Abstract
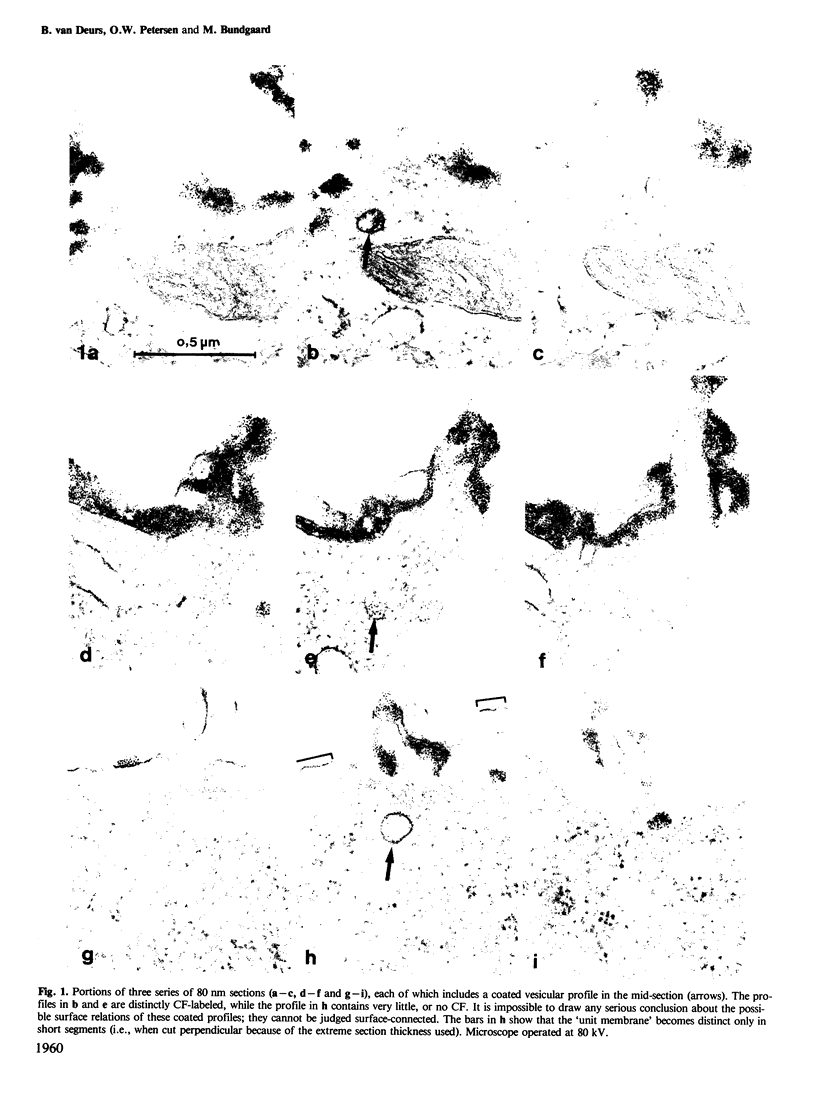
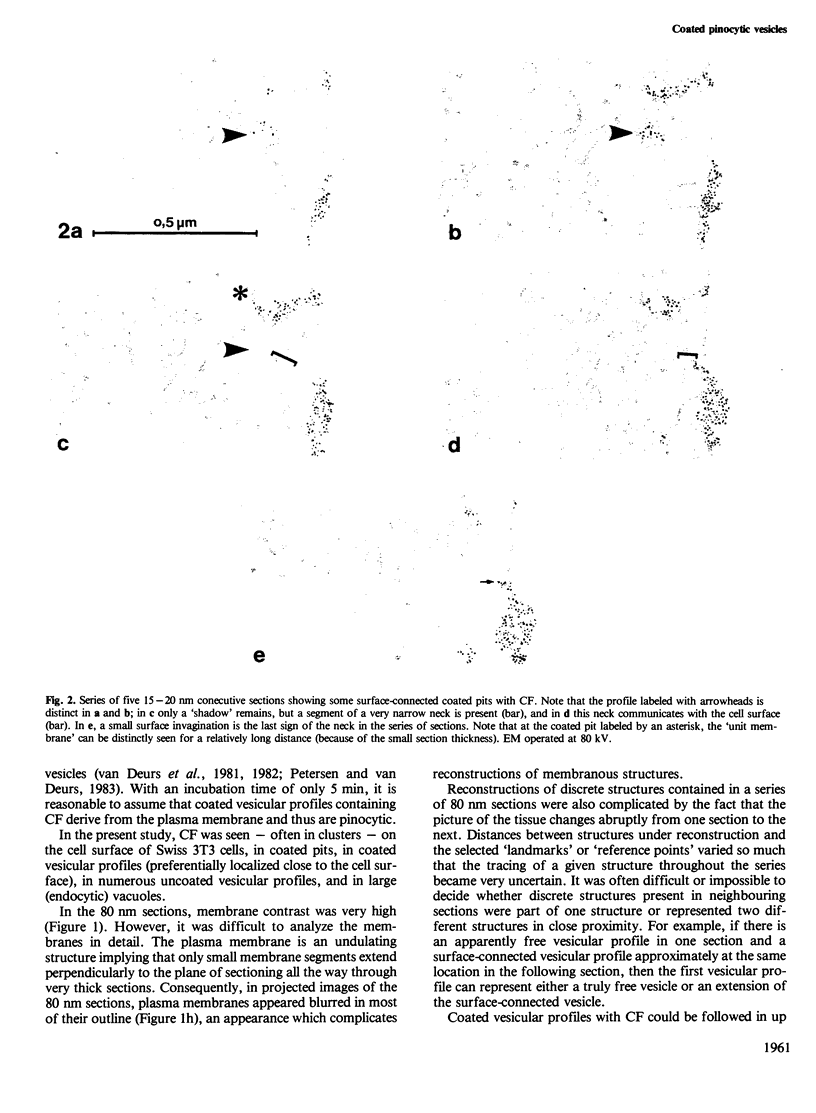
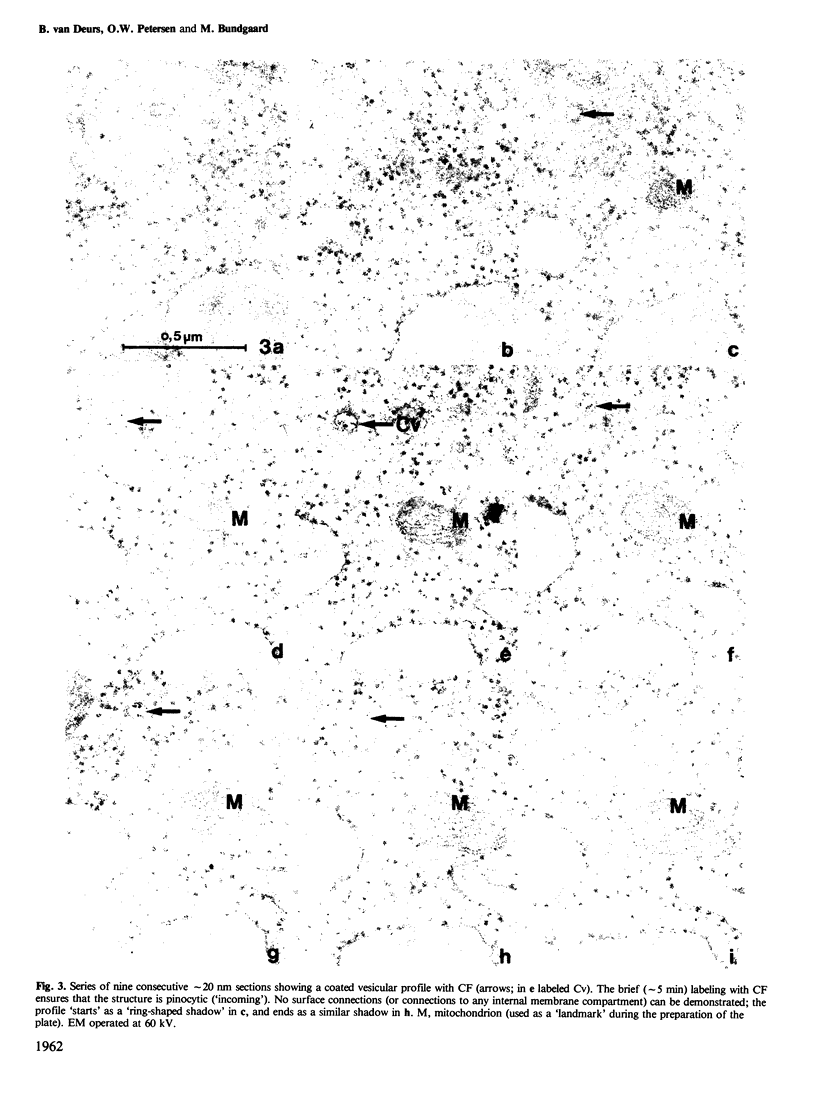
Whether or not free coated vesicles are involved during internalization of ligands bound to the receptors of coated pits is controversial. Free coated vesicles cannot be identified with certainty in random individual thin sections - reconstructions based on consecutive thin sections are required. The thickness of the sections determines the reliability of such reconstructions. In the present study, serial section electron microscopy was applied to Swiss 3T3 cells and the topographical resolution yielded by 80 nm and 20 nm sections was compared. Swiss 3T3 cells in monolayer at 37 degrees C were exposed for 5 min to cationized ferritin (CF) which is a marker of pinocytic vesicles. Subsequently the cells were fixed, pelleted and further processed for electron microscopy. The results showed that reconstructions of coated CF-labeled structures based on consecutive sections of an average thickness of approximately 80 nm could not be performed with certainty. A substantial fraction (25%) of the examined profiles appeared to be free vesicles, but narrow surface connections could easily have been missed in these thick sections. The series of the much thinner 20 nm sections provided a better resolution allowing the narrowest surface connections to be identified. Accordingly, the number of truly free, coated vesicles was much lower than the number of apparently free vesicles in the thick sections. However, free coated vesicles labeled with CF were identified in the consecutive 20 nm sections (4% of the examined profiles).
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bundgaard M. Vesicular transport in capillary endothelium: does it occur? Fed Proc. 1983 May 15;42(8):2425–2430. [PubMed] [Google Scholar]
- Geuze H. J., Slot J. W., Strous G. J., Lodish H. F., Schwartz A. L. Intracellular site of asialoglycoprotein receptor-ligand uncoupling: double-label immunoelectron microscopy during receptor-mediated endocytosis. Cell. 1983 Jan;32(1):277–287. doi: 10.1016/0092-8674(83)90518-4. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Anderson R. G., Brown M. S. Coated pits, coated vesicles, and receptor-mediated endocytosis. Nature. 1979 Jun 21;279(5715):679–685. doi: 10.1038/279679a0. [DOI] [PubMed] [Google Scholar]
- Helenius A., Kartenbeck J., Simons K., Fries E. On the entry of Semliki forest virus into BHK-21 cells. J Cell Biol. 1980 Feb;84(2):404–420. doi: 10.1083/jcb.84.2.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh M., Helenius A. Adsorptive endocytosis of Semliki Forest virus. J Mol Biol. 1980 Sep 25;142(3):439–454. doi: 10.1016/0022-2836(80)90281-8. [DOI] [PubMed] [Google Scholar]
- Maxfield F. R. Weak bases and ionophores rapidly and reversibly raise the pH of endocytic vesicles in cultured mouse fibroblasts. J Cell Biol. 1982 Nov;95(2 Pt 1):676–681. doi: 10.1083/jcb.95.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire P. G., Twietmeyer T. A. Morphology of rapidly frozen aortic endothelial cells. Glutaraldehyde fixation increases the number of caveolae. Circ Res. 1983 Sep;53(3):424–429. doi: 10.1161/01.res.53.3.424. [DOI] [PubMed] [Google Scholar]
- Pastan I. H., Willingham M. C. Journey to the center of the cell: role of the receptosome. Science. 1981 Oct 30;214(4520):504–509. doi: 10.1126/science.6170111. [DOI] [PubMed] [Google Scholar]
- Pastan I. H., Willingham M. C. Receptor-mediated endocytosis of hormones in cultured cells. Annu Rev Physiol. 1981;43:239–250. doi: 10.1146/annurev.ph.43.030181.001323. [DOI] [PubMed] [Google Scholar]
- Pearse B. M., Bretscher M. S. Membrane recycling by coated vesicles. Annu Rev Biochem. 1981;50:85–101. doi: 10.1146/annurev.bi.50.070181.000505. [DOI] [PubMed] [Google Scholar]
- Petersen O. W., van Deurs B. Serial-section analysis of coated pits and vesicles involved in adsorptive pinocytosis in cultured fibroblasts. J Cell Biol. 1983 Jan;96(1):277–281. doi: 10.1083/jcb.96.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury J. L., Condeelis J. S., Satir P. Receptor-mediated endocytosis: machinery and regulation of the clathrin-coated vesicle pathway. Int Rev Exp Pathol. 1983;24:1–62. [PubMed] [Google Scholar]
- Steinman R. M., Mellman I. S., Muller W. A., Cohn Z. A. Endocytosis and the recycling of plasma membrane. J Cell Biol. 1983 Jan;96(1):1–27. doi: 10.1083/jcb.96.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Deurs B., Von Bülow F., Møller M. Vesicular transport of cationized ferritin by the epithelium of the rat choroid plexus. J Cell Biol. 1981 Apr;89(1):131–139. doi: 10.1083/jcb.89.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham M. C., Pastan I. Formation of receptosomes from plasma membrane coated pits during endocytosis: analysis by serial sections with improved membrane labeling and preservation techniques. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5617–5621. doi: 10.1073/pnas.80.18.5617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham M. C., Pastan I. The receptosome: an intermediate organelle of receptor mediated endocytosis in cultured fibroblasts. Cell. 1980 Aug;21(1):67–77. doi: 10.1016/0092-8674(80)90115-4. [DOI] [PubMed] [Google Scholar]
- Willingham M. C., Rutherford A. V., Gallo M. G., Wehland J., Dickson R. B., Schlegel R., Pastan I. H. Receptor-mediated endocytosis in cultured fibroblasts: cryptic coated pits and the formation of receptosomes. J Histochem Cytochem. 1981 Sep;29(9):1003–1013. doi: 10.1177/29.9.6169759. [DOI] [PubMed] [Google Scholar]
- van Deurs B., Nilausen K., Faergeman O., Meinertz H. Coated pits and pinocytosis of cationized ferritin in human skin fibroblasts. Eur J Cell Biol. 1982 Jun;27(2):270–278. [PubMed] [Google Scholar]