Abstract
Background
Prostate diseases are common in males worldwide with high morbidity. Gene therapy is an attractive therapeutic strategy for prostate diseases, however, it is currently underdeveloped. As well known, adeno virus (Ad) is the most widely used gene therapy vector. The aims of this study are to explore transduction efficiency of Ad in prostate cancer cells and normal prostate tissue, thus further providing guidance for future prostate pathophysiological studies and therapeutic development of prostate diseases.
Methods
We produced Ad expressing enhanced green fluorescence protein (EGFP), and characterized the transduction efficiency of Ad in both human and mouse prostate cancer cell lines in vitro, as well as prostate tumor xenograft and wild-type mouse prostate tissue in vivo. Ad transduction efficiency was determined by EGFP fluorescence using microscopy and flow cytometry. Cell type-specific transduction was examined by immunofluorescence staining of cell markers.
Results
Our data showed that Ad efficiently transduced human and mouse prostate cancer cells in vitro in a dose dependent manner. Following intratumoral and intraprostate injection, Ad could efficiently transduce prostate tumor xenograft and the major prostatic cell types in vivo, respectively.
Conclusions
Our findings suggest that Ad can efficiently transduce prostate tumor cells in vitro as well as xenograft and normal prostate tissue in vivo, and further indicate that Ad could be a potentially powerful toolbox for future gene therapy of prostate diseases.
Keywords: Adeno virus, Gene delivery vector, Gene therapy, Prostate cancer, Prostate diseases
Introduction
Prostate is an exocrine gland that is crucial to constituting the male reproductive system, and the functions of prostate are similar in the majority of mammals despite anatomical differences1. Three types of prostate diseases are the major threats for the health of prostate, i.e. prostatitis, benign prostate hyperplasia (BPH) and prostate cancer. Together, these prostate diseases are severely compromising the life quality and life span of males, especially for the aged male population. For example, BPH is one of the top ten most costly diseases among male populations over 50-year old in the USA2, and prostate cancer is the second most diagnosed malignancy and the fifth leading cause for mortality of all cancers in males worldwide3, 4.
To date, many efforts have been made to prevent or to treat prostate diseases, including surgery5, medication6, and radiotherapy7. Nevertheless, highly effective clinical interventions for a variety of prostate diseases are still lacking. For example, although the early stage of prostate cancer can be prevented with hormonal therapy, most hormone-dependent prostate cancers will eventually develop into castration-resistant prostate cancer (CRPC). So far, no effective treatment exists for CRPC. As the genetic basis of prostate diseases was gradually unraveled during the past decades, gene therapy was explored as a therapeutic strategy for prostate diseases, and researchers have demonstrated the feasibility of several gene therapy approaches to treating BPH and prostate cancer in mice using various types of viral gene delivery vectors8.
Adenovirus (Ad) is a medium-sized and nonenveloped virus containing a double-stranded DNA genome, and it was originally isolated from human adenoids9. At present, Ad is the most commonly used viral vectors for gene delivery for many advantages, including large cargo capacity, high efficiency of transduction and high levels of gene expression10.
In this study, we for the first time evaluated the transduction efficiency of Ad in human and mouse prostate cancer cell lines in vitro, as well as in prostate tumor xenograft and mouse prostate gland in vivo following intratumoral and intraprostate injection, respectively, thus opening new avenues for preclinical and clinical development of Ad-based gene therapy for the treatment of prostate diseases.
Materials and methods
Production of Ad vectors
Firstly, enhanced green fluorescence protein (EGFP) driven by chicken beta-actin promoter was cloned into a separate Ad backbone plasmid. E1/E3-deleted Ad type 5 vectors were produced by University of Massachusetts Medical School Viral Vector Core.
Cell culture and viral infection
PC3 cells (cat # CRL-1435) and TRAMP C2 cells (cat # CRL-2731) were purchased from ATCC (Manassas VA, USA), and cultured according to the instructions. Cells were seeded in 24-well plates at a density of 1×104 cells per well. The next day cells were infected with Ad at indicated multiplicity of infection (MOI) from 1 to 105. Two days later, EGFP intensity was measured using a fluorescence plate reader (Synergy HT, BioTek, Winooski VT, USA), and further analyzed by flow cytometry as described below.
Flow cytometry
In total, 10,000 cells from each well were analyzed for EGFP fluorescence signal on a flow cytometer (Guava EasyCyte HT, EMD Millipore, Billerica, MA, USA). Naïve cells were used for gating control. Data were analyzed by the Flowjo software (FLOWJO, Ashland, OR, USA).
Intratumoral injection and Intraprostate injection
C57BL/6 male mice obtained by in-house breeding were used throughout the study. For intratumoral injection, 3 × 106 TRAMP cells in 100 μL of phosphate buffered saline (PBS) were injected into the right flank of C57BL/6 male mice. Six weeks post tumor cell injection, approximately 100 mm3 of tumor were injected with 3.2 × 1010 particles of Ad in 50 μL of PBS. Tumors were harvested 3 days post injection. For intraprostate injection, mice were anesthetized with isoflurane, and the abdominal cavity was opened to expose the prostate gland. Then 3.2×1010 GC of Ad vector diluted in 10 μL of sterile PBS were injected into each of two sites of the anterior prostate (AP) and two sites of the dorsal lateral prostate (DLP) (Supplementary Fig. 1). Following intraprostate injection, prostates were harvested 3 days post injection under a dissection microscope. All animal study protocols were approved by the University of Massachusetts Medical School Institutional Animal Care and Use Committee.
Immunofluorescence staining
Mouse prostates were fixed in 10% buffered formalin overnight at 4°C, and then sequentially dehydrated in 10%, 20% and 30% of sucrose each overnight at 4°C. The samples were embedded in the optimal cutting temperature (O.C.T.) compound (Sakura Finetek, Torrance CA, USA), and stored at −80°C. Eight micron-thick cryo-sections were permeabilized and blocked with 5% bovine serum albumin (BSA) and 1% Triton X-100 in 1× PBS for two hours at 37°C. The sections were incubated with primary antibodies against K8 (1:1000 diluted, cat # 904801, BioLegend, San Diego CA, USA), K5 (1:1000 diluted, cat # 905501, BioLegend), and α-actin (1:100 diluted, cat # ab5694, Abcam, Cambridge MA, USA) overnight at 4°C, and further incubated with secondary antibodies (Life Technologies) for 1 hour at RT in dark. Finally, the sections were mounted with the VECTASHIELD mounting medium containing DAPI (Vector Laboratories, Burlingame CA, USA).
Ad genome copy number and RNA quantification
Tissues were harvested at RT, and stored in RNAlater solution (Life Tech, Carlsbad, CA, USA). Total DNA and RNA were extracted using the AllPrep DNA/RNA Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Multiplexed droplet digital PCR (ddPCR) was performed by QX200 ddPCR system (Bio-rad) using Taqman primers and probes targeting EGFP (Mr00660654_cn, Life Tech, Carlsbad, CA, USA) and the reference gene transferrin receptor (Tfrc) (4458367, Life Tech, Carlsbad, CA, USA). Ad genome copy numbers per diploid genome were calculated through dividing EGFP transgene copy numbers by two times of Tfrc gene copies. Next, approximately 1 μg of total RNA was reverse transcribed into cDNA using a high-capacity cDNA reverse transcription Kit (4374967, Waltham, MA USA), and then subjected to multiplexed ddPCR using Taqman primers and probes targeting EGFP and glyceraldehyde 3-phosphate dehydrogenase (Gapdh) (4352339E, Life Tech, Carlsbad, CA, USA). The quantity of EGFP mRNA was normalized to Gapdh.
Results
Ad efficiently transduce human and mouse prostate cancer cells in vitro
To evaluate the transduction efficiency of Ad in prostate cancer cells in vitro, we infected PC3 and TRAMP-C2 cells, a human and mouse prostate cancer cell line, respectively, with indicated MOI of EGFP-expressing Ad. One day post-infection, we quantitatively profiled transduction efficiency of Ad by measuring the overall EGFP fluorescence intensity (Fig. 1A, B) and the percentage of EGFP-positive cells (Fig. 1C), respectively. The data showed that Ad transduced TRAMP-C2 cells consistently better than PC3 cells with MOI ranging from 1 to 105 (Fig. 1B, C). Taken together, Ad could efficiently transducing human and mouse prostate cancer cells in vitro in a dose-dependent manner.
Fig. 1. Ad efficiently transduces human and mouse prostate cancer cells in vitro.
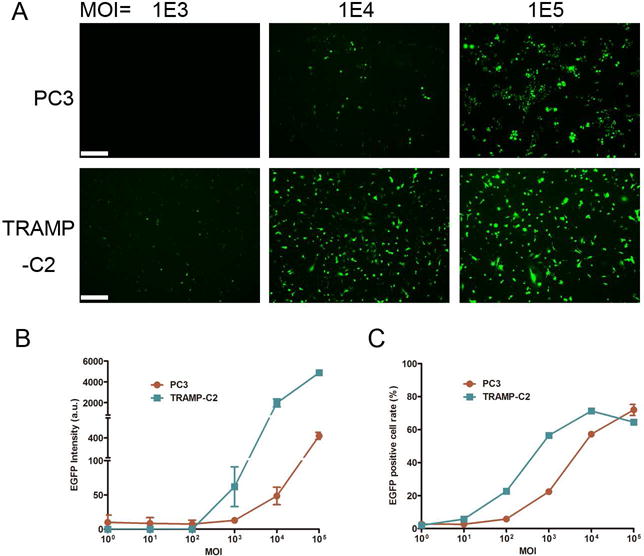
(A) Representative microscopic images showing EGFP fluorescence signal of PC3 and TRAMP-C2 cells infected with indicated MOI of EGFP-expressing Ad vectors. Images were obtained 24 hours post infection. (B) Quantification of EGFP intensity presented in arbitrary unit (a.u.). (C) Percentage of EGFP-positive cells analyzed by flow cytometry.
Ad effectively transduce prostate tumor xenograft in vivo
To further investigate the transduction efficiency of Ad in prostate tumor cells in vivo, we developed a mouse model bearing prostate tumor xenograft. Xenografts were generated by injecting mouse TRAMP-C2 cells into the right flanks of recipient mice. Tumors were harvested 3 days post injection. As shown in Fig. 2A and B, Ad can efficiently transduce xenograft tumor with approximately 0.2 genome copy per diploid genome, and the mRNA expression level of EGFP was also determined using ddPCR (Fig. 2C). These findings suggested that Ad could be used to effectively deliver genes of interest to prostate tumor in vivo.
Fig. 2. Ad efficiently transduces prostate tumor xenograft in vivo.
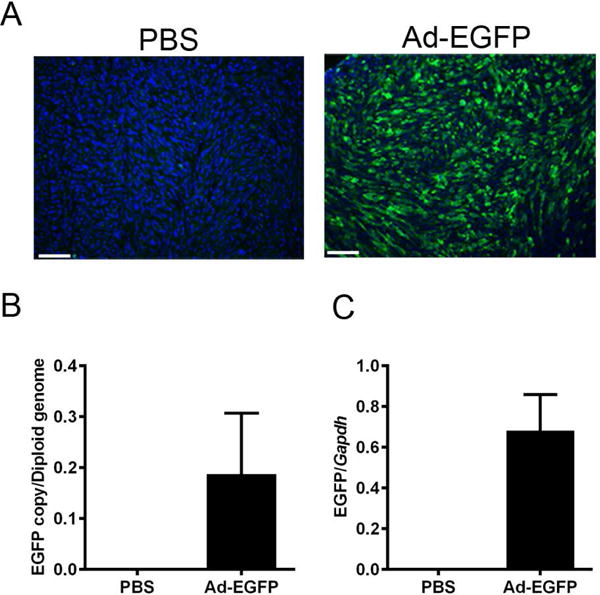
(A) Representative microscopic images showing EGFP fluorescence signal in prostate tumor xenograft. (B) Ad genome copy number and (C) EGFP mRNA level in prostate tumor xenograft after intratumoral injection.
Ad efficiently transduce mouse prostate in vivo following intraprostate injection
To study the transduction efficiency of Ad in normal prostate tissue, we directly injected Ad vectors into mouse prostate (Suppl. Fig. 1). The mouse prostate is divided into two lobes of anterior prostate (AP) and dorsal lateral prostate (DLP) (Suppl. Fig. 1), thus we injected Ad vectors into four sites per prostate, namely the two lobes of AP and two sites of DLP (Suppl. Fig. 1). Prostates were harvested 3 days post injection, AP and DLP cryo-sections were subjected to fluorescence microscopy. We found that Ad could efficiently transduce AP and DLP (Fig. 3A). Moreover, the genome copy number and the EGFP mRNA levels were determined by ddPCR (Fig. 3B and C). These results suggested that Ad could be an efficient viral tool for transient gene delivery to mouse prostate in vivo.
Fig. 3. Ad efficiently transduces mouse AP and DLP following intraprostate injection.
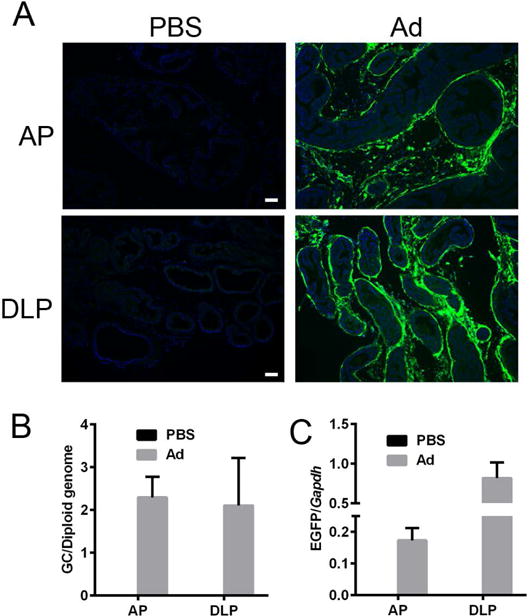
(A) Representative fluorescence images of anterior prostate (AP) and dorsal lateral prostate (DLP) cryo-sections showing the EGFP native fluorescence following injections of PBS or Ad. (B) Biodistribution of Ad genomes and (C) EGFP mRNA level in AP and DLP following intraprostate injection of Ad. Genome copy data are presented in Ad genome copies per diploid genome, and the EGFP mRNA level was normalized by Gapdh.
Ad efficiently transduce mouse prostate cells in vivo
To further characterize the prostatic cell types that were transduced with Ad, we performed immunofluorescence staining of mouse AP and DLP sections with antibodies against cellular markers of major prostate cell types including luminal cells (K8), basal cells (K5) and stromal cells (α-actin for smooth muscle cells). We found that Ad was able to transduce the majority of the three cell types in both AP and DLP. Representative fluorescence microscopic images are shown in Fig. 4. These findings indicated that mouse prostate cells could be comprehensively transduced by Ad in vivo.
Fig. 4. Ad transduces the majority of major prostatic cell types following intraprostate injection.
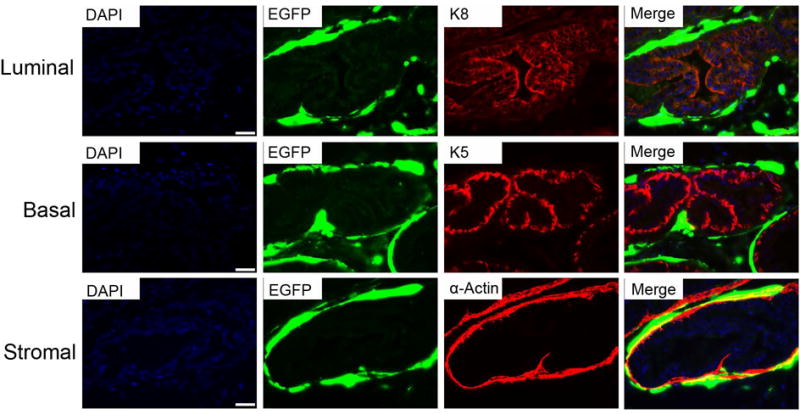
Representative images of immunofluorescence staining of prostate luminal cells (top panel), basal cells (middle panel) and stromal cells (bottom panel), marked by K8, K5 and α-actin staining, respectively.
Discussion
As the most commonly used viral vector, the advantages of Ad, including large cargo capacity, high efficiency of transduction and high levels of gene expression, allowing efficient gene delivery to a broad range of organs in model animals and human10, 11. However, despite the fact that prostate diseases affect a large population of men worldwide, gene therapy for this group of diseases is much underdeveloped. To bridge the knowledge gap regarding vector choice for future studies involving gene delivery to the prostate gland, here we evaluated transduction capability of Ad for both cancerous prostate cells in vitro/in vivo and normal prostate tissue in vivo.
Prostate cancer is the most devastating prostate disease. Researchers previously used Ad for gene delivery into prostate cancer xenograft in mice for mechanistic studies and therapeutic purposes with some success12, 13. Utilizing quantitative measurements of EGFP reporter fluorescence, we demonstrated that Ad can efficiently transduce both human and mouse prostate cancer cells in a dose dependent manner. It should be noted that the in vitro transduction efficiency might be extrapolated to in vivo transduction with caution. Nevertheless, the consistent results obtained from two prostate cancer cell lines are encouraging, and our results suggest that Ad can potentially enhance intra-prostate cancer gene delivery in future studies.
In addition to cancerous prostate cells, gene delivery to non-cancerous prostate tissue in vivo is also highly relevant to pathophysiological studies and therapeutic development. We found that intraprostate injection of Ad vectors could efficiently transduce normal mouse prostate tissue in vivo. Particularly, Ad transduced both AP and DLP efficiently14, 15, and Ad also markedly transduced majority of prostate stromal cells, a cell type that is primarily involved in BPH16. However, the transduction efficacies of Ad vectors in luminal and basal cells were faint. Presumably, the large size (90–100 nm) of adenoviral particles cannot efficiently pass through the barrier of stromal cells. Overall, these findings highlighted the potential application of this gene delivery method.
Conclusion
In sum, we for the first time carried out an extensive characterization of Ad that could efficiently transduce prostate cancer cells in vitro/in vivo and normal prostate tissue in vivo. These findings established a critical foundation for further development of Ad-based gene therapy strategies for treating a wide spectrum of prostate diseases, and also provided a powerful in vitro and in vivo research tool for studying prostate diseases.
Supplementary Material
3.2×1010 particles per injection site of Ad vectors were delivered into four sites as indicated by the syringes, namely the two lobes of AP and two sites in DLP. AP: anterior prostate. DLP: dorsal lateral prostate. SV: seminal vesicle.
Acknowledgments
This work was supported by Public Health Service grants 1R01NS076991-01, P01 HL59407-11, P01AI100263-01 from National Institutes of Health, an internal grant from University of Massachusetts Medical School, and a grant from the National High Technology Research and Development Program (“863” Program) of China (2012AA020810) to G.G..
Footnotes
Conflict of interest
G.G. is a co-founder of Voyager Therapeutics specialized in rAAV-based gene therapy, and holds equity in the company. G.G. is an inventor on patents with potential royalties licensed to Voyager Therapeutics and other biopharmaceutical companies.
References
- 1.Lee CH, Akin-Olugbade O, Kirschenbaum A. Overview of prostate anatomy, histology, and pathology. Endocrinol Metab Clin North Am. 2011;40:565. doi: 10.1016/j.ecl.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 2.Fenter TC, Naslund MJ, Shah MB, et al. The cost of treating the 10 most prevalent diseases in men 50 years of age or older. Am J Manag Care. 2006;12:S90. [PubMed] [Google Scholar]
- 3.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 4.Wong MC, Goggins WB, Wang HH, et al. Global Incidence and Mortality for Prostate Cancer: Analysis of Temporal Patterns and Trends in 36 Countries. Eur Urol. 2016;70:862. doi: 10.1016/j.eururo.2016.05.043. [DOI] [PubMed] [Google Scholar]
- 5.Gilling P. TURP remains a safe and effective alternative for benign prostatic hyperplasia (BPH) surgery. BJU Int. 2014;113:5. doi: 10.1111/bju.12310. [DOI] [PubMed] [Google Scholar]
- 6.Tannock IF, Fizazi K, Ivanov S, et al. Aflibercept versus placebo in combination with docetaxel and prednisone for treatment of men with metastatic castration-resistant prostate cancer (VENICE): a phase 3, double-blind randomised trial. Lancet Oncol. 2013;14:760. doi: 10.1016/S1470-2045(13)70184-0. [DOI] [PubMed] [Google Scholar]
- 7.Zelefsky MJ, Pei X, Chou JF, et al. Dose escalation for prostate cancer radiotherapy: predictors of long-term biochemical tumor control and distant metastases-free survival outcomes. Eur Urol. 2011;60:1133. doi: 10.1016/j.eururo.2011.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okegawa T, Li Y, Pong RC, et al. The dual impact of coxsackie and adenovirus receptor expression on human prostate cancer gene therapy. Cancer Res. 2000;60:5031. [PubMed] [Google Scholar]
- 9.Rowe WP, Huebner RJ, Gilmore LK, et al. Isolation of a cytopathogenic agent from human adenoids undergoing spontaneous degeneration in tissue culture. Proc Soc Exp Biol Med. 1953;84:570. doi: 10.3181/00379727-84-20714. [DOI] [PubMed] [Google Scholar]
- 10.Ginn SL, Alexander IE, Edelstein ML, et al. Gene therapy clinical trials worldwide to 2012 - an update. J Gene Med. 2013;15:65. doi: 10.1002/jgm.2698. [DOI] [PubMed] [Google Scholar]
- 11.Havenga MJ, Lemckert AA, Ophorst OJ, et al. Exploiting the natural diversity in adenovirus tropism for therapy and prevention of disease. J Virol. 2002;76:4612. doi: 10.1128/JVI.76.9.4612-4620.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu W, Neill T, Yang Y, et al. The systemic delivery of an oncolytic adenovirus expressing decorin inhibits bone metastasis in a mouse model of human prostate cancer. Gene Ther. 2015;22:247. doi: 10.1038/gt.2014.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freytag SO, Barton KN, Zhang Y. Efficacy of oncolytic adenovirus expressing suicide genes and interleukin-12 in preclinical model of prostate cancer. Gene Ther. 2013;20:1131. doi: 10.1038/gt.2013.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldstein AS, Huang J, Guo C, et al. Identification of a cell of origin for human prostate cancer. Science. 2010;329:568. doi: 10.1126/science.1189992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X, Kruithof-de Julio M, Economides KD, et al. A luminal epithelial stem cell that is a cell of origin for prostate cancer. Nature. 2009;461:495. doi: 10.1038/nature08361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wasserman NF. Benign prostatic hyperplasia: a review and ultrasound classification. Radiol Clin North Am. 2006;44:689. doi: 10.1016/j.rcl.2006.07.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
3.2×1010 particles per injection site of Ad vectors were delivered into four sites as indicated by the syringes, namely the two lobes of AP and two sites in DLP. AP: anterior prostate. DLP: dorsal lateral prostate. SV: seminal vesicle.


