sandvig K., Sundan A., Olsnes S. Evidence that diphtheria toxin and modeccin enter the cytosol from different vesicular compartments. J Cell Biol. 1984 Mar;98(3):963–970. doi: 10.1083/jcb.98.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S. B., Kawasaki K., Ohnishi S. Hemolytic activity of influenza virus hemagglutinin glycoproteins activated in mildly acidic environments. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3153–3157. doi: 10.1073/pnas.80.11.3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone D. K., Xie X. S., Racker E. An ATP-driven proton pump in clathrin-coated vesicles. J Biol Chem. 1983 Apr 10;258(7):4059–4062. [PubMed] [Google Scholar]
- White J., Matlin K., Helenius A. Cell fusion by Semliki Forest, influenza, and vesicular stomatitis viruses. J Cell Biol. 1981 Jun;89(3):674–679. doi: 10.1083/jcb.89.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro D. J., Fluss S. R., Maxfield F. R. Acidification of endocytic vesicles by an ATP-dependent proton pump. J Cell Biol. 1983 Sep;97(3):929–934. doi: 10.1083/jcb.97.3.929. [DOI] [PMC free article] [PubMed] [Google Scholar]


