Abstract
Purpose
We reviewed the experience of a tertiary cancer centre in the management of adrenocortical carcinoma (acc) treated over 40 years. We also searched the literature for guidelines related to the treatment of acc and for evidence for adjuvant radiation therapy (rt).
Methods
In a retrospective chart review, acc patients treated between January 1974 and December 2013 were identified, and patient demographics and tumour characteristics were extracted. Outcomes data, including dates and sites of failure, vital status, and cause of death, were collected. Overall survival was estimated using the Kaplan– Meier method. A medline search using PubMed, Ovid, and embase was used to review the literature about the role of rt and any available management guidelines for acc.
Results
Of 81 patients identified during the chart review, 39 had confirmed acc. In 32 patients, surgical resection was performed, including in 2 patients with M1 disease. Of those 32 patients, 16 received adjuvant systemic treatment (mitotane or concurrent chemoradiation). Only 6 patients received adjuvant rt, of whom 3 are still alive (2 living with distant failure). At a median follow-up of 3.8 years, 28 patients had died (72%), 10 were living (26%), and 1 had been lost to follow-up. Of the 22 patients for whom failure data were available, 2 experienced local failure, and the rest, distant failure.
Conclusions
The current data are insufficient to make treatment recommendations. Use of collaborative databases and consensus about diagnostic and therapeutic guidelines are warranted for better identification of optimum management. Adjuvant rt could be a reasonable option for R1 disease, but further research is needed.
Keywords: Adrenocortical carcinoma, disease management, adjuvant therapy, radiation therapy
INTRODUCTION
Adrenocortical carcinoma (acc) is a rare disease with an annual incidence of 0.5–2 per million population1,2. Thus, no randomized controlled trials have been conducted so far. As a result, several international networks, such as the European Network for the Study of Adrenal Tumours, have been developed to establish guidelines for the diagnosis and treatment of the disease.
Surgical resection is the main curative treatment for both early and advanced disease; however, local and distant failures are frequent, and survival is poor1. The role of adjuvant radiation therapy (rt) in this disease is ill defined. Ten years ago, the first retrospective trial examining the role of rt in the adjuvant setting reported improved local control with rt3. Subsequent studies reported conflicting results. A study from the MD Anderson Cancer Center reported no benefit4, and another study from the Ann Arbor Group reported a reduced risk of local recurrence with adjuvant rt in conjunction with mitotane after open laparotomy5. Additional consolidated research efforts are needed to address the role of adjuvant rt in acc.
The use of mitotane in the adjuvant setting has become the standard of care in many centres. So far, no randomized controlled trial to support this practice has been conducted. A randomized prospective trial is currently ongoing to answer the question of the efficacy of mitotane in prolonging disease-free survival (dfs) in low-to intermediate-risk acc treated with radical surgical excision [adiuvo (https://clinicaltrials.gov/ct2/show/NCT00777244)]. For advanced acc, mitotane is considered the treatment of choice either alone or in combination with other cytotoxic agents6,7.
In spite of the increasing research efforts related to acc, there is still a need for further collaborative efforts to optimize therapeutic options and to improve outcomes.
PURPOSE
Our aim in the present study was to review the experience of a single tertiary cancer centre in the management of acc treated over a period of 40 years, and to review the literature for available guidelines about the treatment of acc and for available evidence about adjuvant rt in acc.
METHODS
In a retrospective chart review, International Classification of Diseases codes (versions 9 and 10) were used with a major tertiary cancer centre’s database to identify patients with acc treated at the centre between January 1974 and December 2013. The search was then extended to any adrenal malignancy during the same period.
For the identified patients, data were extracted from electronic medical records and physical charts. Those data included
■ demographics (age at time of diagnosis, sex, geographic area, genetic diseases, and family history),
■ diagnosis [date of diagnosis, defined as the date of histopathologic diagnosis (biopsy or surgery); diagnostic imaging; type and date of biopsy; date of referral to the cancer centre; referral service; service at first visit; and presenting symptoms],
■ tumour [laterality, size (gross size if resectable; otherwise, radiologic measurement), histopathology data (type, mitosis count, Ki-67, grade, margin, and staging using the European Network for the Study of Adrenal Tumours proposed staging system1)],
■ treatment [date and type of surgery; type of adjuvant treatment (defined as treatment after surgical resection); rt date, intent, dose, fractionation, and volume; chemotherapy data, including intent and type], and
■ outcome (date and type of failure, vital status, dates of death and last follow-up, cause of death).
Statistical Analysis
Overall survival (os) and dfs were calculated from date of diagnosis. Survival curves were created using the Kaplan– Meier method. Data are presented as percentages, medians, ranges, and means.
The literature review with respect to the role of rt and to guidelines for acc management used a medline search in PubMed, Ovid, and embase with the keywords “adrenocortical carcinoma,” “chemotherapy, adjuvant/or radiotherapy, adjuvant/or combined modality therapy,” and “adjuvant radiation.”
RESULTS
Centre Experience
Of 81 patients identified in the cancer centre’s database during the period of interest, 39 had confirmed acc. The remaining patients were found to have adenoma, pheochromocytoma, or neuroblastoma.
Patient Characteristics
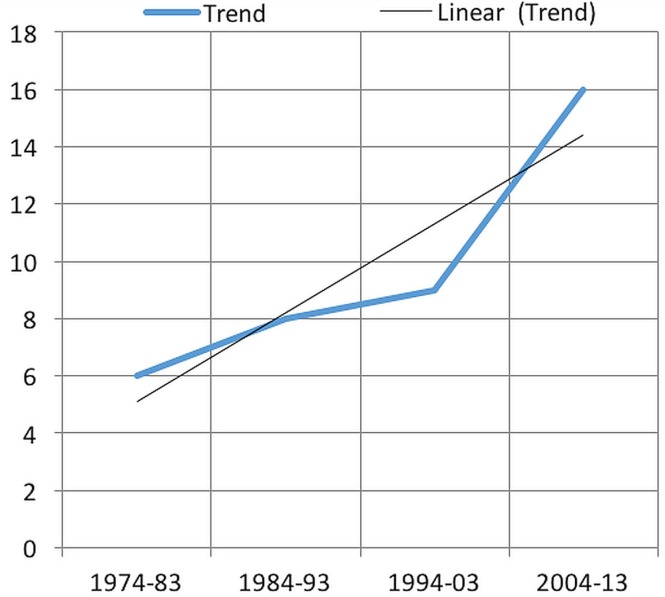
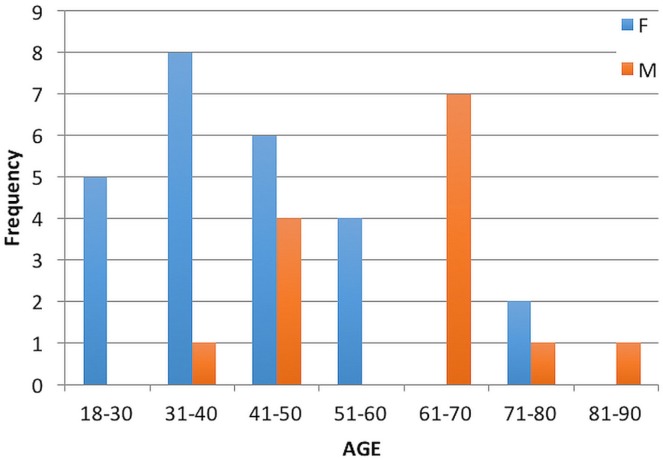
The number of referred patients showed a rising trend over the preceding 10 years (Figure 1). Median age of the identified patients was 46 years (range: 18–82 years), with median age for the women and men being 39 years (range: 19–71 years) and 64 years (range: 35–82 years) respectively (Figure 2). Of the 39 patients, 25 (64%) were women. Genetic syndromes or family histories of endocrine malignancy were absent except for 1 patient with a family history of pheochromocytoma.
FIGURE 1.
Incidence of referrals for adrenocortical carcinoma.
FIGURE 2.
Age frequency for adrenocortical carcinoma, by sex.
The most common presenting symptoms were abdominal pain (n = 15, 38%) and Cushing syndrome (n = 8, 21%). In 8 patients (21%), an incidental adrenal mass was found during investigations for other reasons.
Most patients were initially seen by surgeons, either urologists (n = 10, 26%) or general surgeons (n = 5, 13%). Referrals to the cancer centre came through medical oncologists.
Computed tomography was the main imaging modality for diagnosis and follow-up. Imaging data were available for 27 patients, 6 of whom also underwent magnetic resonance imaging.
Treatment Characteristics and Outcome
Surgery was the main treatment modality in 32 patients. In 5 patients, the tumour was deemed unresectable or medically inoperable, and in 1 patient, surgical resection was attempted, but the procedure was aborted because the tumour was found to be unresectable. Detailed surgical data were unavailable for 10 patients (Table i).
TABLE I.
Patients, tumour, and treatment characteristics
| Characteristic | Value |
|---|---|
| Patients (n) | 39 |
| Sex [n (%) women] | 25 (64.1) |
| Age (years) | |
| Median | 46 |
| Range | 18–82 |
| Symptoms [n (%)] | |
| Cushing syndrome | 8 (20.5) |
| Pain | 15 (38.5) |
| Incidental | 8 (20.5) |
| Unknown | 8 (20.5) |
| Laterality [n (%)] | |
| Right | 17 (43.6) |
| Left | 19 (48.7) |
| Unknown | 3 (7.7) |
| Size (cm) | |
| Median | 12 |
| Range | 3–24 |
| Clinical T stage [n (%)]a | |
| II | 11 (28.2) |
| III | 5 (12.8) |
| IV | 9 (23) |
| Unknown | 14 (35.5) |
| Histopathology [n (%)] | |
| Adrenocortical carcinoma (ACC) | 32 (82) |
| Large-cell ACC | 2 (5.1) |
| Adrenocortical neoplasm uncertain of malignancy | 3 (7.7) |
| Poorly differentiated carcinoma, adrenal carcinoma cannot be excluded | 1 (2.6) |
| Adrenal carcinoma | 1 (2.6) |
| Surgical procedure [n (%)] | |
| Laparoscopic adrenalectomy | 5 (12.8) |
| Open adrenalectomy | 16 (41.0) |
| Attempted adrenalectomy and closed | 1 (2.6) |
| No surgery | 5 (12.8) |
| C4 corpectomy | 1 (2.6) |
| Unknown type | 10 (25.6) |
| No data | 1 (2.6) |
| Radiation [n (%)] | |
| Palliative | 9 (23.1) |
| Adjuvant | 4 (10.3) |
| Adjuvant chemoradiationb | 2 (5.1) |
| Mitotane [n (%)] | |
| Palliative | 4 (10.3) |
| Adjuvant | 6 (15.4) |
By the European Network for the Study of Adrenal Tumors staging system.
Using cisplatin.
Of the 32 patients who underwent surgery, 16 received adjuvant systemic treatment, including 6 who received postoperative mitotane and 3 who received adjuvant chemoradiation. Of the 6 patients who received mitotane, 1 had T4 disease with inferior vena cava thrombosis. That patient was treated by open laparotomy, followed both with adjuvant chemoradiation for positive margins and with mitotane. Subsequently, that patient developed lung and peritoneal metastases and was alive at 2 years after diagnosis. Another patient had T3 disease treated by laparoscopic surgery, followed with adjuvant mitotane. That patient experienced local recurrence at the tumour bed 8 months after surgery and died of pneumonia. The other 4 patients had T2 disease. Of those 4 patients, 1 received adjuvant radiation in addition to mitotane, but experienced systemic failure in the liver, and was alive at 3 years after diagnosis. The other T2 patients received mitotane alone as an adjuvant therapy; 2 experienced systemic failure in lung or liver, and 1 patient stayed disease-free.
Only 6 patients (15%) received adjuvant rt. One experienced local failure, underwent salvage surgery, and remained disease-free. Another 3 patients received adjuvant concurrent chemoradiation with cisplatin and were still alive (2 with distant failure).
Of the 8 patients who presented with Cushing syndrome (21%), 4 had T2 disease, 2 had T3 disease (1 with M1), and 2 had T4 disease (1 with M1). Surgery was the main treatment in 7, and 3 received adjuvant radiation, 1 of whom also received concurrent cisplatin. Of 3 irradiated patients, 2 are still alive (os: 90 months free of disease, and 21.3 months living with disease); the 3rd, although free of acc, died of an intracranial bleed after developing acute myelogenous leukemia (os: 11.5 months).
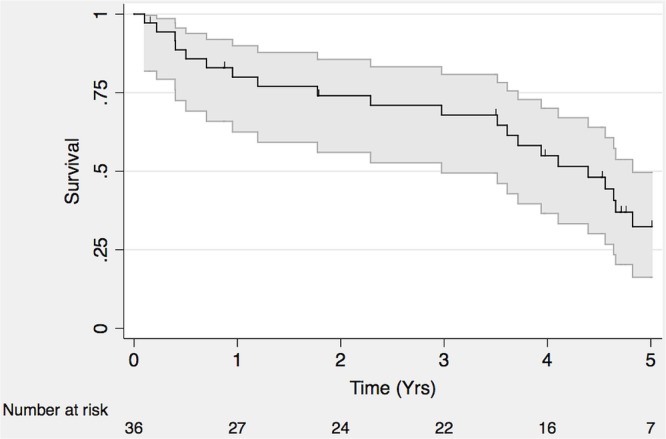
At a median follow-up of 3.8 years, 28 patients had died (72%), 10 were alive (26%), and 1 had been lost to follow-up (Figure 3). Of the 22 patients with available failure data, 2 experienced local failure, and the rest experienced distant failure.
FIGURE 3.
Overall survival in adrenocortical carcinoma.
Adjuvant RT Evidence and Guidelines
The literature search found 423 publications. After excluding pediatric acc and studies or review articles focusing on systemic therapy, we were left with seventy-one articles. Those articles included literature reviews, dosimetry, and basic science related to rt, among which were eighteen retrospective studies testing the role of rt in the management of acc. Of those eighteen studies, three were duplicate reports; one dealt with radiation-related toxicity; and one was an abstract containing insufficient data for analysis (Table ii). The search also found five guidelines from the European Society for Medical Oncology (esmo), the French National Cancer Institute, the European Association of Urology, the Italian Society of Endocrinology, and the European Society of Endocrine Surgeons8–12.
TABLE II.
Retrospective studies of adjuvant radiation therapy
| Reference | Pts (n) | Systemic treatment [n (%)] | Radiation | Local control [n (%)] | |
|---|---|---|---|---|---|
|
| |||||
| Type [n (%)] | Dose (Gy) | ||||
| Percarpio and Knowlton 197627 | 4 | Not reported | Adjuvant | 28–40 | 1 (25) |
| Nader et al., 198328 | 10 | Not reported | Adjuvant [5 (50)] | Not reported | 2 (20) |
| Markoe et al., 199129 | 5 | Chemotherapy or mitotane [2 (40)] | Adjuvant | 42–60 | 3 (60) |
| Pommier and Brennan, 199230 | 3 | Not reported | Adjuvant | 39–45 | 0/3 |
| Crucitti et al., 199631 | 11 | Chemoradiation [5 (45.5)] | Adjuvant | 30–52 | Not reported |
| Jacob et al., 200032 | 5 | Chemoradiation [2 (40)] | Adjuvant | 40–45 | 5 (100) |
| Fassnacht et al., 20063 | 14 | 5 (35.7) | Adjuvant | 41.4–54 | 12 (85.7) |
| Hermsen et al., 201033 | 3 | Not reported | Adjuvant | Not reported | 3 (100) |
| Sabloch et al., 201134 | 10 | No | Adjuvant [10 (38.5)] | 45–57 | 8 (80) |
| 16 | Definitive [16 (61.5)] | 22.5–73.51 | 5 (31.3) | ||
| Habra et al., 20134 | 16 | 4 (25) | Adjuvant | 36–59.4 | 9 (56.3) |
| Ho et al., 201335 | 14 | Chemoradiation 7 (50) | Adjuvant [2 (14.3)] | 17.5–60 | Of the 2 adjuvant patients, both experienced out-of-field recurrence |
| Palliative [12 (85.7)] | 50.4–60 | ||||
| Else et al., 20145 | 59 | Mitotane [42 (71.2) radiation and mitotane] | Adjuvant | Not reported | Not reported. Significant interaction observed between adjuvant mitotane and adjuvant radiation for recurrence-free survival when integrated in one model (hazard ratio: 0.4; p=0.03) |
| Sabolch et al., 201526 | 20a | Mitotane concurrent with radiation | Adjuvant | 45–60 | Adjuvant radiation: 19 (95) Control groupa: 8 (40) (p=0.0005) |
The 20 patients treated with adjuvant radiation were matched to 20 surgical control subjects not treated with radiation. Matching was based on stage, grade, margins, and adjuvant mitotane.
DISCUSSION
The rarity of acc explains the limited number of patients and publications related to the disease. Our study reports the experience of a single cancer centre in the diagnosis and treatment of acc. During a period of 40 years, only 39 patients at the centre were found to have true acc, and all but 1 come from the same Canadian province. In the most recent 10 years, the number of patients being seen has been higher, which might be a result of increased accuracy in diagnosis or a real increase in the incidence of the disease. Our results reflect the poor outcomes for those patients; however, our cohort included patients diagnosed and treated decades in the past. Long-term follow-up of a more recent cohort is therefore necessary.
Most of our patients were women, as has previously been reported. The median age of 46 years in our cohort is similar to that in the German acc registry13 and a large French single-centre series14; however, it is younger than the mean age of 55 reported from the U.S. Surveillance, Epidemiology, and End Results database15.
All patients had sporadic acc. The only established risk factor for acc is genetic predisposition16. Chart review at our institution did not show any genetic counselling or testing, although based on the extracted data, 5 patients had experienced a prior cancer (non-melanoma skin cancer, melanoma, high-grade histiocytoma, optic nerve glioma, prostate carcinoma). One patient had a family history of pheochromocytoma. Fortunately, our centre now has an evolving genetic counselling service, and acc is one of the indications for referral to that service. Those referrals will be helpful for future studies, especially with an established prospective database.
In a study by Chompret and colleagues17 at a children’s hospital, in which a complete family history was obtained, the authors reported that in only a small proportion of patients could the disease be attributed to inherited TP53 germline mutation (associated with a high risk of developing acc). They also reported that de novo germline mutation can be found in the absence of family history. According to a comprehensive review by Else and colleagues16, TP53 mutation testing should be considered in all acc patients, and rt should be considered with caution because of the possibly higher incidence of second malignancy. Other reported risk factors are smoking in men and contraceptive pills in women18.
Most of the patients were initially seen by a surgeon and were treated primarily with surgery. The main procedure was open adrenalectomy. One of the surgically treated patients had metastatic disease and experienced an os duration of 47 months, which is consistent with data suggesting better survival outcomes even with distant or recurrent disease19,20. Laparoscopic adrenalectomy was performed in 5 patients (13%), of whom 2 experienced local failure, and 1, distant failure. According to the esmo guideline, opened adrenalectomy is the standard of care for stages i and ii and selected stage iii patients; however, laparoscopic adrenalectomy is considered safe and effective for pheochromocytoma and incidentaloma. The guideline emphasizes the importance of performing the procedure in a centre experienced in both laparoscopic procedure and oncologic surgery8. That advice is consistent with prior studies showing a higher risk for peritoneal carcinomatosis with a laparoscopic approach21,22.
Histopathologic reporting was variable; comments on mitosis were not consistent, if present at all; and Ki-67 testing was not routine.
Adjuvant Systemic Therapy
There is no guideline for adjuvant therapy for acc, and no consensus for the use of either or both of mitotane and rt. The role of adjuvant treatment has not been determined, and its use is largely individualized. Mitotane is the key adjuvant systemic therapy in management of acc, a choice that is based on retrospective data showing improved dfs, with or without improvement in os23,24. In a multi-institutional retrospective study conducted in 8 Italian centres and 47 German centres, better recurrence-free survival (rfs) was reported in favour of adjuvant mitotane. Only 4 Italian centres were using adjuvant mitotane as the standard of care, and patients treated there (n = 47) were compared with a control group from the other 4 Italian centres (n = 55) and with patients in the German centres (n = 75). On multivariate analysis, significant improvements in both dfs and os were observed. After adding 75 German patients to the control arm as a second comparator, outcome was still significantly better in favour of adjuvant mitotane. However, no randomized trial has been published; an ongoing randomized prospective trial [adiuvo (https://clinicaltrials.gov/ct2/show/NCT00777244)] will answer the question of the efficacy of mitotane in prolonging dfs in low-to intermediate-risk acc treated with radical surgical excision.
At the 2nd Annual International Adrenal Cancer Symposium in 2008, an international panel of physicians and experts in acc concluded that the most important predictors of outcome are the completeness of surgical resection (R0) and the proliferative index. Patients are classified as low-or intermediate-risk with an R0 resection or a Ki-67 index of less than 10%; they are classified as high-risk with an R1 resection or a Ki-67 index exceeding 10%. The panel therefore recommended mandatory adjuvant mitotane for R1 resections and for Ki-67 indices exceeding 10%. No clear recommendation emerged regarding stage iii disease with an R0 resection25.
In the present study, 6 patients received adjuvant mitotane, 3 of whom had Ki-67 indices exceeding 10% with negative margins; 1 patient had a positive margin. Of those 6, 4 are still alive; 1 died of pneumonia; and 1 was lost to follow-up.
Adjuvant RT
The role of rt was mainly palliative in our identified population. Adjuvant rt was given to 6 patients (15%). Cisplatin was added to rt in 3 patients, all of whom are still alive (2 with distant failure, 1 free of disease for 7 years since the rt).
The role of adjuvant rt is still unclear. Several retrospective studies have evaluated the benefit of rt after surgery, with conflicting results. The oldest trial found in our search dated to 1974. So far, no randomized trial has tried to answer the question. One of the tertiary centres making a consistent effort to study acc is University Hospital at Michigan University in Ann Arbor, MI, U.S.A. A recent retrospective single-centre study by Else and colleagues5 tried to identify prognostic factors to guide the adjuvant therapy decision in acc. The study identified 391 acc patients treated between January 1979 and December 2013. Median os in the cohort was 35.2 months. Cortisol production, tumour stage, and tumour grade were found to be negative prognostic factors, with the highest hazard ratio for death (4.8) being associated with stage iv disease. Of the patients in that study, 40% and 21% received adjuvant mitotane and rt respectively. Cox regression models that included either adjuvant modality showed that only mitotane was associated with improved rfs (hazard ratio: 0.7; p < 0.05). When both adjuvant modalities were included in the model, a significant interaction was observed (hazard ratio: 0.4; p < 0.05), which might reflect a benefit of adding rt to mitotane. Neither adjuvant modality had any effect on os. The study concluded that, to prolong rfs, adjuvant therapy—particularly with combined mitotane—should be considered. The study found no significant difference in patient characteristics between the group that received adjuvant therapy and the group that did not, but there was a trend (nonsignificant) toward the presence of more adverse features in the group treated with adjuvant therapy.
In another retrospective study from the same centre, 360 acc patients were identified between 1991 and 2011. Of those 360 patients, 20 with localized acc and an R0 or R1 resection received adjuvant rt and were matched with 20 control subjects. There were no statistical differences between the treatment and control groups in terms of patient or tumour characteristics. The hazard ratio for local recurrence was 12.59 (p = 0.0005), but there were no statistically significant differences between the groups in terms of rfs or os26. Table ii summarizes the thirteen retrospective studies found in our search.
In our patient cohort, 6 patients received adjuvant rt, 1 of whom experienced local recurrence that was surgically salvaged, with the patient still being alive 7 years later. Another 2 patients did not experience any failure (1 died of an intracranial bleed, and the other is still alive and disease-free). Of 3 patients who experienced distant failure (bone, liver, lung), 2 are still alive.
Our data are very limited in term of patient numbers and availability of information. We were not able to perform a survival analysis by treatment modality; however, the crude numbers at least suggest better local control with adjuvant rt.
Several groups have started to lay down guidelines for the diagnosis and management of acc so that management plans are consistent and comparable. A clinical practice guideline by esmo, published in 2012, recommends considering adjuvant mitotane for acc patients having R1, Rx, or high-risk features as a Ki-67 index exceeding 10%. For R1 and Rx, adjuvant rt could also be considered8.
The guideline from the Cancer Committee of the French Association of Urology concluded that surgery is the first-line treatment and that adjuvant rt is recommended for stage i or ii R1 resections, concomitant with mitotane within 3 months after surgery, even though sufficient evidence to support that approach is not currently available10.
The evidence and the guidelines are building up in the direction of supporting adjuvant treatment and suggesting that adding rt to mitotane is at least improving local control. However, a randomized trial or at least a large population-based prospective study is still needed. A national or international prospective database would be helpful in overcoming the problem of the rarity of this disease and in trying to develop a consensus concerning the diagnosis and management of acc to ensure consistency in the data collected.
Understandably, our study has the limitations of a retrospective study. Also, treatment and follow-up data were limited and sometimes unavailable. Those data issues limited the analysis. Furthermore, the study had to span a long time period to achieve an acceptable sample size, but that timeline comes with variability in imaging, surgery, and rt techniques, as well as in histopathology reporting and the accuracy of pathology diagnosis techniques. However, based on the available retrospective data and the available guidelines, including the guideline from esmo, adjuvant rt is a reasonable option for R1 and Rx patients. Further research with a larger sample size is warranted.
CONCLUSIONS
The current data are insufficient to make treatment recommendations. A collaborative database and consensus about diagnostic and therapeutic guidelines is warranted for better identification of optimal management. Adjuvant rt could be a reasonable option for R1 and Rx disease, but further research is needed.
ACKNOWLEDGMENTS
The authors thank Ms. Michelle Thomas for her dedicated efforts in helping with data collection.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Fassnacht M, Johanssen S, Quinkler M, et al. on behalf of the German Adrenocortical Carcinoma Registry Group and the European Network for the Study of Adrenal Tumors Limited prognostic value of the 2004 International Union Against Cancer staging classification for adrenocortical carcinoma: proposal for a revised TNM classification. Cancer. 2009;115:243–50. doi: 10.1002/cncr.24030. [DOI] [PubMed] [Google Scholar]
- 2.Kebebew E, Reiff E, Duh QY, Clark OH, McMillan A. Extent of disease at presentation and outcome for adrenocortical carcinoma: have we made progress? World J Surg. 2006;30:872–8. doi: 10.1007/s00268-005-0329-x. [DOI] [PubMed] [Google Scholar]
- 3.Fassnacht M, Hahner S, Polat B, et al. Efficacy of adjuvant radiotherapy of the tumor bed on local recurrence of adrenocortical carcinoma. J Clin Endocrinol Metab. 2006;91:4501–4. doi: 10.1210/jc.2006-1007. [DOI] [PubMed] [Google Scholar]
- 4.Habra MA, Ejaz S, Feng L, et al. A retrospective cohort analysis of the efficacy of adjuvant radiotherapy after primary surgical resection in patients with adrenocortical carcinoma. J Clin Endocrinol Metab. 2013;98:192–7. doi: 10.1210/jc.2012-2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Else T, Williams AR, Sabolch A, Jolly S, Miller BS, Hammer GD. Adjuvant therapies and patient and tumor characteristics associated with survival of adult patients with adrenocortical carcinoma. J Clin Endocrinol Metab. 2014;99:455–61. doi: 10.1210/jc.2013-2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gonzalez RJ, Tamm EP, Ng C, et al. Response to mitotane predicts outcome in patients with recurrent adrenal cortical carcinoma. Surgery. 2007;142:867–75. doi: 10.1016/j.surg.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Barzon L, Fallo F, Sonino N, Daniele O, Boscaro M. Adrenocortical carcinoma: experience in 45 patients. Oncology. 1997;54:490–6. doi: 10.1159/000227608. [DOI] [PubMed] [Google Scholar]
- 8.Berruti A, Baudin E, Gelderblom H, et al. on behalf of the esmo Guidelines Working Group Adrenal cancer: esmo clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(suppl 7):vii131–8. doi: 10.1093/annonc/mds231. [DOI] [PubMed] [Google Scholar]
- 9.Henry JF, Peix JL, Kraimps JL. Positional statement of the European Society of Endocrine Surgeons (eses) on malignant adrenal tumors. Langenbeck Arch Surg. 2012;397:145–6. doi: 10.1007/s00423-011-0893-5. [DOI] [PubMed] [Google Scholar]
- 10.Sebe P, Rigaud J, Avances C, et al. on behalf of les membres de l’afce and les membres du ccafu ccafu’s contribution to the French National Cancer Institute’s reference frame: adrenal malignant tumors [French] Prog Urol. 2013;23(suppl 2):S167–74. doi: 10.1016/S1166-7087(13)70054-X. [DOI] [PubMed] [Google Scholar]
- 11.Zini L, Porpiglia F, Fassnacht M. Contemporary management of adrenocortical carcinoma. Eur Urol. 2011;60:1055–65. doi: 10.1016/j.eururo.2011.07.062. [DOI] [PubMed] [Google Scholar]
- 12.Stigliano A, Chiodini I, Giordano R, et al. Management of adrenocortical carcinoma: a consensus statement of the Italian Society of Endocrinology (sie) J Endocrinol Investig. 2016;39:103–21. doi: 10.1007/s40618-015-0349-9. [DOI] [PubMed] [Google Scholar]
- 13.Fassnacht M, Allolio B. Clinical management of adrenocortical carcinoma. Best Pract Res Clin Endocrinol Metab. 2009;23:273–89. doi: 10.1016/j.beem.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 14.Luton JP, Cerdas S, Billaud L, et al. Clinical features of adrenocortical carcinoma, prognostic factors, and the effect of mitotane therapy. N Engl J Med. 1990;322:1195–201. doi: 10.1056/NEJM199004263221705. [DOI] [PubMed] [Google Scholar]
- 15.Bilimoria KY, Shen WT, Elaraj D, et al. Adrenocortical carcinoma in the United States: treatment utilization and prognostic factors. Cancer. 2008;113:3130–6. doi: 10.1002/cncr.23886. [DOI] [PubMed] [Google Scholar]
- 16.Else T, Kim AC, Sabolch A, et al. Adrenocortical carcinoma. Endocrine Rev. 2014;35:282–326. doi: 10.1210/er.2013-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chompret A, Brugieres L, Ronsin M, et al. P53 germline mutations in childhood cancers and cancer risk for carrier individuals. Br J Cancer. 2000;82:1932–7. doi: 10.1054/bjoc.2000.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsing AW, Nam JM, Co Chien HT, McLaughlin JK, Fraumeni JF., Jr Risk factors for adrenal cancer: an exploratory study. Int J Cancer. 1996;65:432–6. doi: 10.1002/(SICI)1097-0215(19960208)65:4<432::AID-IJC6>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 19.Erdogan I, Deutschbein T, Jurowich C, et al. on behalf of the German Adrenocortical Carcinoma Study Group The role of surgery in the management of recurrent adrenocortical carcinoma. J Clin Endocrinol Metab. 2013;98:181–91. doi: 10.1210/jc.2012-2559. [DOI] [PubMed] [Google Scholar]
- 20.Hermsen IG, Kerkhofs TM, den Butter G, et al. Surgery in adrenocortical carcinoma: importance of national cooperation and centralized surgery. Surgery. 2012;152:50–6. doi: 10.1016/j.surg.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 21.Miller BS, Ammori JB, Gauger PG, Broome JT, Hammer GD, Doherty GM. Laparoscopic resection is inappropriate in patients with known or suspected adrenocortical carcinoma. World J Surg. 2010;34:1380–5. doi: 10.1007/s00268-010-0532-2. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez RJ, Shapiro S, Sarlis N, et al. Laparoscopic resection of adrenal cortical carcinoma: a cautionary note. Surgery. 138:1078–85. doi: 10.1016/j.surg.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 23.Terzolo M, Angeli A, Fassnacht M, et al. Adjuvant mitotane treatment for adrenocortical carcinoma. N Engl J Med. 2007;356:2372–80. doi: 10.1056/NEJMoa063360. [DOI] [PubMed] [Google Scholar]
- 24.Grubbs EG, Callender GG, Xing Y, et al. Recurrence of adrenal cortical carcinoma following resection: surgery alone can achieve results equal to surgery plus mitotane. Ann Surg Oncol. 2010;17:263–70. doi: 10.1245/s10434-009-0716-x. [DOI] [PubMed] [Google Scholar]
- 25.Berruti A, Fassnacht M, Baudin E, et al. Adjuvant therapy in patients with adrenocortical carcinoma: a position of an international panel. J Clin Oncol. 2010;28:e401–2. doi: 10.1200/JCO.2009.27.5958. [DOI] [PubMed] [Google Scholar]
- 26.Sabolch A, Else T, Griffith KA, et al. Adjuvant radiation therapy improves local control after surgical resection in patients with localized adrenocortical carcinoma. Int J Radiat Oncol Biol Phys. 2015;92:252–9. doi: 10.1016/j.ijrobp.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 27.Percarpio B, Knowlton AH. Radiation therapy of adrenal cortical carcinoma. Acta Radiol Ther Phys Biol. 1976;15:288–92. doi: 10.3109/02841867609131965. [DOI] [PubMed] [Google Scholar]
- 28.Nader S, Hickey RC, Sellin RV, Samaan NA. Adrenal cortical carcinoma. A study of 77 cases. Cancer. 1983;52:707–11. doi: 10.1002/1097-0142(19830815)52:4<707::AID-CNCR2820520424>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 29.Markoe AM, Serber W, Micaily B, Brady LW. Radiation therapy for adjunctive treatment of adrenal cortical carcinoma. Am J Clin Oncol. 1991;14:170–4. doi: 10.1097/00000421-199104000-00015. [DOI] [PubMed] [Google Scholar]
- 30.Pommier RF, Brennan MF. An eleven-year experience with adrenocortical carcinoma. Surgery. 1992;112:963–70. [PubMed] [Google Scholar]
- 31.Crucitti F, Bellantone R, Ferrante A, Boscherini M, Crucitti P. The Italian Registry for Adrenal Cortical Carcinoma: analysis of a multiinstitutional series of 129 patients. The acc Italian Registry Study Group. Surgery. 1996;119:161–70. doi: 10.1016/S0039-6060(96)80164-4. [DOI] [PubMed] [Google Scholar]
- 32.Jacob R, Madhavan J, Jyothirmayi R, Nair MK. Adrenocortical carcinoma: is adjuvant therapy indicated? A single institution experience. Int J Clin Oncol. 2000;5:104–8. doi: 10.1007/s101470050099. [DOI] [Google Scholar]
- 33.Hermsen IG, Groenen YE, Dercksen MW, Theuws J, Haak HR. Response to radiation therapy in adrenocortical carcinoma. J Endocrinol Investig. 2010;33:712–14. doi: 10.1007/BF03346675. [DOI] [PubMed] [Google Scholar]
- 34.Sabolch A, Feng M, Griffith K, Hammer G, Doherty G, Ben-Josef E. Adjuvant and definitive radiotherapy for adrenocortical carcinoma. Int J Radiat Oncol Biol Phys. 2011;80:1477–84. doi: 10.1016/j.ijrobp.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 35.Ho J, Turkbey B, Edgerly M, et al. Role of radiotherapy in adrenocortical carcinoma. Cancer J. 2013;19:288–94. doi: 10.1097/PPO.0b013e31829e3221. [DOI] [PMC free article] [PubMed] [Google Scholar]