Abstract
Cannabidiol (CBD), the main nonpsychoactive constituent of Cannabis sativa, has shown a wide range of therapeutically promising pharmacological effects either as a sole drug or in combination with other drugs in adjunctive therapy. However, the targets involved in the therapeutic effects of CBD appear to be elusive. Furthermore, scarce information is available on the biological activity of its human metabolites which, when formed in pharmacologically relevant concentration, might contribute to or even account for the observed therapeutic effects. The present overview summarizes our current knowledge on the pharmacokinetics and metabolic fate of CBD in humans, reviews studies on the biological activity of CBD metabolites either in vitro or in vivo, and discusses relevant drug–drug interactions. To facilitate further research in the area, the reported syntheses of CBD metabolites are also catalogued.
Key words: : biological activity, cannabidiol, metabolites, pharmacokinetics, synthesis
Introduction
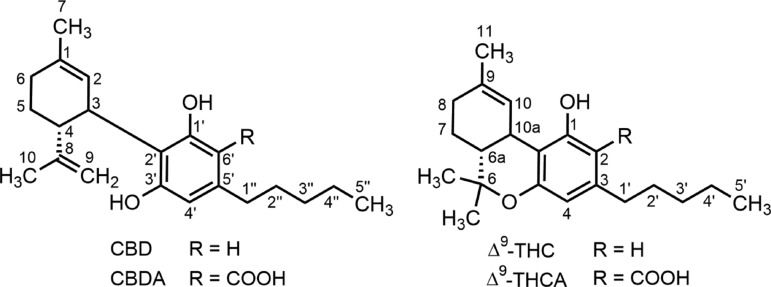
Cannabidiol (CBD; Fig. 1) is one of the chemically and phytogenetically related phenolic terpenes derived from hemp (Cannabis sativa L). It was first obtained in pure form in 1940 simultaneously from fiber-type American hemp1 and from psychotropic Egyptian hashish.2 The chemical structure of CBD was determined by Mechoulam and Shvo in 1963.3 CBD is one of the 142 phytocannabinoids that have been isolated so far from hemp.4 Strictly speaking, however, CBD is an artifact: the genuine natural product is cannabidiolic acid (CBDA; Fig. 1), which under the influence of heat is decarboxylated into CBD in the plant material. Likewise, another major phenolic terpene of hemp, Δ9-tetrahydrocannabinol (THC)5 is formed from the corresponding carboxylic acid (THCA; Fig. 1).
FIG. 1.
Chemical structures and numbering system for CBD and Δ9- THC type cannabinoids. CBD, cannabidiol; THC, tetrahydrocannabinol.
Although CBD was isolated and characterized first, THC has been investigated more thoroughly: THC is responsible for the unique psychoactivity of marijuana, or cannabis, which is an internationally controlled substance, nevertheless widely used for recreational purposes or, more recently, for self-medication.6 Synthetic THC has been available for three decades as a medicine, and pharmaceutical-grade herbal cannabis, as well as formulations of cannabis extracts containing THC and CBD in well-defined ratios, has also been registered as medicines in several countries (see chapters of Part 3 of Pertwee6). Due to its unique psychoactivity and therapeutic potential, both associated with the activation of cannabinoid (CB) receptors, as well as for forensic reasons, the pharmacokinetics and pharmacodynamics of THC is much better understood than those of the nonpsychoactive CBD, which for decades has been a neglected phytocannabinoid.
The chemistry and pharmacology of CBD, as well as the various molecular targets, including CB receptors and other components of the endocannabinoid system it interacts with, have adequately been reviewed,7–11 while the pharmacology of CBD analogs, with emphasis on anti-inflammatory effects, was the subject of a recent overview.12
In the recent decade, preclinical studies, human case reports, and a plethora of anecdotal accounts, recognizing the relative safety of CBD, have prompted the exploration of the therapeutic potential of CBD against a range of diseases.13–18 In particular, the promise of CBD in treating cancer and drug-resistant epilepsy in children has recently brought this natural product into the focus of the scientific community, clinicians, the media, as well as politicians and regulatory agencies.19–24 Consequently, the US Food and Drug Administration and the European Medicines Agency have granted CBD preparations the “Orphan Drug” designation for use in the treatment of epilepsy in children (Dravet and Lennox-Gastaut syndromes) and neonatal asphyxia, and clinical trials sponsored by GW Pharma Ltd. have been started in these indication areas.25,25a,25b
While some information on the pharmacokinetics of CBD in experimental animals and humans is available,26–29 the biological activity of CBD metabolites has received scant attention.30 The purpose of this review is to summarize our current knowledge of the human pharmacokinetics of CBD with particular emphasis on the biological properties of established or putative human metabolites of CBD. We also indicate several gaps in our knowledge on CBD metabolites, which should be filled by further research that aims to expand the therapeutic use of CBD-based medications. Forensic studies reporting on CB levels as detected in the urine, blood, or saliva of smokers of cannabis cigarettes or of users of various medicinal cannabis preparations have been excluded (for recent reviews, see Huestis28 and Huestis and Smith29). To facilitate further research in the area, the synthetic routes reported for CBD metabolites and their close structural analogs are also catalogued.
Human Pharmacokinetics of CBD Upon Various Administration Routes
Extensive studies in animals, including rodents and the dog, indicate that a large portion of the administered CBD is excreted intact or as its glucuronide.26,27 Due to extensive Phase I metabolism, the pharmacokinetics of CBD is complex and the bioavailability of oral CBD is low across species.26–29 In general, the most abundant metabolites are hydroxylated 7-COOH derivatives of CBD (Fig. 2) that are excreted either intact or as glucuronide conjugates. The route of administration affects the pharmacokinetics of CBD and high intra- and intersubject variability is common in humans as the following paragraphs demonstrate.
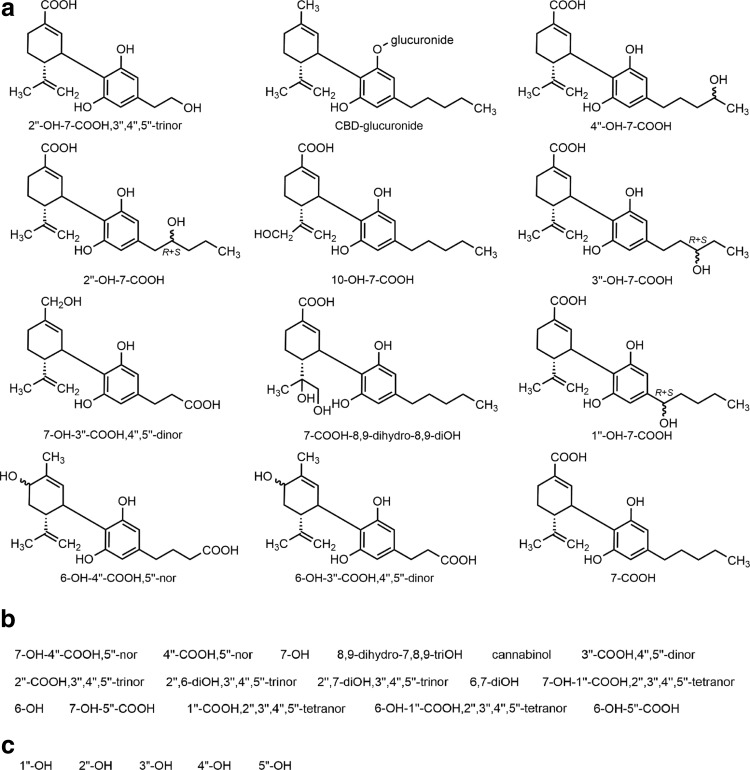
FIG. 2.
(a) Chemical structures of major (>1%) metabolites of CBD identified by GC-MS in anhydrolyzed urine of a dystonic patient treated chronically with the drug.59 The compounds are listed according to their relative amounts (decreasing from upper left to lower right) based on peak areas of the gas chromatogram. Additional major metabolite was Δ8-THC (1.97%; structure not shown). In the structural diagrams, wavy bonds indicate stereogenic center with R+S indicating that the two epimers were chromatographically separated; otherwise, stereochemistry is unknown. (b) List of minor (<1%) urinary metabolites present in the urine of the same patient; additional excreted compound present was Δ9-THC (0.69%), which has also been identified in artificial gastric juice.128 For numbering, see Figure 1. The chemical structures of the minor metabolites are given in Supplementary Figure S1. (c) CBD metabolites identified in human liver microsomal preparation.60 Their chemical structures are given in Supplementary Figure S1. For convenience, the shorthand notation adopted here indicates only the newly formed functional group(s) present in the metabolite of CBD.
In five individuals each smoking a single cigarette containing ∼19 mg [2H]CBD, the average peak blood plasma level of CBD was 110 ng/mL (range: 42–191 ng/mL) recorded at 3 min postdose; the mean half-life was 31±4 h and the average systemic availability was 31% (range: 11–45%).31
In an early study with healthy volunteers who were given 20 mg [3H]CBD by intravenous (i.v.) injection, 7-COOH-CBD was the most abundant metabolite in the plasma, while 7-OH-CBD was only a minor biotransformation product (in the original publication, the compounds are referred to as 11-carboxy-CBD and 11-hydroxy-CBD, respectively).32 In the urine, unchanged CBD and, to a lesser extent, conjugated CBD were the main excretion products and about 16% of the total radioactivity was eliminated in 72 h by this route of excretion. It was also observed that 33% of the total radioactivity, again mostly unchanged CBD accompanied by several oxygenated metabolites, including mono- and dihydroxylated and monocarboxylic derivatives of CBD, was excreted in the feces within 72 h. In a subsequent and more detailed investigation, five young marijuana smokers were given 20 mg [2H]CBD by i.v. injection.31 At 3 min following drug administration, the CBD plasma levels peaked at 686 ng/mL (range: 356–962 ng/mL), which rapidly dropped to 48 ng/mL (range: 37–61 ng/mL) after 1 h; the mean half-life was 24±6 h.
In 12 subjects, oral administration of chocolate cookies spiked with a blend of 40 mg CBD+20 mg THC resulted in low peak plasma levels of ∼5 ng/mL for each drug at 1.5–3 h.33 Similar low peak plasma levels with a mean of 0.93 ng/mL (range: 0.3–2.6 ng/mL) were noted in 24 volunteers 1 h after oral ingestion of gelatin capsules with cannabis extract containing 5.4 mg CBD+10 mg THC.34 Interesting results were obtained from experiments in which capsules filled with either unheated or heated cannabis extracts containing 10 mg THCtotal (THC+THCA) and 10–15 mg CBDtotal (CBD+CBDA) estimated when fresh: pharmacokinetic analysis of the blood of patients ingesting two such capsules showed mean peak plasma CBD concentrations four times higher in the unheated extract than in the heat-treated extract (1.24 ng/mL at 1.17 h vs. 0.30 ng/mL at 0.83 h, respectively).35 The results suggest that the use of unheated cannabis extract rich in acidic phytocannabinoids may beneficially affect the uptake and metabolism of CBD or other phytocannabinoids.
In another investigation, repeated oral administration of daily doses of 700 mg of CBD to 14 Huntington's disease patients did not result in elevated mean blood concentrations; in a 6-week trial, plasma levels of the drug remained in a relatively constant but low range of 5.9–11.2 ng/mL throughout the trial, averaged 1.5 ng/mL 1 week after CBD administration was discontinued and virtually undetectable by gas chromatography coupled with mass spectrometry (GC-MS) thereafter; the elimination half-life of CBD ranged from 2 to 5 days.36,37 In this study, CBD was found to be neither symptomatically beneficial nor toxic. During a functional magnetic resonance imaging investigation of the effects of THC and CBD on regional brain function in 15 healthy volunteers, respective mean blood concentrations of 4.7±7 and 17±29 ng/mL of CBD were recorded at 1 and 2 h following a single oral dose of 600 mg CBD.38
A recent study examined the safety and pharmacokinetics of 400 and 800 mg of CBD coadministered with various doses of the potent opioid analgesic fentanyl to 17 healthy individuals.39 In a representative session, 3 h after the oral administration of 800 mg of CBD and 2 h after fentanyl injection (0.5 μg/kg i.v.), the highest plasma concentration of CBD was 221±36 ng/mL; the mean peak urinary CBD concentration was recorded at 4 h after CBD intake and estimated to be 3.7 ng/mL.
As a part of a series of trials with “Cannabis Based Medicine Extracts” such as Sativex®, the pharmacokinetics of a total dose of 20 mg CBD in sublingual drops was studied in six healthy subjects.40 In a representative experiment, the mean of the peak plasma concentration of CBD was 2 ng/mL at 130 min postdose. Similar values were obtained for a mixture of 20 mg THC+20 mg CBD applied either in sublingual drops or as aerosol; when applied through a nebulizer (10 mg THC+10 mg CBD), however, the peak plasma level was 9.5 ng/mL at 36 min after administration and the half-life of CBD in plasma was 66 min.
In a separate study with nine cannabis smokers, oromucosal application of low (5.4 mg THC+5.0 mg CBD) and high (16.2 mg THC+15.0 mg CBD) Sativex doses resulted in median peak plasma CBD concentrations of 1.2 ng/mL (range: 0.6–3.9 ng/mL) at 3.6 h (range: 1.0–5.5 h) postdose and 3.7 ng/mL (range: 2.0–20.5 ng/mL) at 4.5 h (range: 1.2–5.6 h) postdose, respectively41 (see also Stott et al.42).
The human skin permeation of CBD solutions was investigated in vitro and CBD concentrations as high as 6.1 mg per gram skin preparation could be achieved under certain experimental conditions.43 Various CBD formulations for transdermal and intranasal delivery have also been studied in rodent models.44,45
While first-pass metabolism could be avoided by rectal administration of suppository formulation of CBs, as it has been demonstrated for THC,46,47 relevant studies with CBD appear to be lacking.
Finally, an analysis of in vivo distribution of CBs in five postmortem cases indicated relatively high CBD concentrations in bile (up to 63 ng/mL) and muscle (up to 32 ng/g) tissues, and it was noted that the CBD content of the brain was unexpectedly high (up to 6.7 ng/g)48 (see also Fabritius et al.49). In these cases, however, factors influencing CB pharmacokinetics were unknown.
No information is available for tissue distribution of CBD or its metabolites in living humans and relevant animal studies are scarce. In rats, analysis of blood and brain 21.5 h after intragastric administration of 23.4 mg/kg of [3H]CBD in olive oil solution showed respective tissue concentrations of unchanged [3H]CBD of 20.2 ng/mL and 6.4 ng/g; the hepatic concentration of the drug was higher throughout the experiment and 20.8 ng/g was recorded even 84 h after treatment.50 In another test series also with rats, analysis of brain parts 5 min after administration of [3H]CBD (1 mg/kg i.v.) revealed an even distribution of the radiolabel (CBD+its unspecified metabolites) at about 1 ng/mg as initial peak concentrations throughout all brain regions examined.51
A recent study compared plasma and brain levels of CBD after oral or intraperitoneal (i.p.) administrations of the drug in Cremophor at 120 mg/kg to rats and mice.52 Following i.p. administration, mice had a higher CBD plasma level than rats (14.3 and 2.6 μg/mL, respectively), whereas oral dosing resulted in a similar peak plasma concentration in both species (∼2 μg/mL). Oral administration offered six times higher brain peak CBD concentrations in rats than in mice (8.6 vs. 1.3 μg/g). It was also noted that oral administration of CBD (120 mg/kg) dissolved in the micelle-forming Solutol resulted in enhanced absorption of the drug compared to the solution based on the emulsion-forming surfactant Cremophor as evidenced by higher peak concentrations and prolonged exposures in blood (3.2 μg/mL at 6 h and 2 μg/mL at 2 h, respectively) and brain (12.6 μg/mL at 4 h and 8.6 μg/mL at 4 h, respectively). The effects of cosolvents and excipients on pharmacokinetics, involving cytochrome P450 (CYP450) oxidases and P-glycoprotein efflux transporters, of lipophilic substances in general have been extensively investigated.53,54
It must be noted that none of the above studies reported on the metabolic fate of CBD and no information is available on the human pharmacokinetics of the metabolites. (For an early mouse study indicating slow elimination of unidentified polar metabolites, see Karler et al.55).
Human Metabolites of CBD
The first demonstration of CB biotransformation in humans appears to be the study of Christiansen and Rafaelsen56 who in 1969 reported the separation by thin layer chromatography several polar CB derivatives from the urine of 10 volunteers drinking cannabis “tea”; however, no attempts were made to identify the substances. Subsequently, the use of sophisticated analytical techniques, especially GC-MS and, occasionally, reliance on synthetic standards for structure confirmation allowed the unequivocal identification of CB metabolites in humans (see below).
The first CBD metabolites to be identified were isolated from rat liver homogenate and their structures were determined as a primary alcohol derived from the oxidation at the allylic C-7 methyl group on the cyclohexene moiety (7-OH-CBD) and a secondary alcohol resulting from the oxidation of the central (C-3′′) methylene group of the pentyl side chain (3′′-OH-CBD; for numbering, see Fig. 1) in 1973.57,57a Since then, biotransformation studies in mammals, including humans, using various types of CBD administration have indicated considerable species variability.26,58,59 All studies in vivo appear to have been restricted to the characterization of urinary CBD metabolites.
Being a good substrate of CYP450 mixed function oxidases, CBD undergoes extensive hydroxylation at multiple sites and further oxidations result in a complex metabolic pattern; altogether, some 100 CBD metabolites have been identified from various organisms.26 In general, the major metabolites of CBD were derivatives of CBD-7-oic acid (7-COOH-CBD) further oxidized at the side chain (Fig. 2a, b).
Following initial excretion studies,32,58 about 40 oxygenated human Phase I metabolites have been characterized.26,58–60 The urinary excretion profile of CBD metabolism has been reported only for a single case, which involved a dystonic patient chronically treated with 600 mg daily oral doses of CBD.58,59 The characterization of the metabolites relied on GC-MS analysis using an array of derivatization techniques. The chemical structures of the metabolites present in the urine larger than 1% of the total CB content are shown in Figure 2a, while minor metabolites found in trace amounts in the urine of the patient are listed by their short name in Figure 2b. The O-glucuronide conjugate of CBD was one of the most abundant urinary excretion products (13.3%), while the concentration of intact CBD was 12.1% of the total excreted CB. The two nonoxidized CB identified were Δ8-THC (structure not shown) and Δ9-THC, both presumably formed by cyclization of CBD, while cannabinol originating from aromatization of the Δ8/9-THC species was also present in the urine. A further nonoxidized CB, probably a cyclized monophenol, was also detected at 1.2% but its structure was not elucidated. Figure 2c lists five side-chain monohydroxylated CBD metabolites, which were recently identified in a human liver preparation in vitro60; all of them had been known from previous animal studies.26
Interestingly, inspection of the chemical structures of the abundant 7-COOH-CBD derivatives, which account for about the half of the metabolites identified in the above study, reveals the branched alkyl chain of (2E)-2-propylpent-2-enoic acid (Δ2(E)-valproate) embedded in the cyclohexenecarboxylic acid moiety of such acidic CBD metabolites. Δ2(E)-Valproate is the major active metabolite of valproic acid, and unlike the parent saturated acid, its anticonvulsant properties are not compromised by hepatotoxicity and teratogenicity, and is well tolerated in humans.61 Whether the 7-COOH-CBD metabolite species with a Δ2(E)-valproate-like structure are involved in the antiepileptic activity of CBD remains to be established.
The enzymatic processes responsible for the formation of the metabolites involve CYP450 oxidases, glucuronyl transferases and sulfotransferases, of which the CYP450 enzyme family has only been thoroughly studied.
Experiments in vitro with seven recombinant human CYP450 isoforms indicated 6α-OH-, 6β-OH-, 7-OH-, and 4′′-OH-CBD as main monohydroxylated metabolites.60 Specifically, CYP1A1 is involved in the formation of 6α/β-OH-, 7-OH-, and 1′′-OH-CBD; CYP1A2 is responsible for the formation of 6α/β-OH-CBD, as well as of 1′′-, 2′′-, 3′′-, and 4′′-OH-CBD species; CYP2C19 is involved in the formation mostly of 6α-OH-, 7-OH-, and 4′′-OH-CBD; CYP2D6 appears to be the main isoform responsible for the formation of 6α/β-OH-CBD as well as of 7-OH-, 4′′-OH-, and 5′′-OH-CBD; CYP3A4 efficiently catalyzes the formation of 6α/β-OH-CBD, although the 7-OH-, 2′′-OH-, 4′′-OH-, and 5′′-OH-CBD metabolites are also produced by this isoform; CYP3A5 is involved in the formation mainly of 6α/β-OH-CBD although the 7-OH-, 2′′-OH-, 3′′-OH-, and 4′′-OH-CBD metabolites are also produced. The role of CYP2A9 appears to be minor, but 6α/β-OH-, 7-OH-, 4′′-OH-, and 5′′-OH-CBD could be formed by this isoform.
Glucuronidation of CBD at the phenolic oxygen is a major Phase II biotransformation in humans,59,62 but hydroxylated metabolites of CBD may also be substrates. Sulfation of CBD species may also occur but such conjugates remain unknown. Studies should thus use appropriate hydrolysis before Phase I metabolite quantification.63
Finally, it should be mentioned that interindividual differences in the expression and function of CYP450 enzymes may considerably affect the pharmacokinetics of CBD and its metabolites, and this could be relevant in the therapeutic action and any possible adverse effects of CBD-containing preparations. For CBD, essentially no relevant information is available and we are aware only of one publication that deals with the genetic polymorphisms in CYP2C9 and CYP3A5 as related to THC metabolism in humans.64
CBD-derived and metabolite-like substances
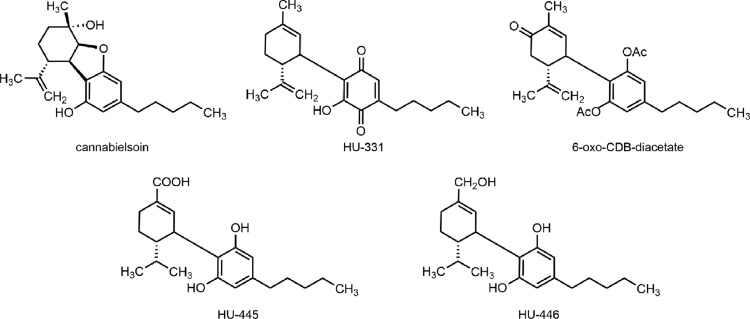
One of the interesting metabolites of CBD is cannabielsoin (Fig. 3), which was obtained first photochemically from CBD,65 and has not been isolated from humans but has been identified in guinea pigs.66,67 Furthermore, a hydroxyquinone derivative of CBD (HU-331; Fig. 3), which was again obtained first by synthesis,68 has been postulated to be a short-lived (re)active oxidative metabolite of CBD (see below).
FIG. 3.
Chemical structures of CBD-derived substances of biological interest.
Recently, the side-chain hydrogenated form of the minor human metabolite 7-COOH-CBD, that is, 8,9-dihydro-7-COOH-CBD (HU-445; Fig. 3), has been synthesized.69 The dihydrogenated product of the minor human metabolite 7-OH-CBD, that is, 8,9-dihydro-7-OH-CBD (HU-446; Fig. 3) has also been prepared and found to have anti-inflammatory properties in vitro with negligible affinity toward CB1 and CB2 receptors.70
Biological Activity Studies with CBD Metabolites
Studies in vitro
An early study71 on the inhibition of the binding of [3H]-5′-trimethylammonium-Δ8-THC to rat brain neuronal membrane homogenate by a series of CBs showed 7-OH-CBD (corresponding to 10-OH-CBD in the original publication) to be slightly less active than CBD (Ki=94 and 73.1 nM, respectively; THC: Ki=27 nm). Subsequent CB receptor binding studies72–74 with CBD enantiomers and their derivatives revealed that (−)-CBD (natural enantiomer) and its 7-OH and 7-COOH metabolites were devoid of receptor affinity (Ki > 10,000 nM); however, synthetic (+)-CBD was a modest receptor ligand with Ki values of 842 and 203 nM for the CB1 and CB2 receptor, respectively. The 7-OH and 7-COOH derivatives of (+)-CBD had high affinity to the CB1 receptor with respective Ki values of 5.3 and 13.2 nM; these two unnatural compounds also bound to the CB2 receptor with respective Ki values of 322 and 156 nM. Furthermore, while both CBD enantiomers proved to be equipotent full agonists of the type-1 vanilloid receptor (EC50=3500 nM), the 7-OH and 7-COOH metabolites of (−)-CBD were inactive.72 Furthermore, (−)-CBD and (+)-CBD, as well as the natural metabolite 7-OH-CBD, inhibited fatty acid amide hydrolase with IC50 values of 27.5, 63.5, and 34.0 μM, respectively, but the 7-COOH-CBD metabolite was inactive (IC50 > 100 μM); also, anandamide uptake by rat basophilic leukemia cells was inhibited by (−)-CBD and by its 7-OH metabolite with IC50 values of 22.0 and ∼50 μM, respectively, as well as by (+)-CBD (IC50=17.0 μM) but 7-COOH-CBD was inactive (IC50 > 50 μm).72
With regard to metabolic enzymes, not only CBD but also its 6α/β-OH, 6-oxo (not observed species in humans), and 10-OH metabolites effectively inactivated mouse liver microsomal CYP2C and CYP3A oxidases isolated from mice treated i.p. with 120 mg/kg CBD.75 The key role of the resorcinol moiety of CBD (and of its metabolites) in the inhibition has been established.75,76 A recent patent described 7-OH- and 7-COOH-CBD as anti-inflammatory substances in the mouse and as dose-dependent inhibitors in vitro of the generations of nitric oxide and reactive oxygen species as well as the production of TNF-α.77
The atypical quinone HU-331 was found to inhibit mouse hepatic microsomal CYP450 enzymes,78 CYP3A11 in particular,79 as well as induced apoptosis of splenocytes isolated from mice.80 Several lines of evidence have suggested that the inhibition (inactivation) is due to the adduct formation between this reactive quinone and the cysteine thiols at the active site of the enzyme. HU-331 also has antiangiogenic properties and is a selective inhibitor of topoisomerase II.81,82 Being more effective and safer than the prominent antitumor agent doxorubicin, HU-331 has been extensively investigated as a potential anticancer drug.83
Studies in animals
There have been only a few in vivo investigations with selected monooxygenated metabolites. In the standard mouse “tetrade” test using i.p. injection of 20 mg/kg of the compounds, Fride et al. reported84 that CBD was inactive that reflects its lack of affinity to central CB1 and CB2 receptors (see Bisogno et al.72); 7-COOH-CBD caused slight hypothermia (−1.1°C) and a minimal inhibition of intestinal motility (defecation). Interestingly, unnatural (+)-CBD and its 7-OH and 7-COOH derivatives potently inhibited defecation indicating peripheral activity perhaps through a CB receptor-independent mechanism; furthermore, weak antinociceptive effects for 7-OH-(+)-CBD were also noted.73,77 As mentioned before, antinociceptive as well as anti-inflammatory effects in mice of 7-OH-CBD and 7-COOH-CBD have been described in a patent but these experiments used chemicals as noxious stimuli.77
Upon i.v. administration to rabbits, CBD (1 mg per animal) had no activity in lowering intraocular pressure but 10-OH-CBD (2 mg per animal) was effective with a slow onset of action; nevertheless, THC was more potent at the 1 mg dose in the study.85
Cannabielsoin (at ≤10 mg/kg i.v.) “showed no CNS activity” in rodents,86 and did not affect body temperature or pentobarbital-induced sleep time in mice.66,67 In rabbits, a 5 mg/kg i.v. dose of cannabielsoin reduced intraocular pressure; CBD had no effect at 10 mg/kg, while THC was active at 1 mg/kg.87
HU-331, which has been postulated to be a short-lived (re)active oxidative metabolite of CBD with CYP450 inhibitory properties,79,80 has been extensively investigated in rodents due to its anticancer activity.83
Finally, CBD as well as the synthetic 6-oxo-CBD-diacetate (Fig. 3), which could hydrolyze in vivo to the corresponding phenolic 6-oxo-CBD, showed anticonvulsant activity and prolonged pentobarbital sleep time when administered to mice at 200 mg/kg (i.p.)88; furthermore, CBD as well as the synthetic 10-OH-CBD-triacetate (structure not shown) reduced spontaneous motor activity in this trial. Although 6-oxo-CBD has not been detected in humans as a metabolite, its glycoside was identified in CBD-treated dogs.89
Human studies
There are no publications describing the biological activity of CBD metabolites in humans.
Interaction with other drugs
The pharmacological actions of CBD on receptors, ion channels, cellular uptake processes, and enzymes have recently been reviewed9–11 and are not reiterated here. Since CBD is often administered concomitantly with other medicines, for example, as an adjunct in the therapy of certain diseases, drug–drug interactions should be taken into account. What follows is a brief summary of such effects possibly having relevance in the clinical use of CBD. The contentious issue of CBD–THC interaction, however, is not discussed here (for a brief summary, see the relevant section in a recent review90).
The first pharmacological effect to be observed for CBD was, in fact, related to drug interaction. Already in 1942, it was noted by Adams91 that “cannabidiol, which is devoid of the marihuana effect upon man, showed the highest potency in this [mouse sleep prolongation] test” (that is extending the hypnotic effects of certain barbiturates); such a synergism was later shown to be related to the inhibition by CBD of mouse hepatic microsomal metabolism as demonstrated for phenazone (antipyrine).92 In young men with marijuana use experience, however, CBD at the low acute smoked dose of 0.5 mg/kg failed to affect the plasma level of secobarbital.93 Yet, a subsequent human pharmacokinetics study found that upon oral administration in a 6×100 mg daily dose regimen, CBD significantly increased the bioavailability and prolonged the elimination half-time of hexobarbital.94 It has also been proposed that the observed interference with drug metabolism in vivo could be due to one or more metabolites and not to the parent CB.55
It has repeatedly been demonstrated that CBD is not only a substrate but also an inhibitor of CYP450 enzymes, and thus, it could interfere with the metabolism of other xenobiotics, including THC and medicinal products.27,34,76,90,95 Moreover, prolonged CBD administration may induce specific CYP450 isoenzymes, as it has been shown in mouse liver CYP3A and CYP2B1096 as well as for human CYP1A1 in vitro.97 Also, 6α-OH-CBD, but not 6-oxo-CBD, was found to be an effective inducer of CYP2B10.96 The resorcinol moiety apparently plays a pivotal role in CYP450 induction.
The CYP450-mediated metabolism of anandamide in vitro by liver microsomes isolated from mice treated with CBD (120 mg/kg i.p.) was shown to be inhibited, but the physiological significance of this finding has not been explored.98 The involvement of CBD in the regulation of the endocannabinoid system has recently been reviewed.10
During a Phase I study with healthy male subjects, potential drug–drug interactions of THC/CBD oromucosal spray (Sativex, nabiximols) in combination of CYP450 inducers and inhibitors were assessed using various dose regimens.99 The antibiotic rifampicin, an inducer of CYP3A4 involved in the metabolism of CBD, significantly reduced the peak plasma concentration of CBD, while the antifungal ketoconazole, a CYP3A4 inhibitor, nearly doubled the peak plasma concentration of CBD; however, the moderate CYP2C19 inhibitor omeprazole, a proton-pump inhibitor used to treat gastroesophageal reflux disease, did not significantly alter the pharmacokinetics of CBD. The presence and role of CBD metabolites in the observed drug interactions have not been reported.
Recently, an 8-week trial studied the interaction of the anticonvulsant drug clobazam and CBD in 13 children with refractory epilepsy.100 Oral CBD treatment started with 5 mg/kg per day with a weekly increase of 5 mg/kg per day up to 25 mg/kg per day. It was observed that coadministration of the CB increased clobazam plasma level by 60%±80%, while the level of its active metabolite norclobazam increased by 500%±300%. This allowed the reduction of the initial average 1 mg/kg daily clobazam dose in most subjects thereby alleviating consequential side effects. Since both the bioactivation by demethylation of clobazam and the inactivation by hydroxylation of clobazam/norclobazam involve, respectively, CYP3A4 and CYP2C19 isoenzymes, which are also implicated in CBD metabolism, interactions with CBD and/or its metabolites in such combination therapies should be considered.
Furthermore, CBD has been shown to interact in vitro with P-glycoprotein efflux transporters involved in multidrug resistance, and thus, it may affect the pharmacokinetics of anticancer drugs.101–103 Also, the placental permeability in pregnant women who consume CBD-containing preparations may also be influenced.104 Again, no relevant information is available for the metabolites of CBD.
Synthesis of CBD Metabolites
The identification of CBD metabolites has typically relied on mass spectral fragmentation patterns,105 and structural confirmation by synthesis was done only in a few cases; nevertheless, essentially all single-site modified CBD metabolites have been prepared. The aim of most of these syntheses was merely to verify the chemical structure of a metabolite and not to provide material for bioassays. The few exceptional studies were discussed in the preceding paragraphs.
Analytical characterizations of and synthetic methodologies for all five metabolites hydroxylated at the pentyl side chain were described in the early 1970s.57,57a,106,107 The syntheses of the epimeric 6α- and 6β-OH-CBD108 as well as the syntheses of the 7-OH-CBD74,108–110 and the nonhuman metabolite species 10-OH-CBD109,111 have been reported. The rabbit metabolite 8,9-diOH-CBD was obtained by incubating 8,9-epoxy-CBD112 with guinea pig hepatic microsomes.113 Ring- and side-chain hydroxylated derivatives, namely 3′′-OH-CBD,4′′,5′′-dinor, 4′′-OH-CBD, 4′′,6α-diOH-CBD, and 3′′,6-diOH-CBD,4′′,5′′-dinor, have also been produced in milligram quantities by microbial oxidation.114 The synthesis of the 7-COOH metabolite of CBD has been described.74,115 Synthesis of the side-chain terminal carboxylic acid (5′′-COOH, identified only in animals116–119) has also been described.120 A patent described the methyl ester of 2-COOH-CBD,3,′′4,′′5′′-trinor.121 The glucuronide of CBD has been prepared using a glucuronyltransferase.122 Cannabielsoin, a putative CBD metabolite may be obtained from CBD by several synthetic routes,86,123,124 as well as by biotransformation using tissue cultures of C. sativa and Saccharum officinarum.125 The 6-oxo-CBD derivatives, which may serve as precursors to the 6α/β-OH-CBD species, were prepared from CBD by allylic oxidation of the corresponding CBD derivative.88,108
Chemical syntheses of metabolites oxidized at multiple sites have not been published.
Summary
Several drugs used in therapy are metabolically converted into active metabolites and interindividual variations in the generation and pharmacokinetics of such active species may cause variability in the response to treatment by different individuals.126 The use of relatively high daily doses of CBD in human clinical trials as well as in self-medicating patients is not uncommon. For example, in a 30-day CBD monotherapy study, an escalating oral dose reaching 1280 mg/day was administered.127 Although information is lacking, the metabolites formed from CBD are assumed to be present in the body at pharmacologically relevant concentrations. Pharmacological studies with such metabolites are scarce yet suggest interesting biological activities, which are unrelated or not directly related to CB receptors. Thus, intriguing questions arise:
Could any of the pharmacological effects observed for CBD be attributed to its metabolites?
Are there any drug–drug interactions that affect the outcome of the therapeutic effects of other, non-CB medicines used concomitantly with CBD?
Could any of the metabolites be used as templates for the development of novel therapeutic agents?
The pharmacological characterization of CBD metabolites both in vitro and in vivo is timely and necessary to shed light on the multifaceted, perplexing, or sometimes even contradictory biological properties observed for the parent CB. The understanding of the clinical significance of these abundant metabolites in the proven therapeutic effects of CBD-containing preparations warrants further studies.
Supplementary Material
Abbreviations Used
- CB
cannabinoid
- CBD
cannabidiol
- CBDA
cannabidiolic acid
- i.p.
intraperitoneal
- i.v.
intravenous
- THC
tetrahydrocannabinol
- TNF-α
tumor necrosis factor alpha
Acknowledgment
Michael Evans-Brown is gratefully acknowledged for linguistic advice.
Author Disclosure Statement
No competing financial interest.
References
- 1.Adams R, Hunt M, Clark JH. Structure of cannabidiol, a product isolated from the marihuana extract of Minnesota wild hemp. I. J Am Chem Soc. 1940;62:196–200 [Google Scholar]
- 2.Jacob A, Todd AR. Cannabis indica. Part II. Isolation of cannabidiol from Egyptian hashish. Observations on the structure of cannabinol. J Chem Soc. 1940;649–653 [Google Scholar]
- 3.Mechoulam R, Shvo Y. Hashish–I. The structure of cannabidiol. Tetrahedron. 1963;19:2073–2078 [DOI] [PubMed] [Google Scholar]
- 4.ElSohly M, Gul W. Constituents of Cannabis sativa. In: Handbook of Cannabis (Pertwee RG, ed.). Oxford University Press: Oxford, 2014, pp. 3–22 [Google Scholar]
- 5.Gaoni Y, Mechoulam R. Isolation, structure, and partial synthesis of an active constituent of hashish. J Am Chem Soc. 1964;86:1646–1647 [Google Scholar]
- 6.Pertwee R. (ed.). Handbook of Cannabis. Oxford University Press: Oxford, 2014 [Google Scholar]
- 7.Mechoulam R, Hanuš L. Cannabidiol: an overview of some chemical and pharmacological aspects. Part I.: chemical aspects. Chem Phys Lipids. 2002;121:35–43 [DOI] [PubMed] [Google Scholar]
- 8.Mechoulam R, Peters M, Murillo-Rodriguez E, et al. . Cannabidiol–recent advances. Chem Biodivers. 2007;4:1678–1692 [DOI] [PubMed] [Google Scholar]
- 9.Cascio MG, Pertwee RG. Known pharmacological actions of nine nonpsychotropic phytocannabinoids. In: Handbook of Cannabis (Pertwee RG, ed.). Oxford University Press: Oxford, 2014, pp. 137–156 [Google Scholar]
- 10.McPartland JM, Duncan M, Di Marzo V, et al. . Are cannabidiol and Δ9-tetrahydrocannabivarin negative modulators of the endocannabinoid system? A systematic review. Br J Pharmacol. 2015;172:737–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ibeas Bih C, Chen T, Nunn AVW, et al. . Molecular targets of cannabidiol in neurological disorders. Neurotherapeutics. 2015;12:699–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burstein S. Cannabidiol (CBD) and its analogs: a review of their effects on inflammation. Bioorg Med Chem. 2015;23:1377–1385 [DOI] [PubMed] [Google Scholar]
- 13.Bergamaschi MM, Costa Queiroz RH, Crippa JAS, et al. . Safety and side effects of cannabidiol, a Cannabis sativa constituent. Curr Drug Saf. 2011;6:237–249 [DOI] [PubMed] [Google Scholar]
- 14.Zhornitsky S, Potvin S. Cannabidiol in humans—the quest for therapeutic targets. Pharmaceuticals. 2012;5:529–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernández-Ruiz J, Sagredo O, Pazos MR, et al. . Cannabidiol for neurodegenerative disorders: important new clinical applications for this phytocannabinoid? Br J Clin Pharmacol. 2013;75:323–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Massi M, Solinas M, Cinquina V, et al. . Cannabidiol as potential anticancer drug. Br J Clin Pharmacol. 2013;75:303–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oláh A, Tóth BI, Borbíró I, et al. . Cannabidiol exerts sebostatic and antiinflammatory effects on human sebocytes. J Clin Invest. 2014;124:3713–3724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.ClinicalTrials.gov Search results: Cannabidiol. Available at: https://clinicaltrials.gov/ct2/results?term=cannabidiol&Search=Search
- 19.Gloss D, Vickrey B. Cannabinoids for epilepsy. Cochrane Database Syst Rev. 2012;6:Ar–t.. No. CD009270. [DOI] [PubMed] [Google Scholar]
- 20.Devinsky O, Cilio MR, Cross H, et al. . Cannabidiol: pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia. 2014;55:791–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wright S, Sommerville K, Jones NA, et al. . Cannabidiol. Epilepsy Res. 2015;111:111–114 [Google Scholar]
- 22.Volkow ND. The biology and potential therapeutic effects of cannabidiol. Testimony before the US Senate Caucus on International Narcotics Control “Cannabidiol: barriers to research and potential medical benefits”, June 24 2015, DHHS, NIH-NIDA, Washington, D.C. Available at: www.drugcaucus.senate.gov/sites/default/files/Volkow.pdf (accessed January31, 2016)
- 23.U.S. Food and Drug Administration. Warning letters and test results. 2015. Available at: www.fda.gov/newsevents/publichealthfocus/ucm435591.htm (accessed January31, 2016)
- 24.Nature Outlook—Cannabis. 525(7570) Supplement Issue 23 September 2015. Available at: www.nature.com/nature/outlook/cannabis/index.html (accessed January31, 2016)
- 25.ClinicalTrials.gov Epidiolex and Drug Resistant Epilepsy in Children (CBD). Available at: https://clinicaltrials.gov/ct2/show/NCT02397863?term=epidiolex (accessed January31, 2016)
- 25a.European Medicines Agency. Community register of orphan medicinal products: Cannabidiol. Available at: http://ec.europa.eu/health/documents/community-register/html/o1339.htm (accessed January31, 2016)
- 25b.European Medicines Agency. Community register of orphan medicinal products: Cannabidiol. Available at: http://ec.europa.eu/health/documents/community-register/html/o1520.htm (accessed January31, 2016)
- 26.Harvey DJ. Metabolism and pharmacokinetics of the cannabinoids. In: Biochemistry and physiology of substance abuse (Watson RR, ed.). CRC Press: Boca Raton, 1991, pp. 279–365 [Google Scholar]
- 27.Hawksworth G, McArdle K. Metabolism and pharmacokinetics of cannabinoids. In: The Medicinal Uses of Cannabis and Cannabinoids (Guy GW, Whittle BA, Robson PJ, eds.). Pharmaceutical Press: London, 2004, pp. 205–228 [Google Scholar]
- 28.Huestis MA. Human cannabinoid pharmacokinetics. Chem Biodivers. 2007;4:1770–1804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huestis MA, Smith ML. Cannabinoid pharmacokinetics and disposition in alternative matrices. In: Handbook of Cannabis (Pertwee RG, ed.). Oxford University Press: Oxford, 2014, pp. 296–316 [Google Scholar]
- 30.Ujváry I, Grotenhermen F. 11-Nor-9-carboxy-Δ9-tetrahydrocannabinol—a ubiquitous yet underresearched cannabinoid. A review of the literature. Cannabinoids. 2014;9:1–8. Available at: www.cannabis-med.org/data/pdf/en_2014_01_1.pdf (accessed January31, 2016) [Google Scholar]
- 31.Ohlsson A, Lindgren J-E, Andersson S, et al. . Single-dose kinetics of deuterium-labelled cannabidiol in man after smoking and intraveous administration. Biomed Environ Mass Spectrom. 1986;13:77–83 [DOI] [PubMed] [Google Scholar]
- 32.Wall ME, Brine DR, Perez-Reyes M. Metabolism of cannabinoids in man. In: The Pharmacology of Marihuana (Braude MC, Szara S, eds.). Raven Press: New York, 1976, pp. 93–113 [Google Scholar]
- 33.Agurell S, Carlsson S, Lindgren JE, et al. . Interactions of Δ1-tetrahydrocannabinol with cannabinol and cannabidiol following oral administration in man. Assay of cannabinol and cannabidiol by mass fragmentography. Experientia. 1981;37:1090–1092 [DOI] [PubMed] [Google Scholar]
- 34.Nadulski T, Pragst F, Weinberg G, et al. . Randomized, double-blind, placebo-controlled study about the effects of cannabidiol (CBD) on the pharmacokinetics of Δ9-tetrahydrocannabinol (THC) after oral application of THC verses standardized cannabis extract. Ther Drug Monit. 2005;27:799–810 [DOI] [PubMed] [Google Scholar]
- 35.Eichler M, Spinedi L, Unfer-Grauwiler S, et al. . Heat exposure of Cannabis sativa extracts affects the pharmacokinetic and metabolic profile in healthy male subjects. Planta Med. 2012;78:686–691 [DOI] [PubMed] [Google Scholar]
- 36.Consroe P, Kennedy K, Schram K. Assay of plasma cannabidiol by capillary gas chromatography/ion trap mass spectroscopy following high-dose repeated daily oral administration in humans. Pharmacol Biochem Behav. 1991;40:517–522 [DOI] [PubMed] [Google Scholar]
- 37.Consroe P, Laguna J, Allender J, et al. . Controlled clinical trial of cannabidiol in Huntington's disease. Pharmacol Biochem Behav. 1991;40:701–708 [DOI] [PubMed] [Google Scholar]
- 38.Fusar-Poli P, Crippa JA, Bhattacharyya S, et al. . Distinct effects of Δ9-tetrahydrocannabinol and cannabidiol on neural activation during emotional processing. Arch Gen Psychiatry. 2009;66:95–105 [DOI] [PubMed] [Google Scholar]
- 39.Manini AF, Yiannoulos G, Bergamaschi MM, et al. . Safety and pharmacokinetics of oral cannabidiol when administered concomitantly with intravenous fentanyl in humans. J Addict Med. 2015;9:204–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guy GW, Flint ME. A single centre, placebo-controlled, four period, crossover, tolerability study assessing, pharmacodynamic effects, pharmacokinetic characteristics and cognitive profiles of a single dose of three formulations of Cannabis Based Medicine Extracts (CBMEs) (GWPD9901) plus a two period tolerability study comparing pharmacodynamic effects and pharmacokinetic characteristics of a single dose of a Cannabis Based Medicine Extract given via two administration routes (GWPD9901 Ext). J Cannabis Ther. 2003;3:35–77 [Google Scholar]
- 41.Karschner EL, Darwin WD, Goodwin RS, et al. . Plasma cannabinoid pharmacokinetics following controlled oral Δ9-tetrahydrocannabinol and oromucosal cannabis extract administration. Clin Chem. 2011;57:66–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stott CG, White L, Wright S, et al. . A phase I study to assess the single and multiple dose pharmacokinetics of THC/CBD oromucosal spray. Eur J Clin Pharmacol. 2013;69:1135–1147 [DOI] [PubMed] [Google Scholar]
- 43.Stinchcomb AL, Valiveti S, Hammell DC, et al. . Human skin permeation of Δ8-tetrahydrocannabinol, cannabidiol and cannabinol. J Pharm Pharmacol. 2004;56:291–297 [DOI] [PubMed] [Google Scholar]
- 44.Lodzki M, Godin B, Rakou L, et al. . Cannabidiol–transdermal delivery and anti-inflammatory effect in a murine model. J Control Release. 2003;93:377–387 [DOI] [PubMed] [Google Scholar]
- 45.Paudel KS, Hammell DC, Agu RU, et al. . Cannabidiol bioavailability after nasal and transdermal application: effect of permeation enhancers. Drug Dev Ind Pharm. 2010;36:1088–1097 [DOI] [PubMed] [Google Scholar]
- 46.Mattes RD, Engelman K, Shaw LM, et al. . Cannabinoids and appetite stimulation. Pharmacol Biochem Behav. 1994;49:187–189 [DOI] [PubMed] [Google Scholar]
- 47.Brenneisen R, Egli A, ElSohly MA, et al. . The effect of orally and rectally administered delta-9-tetrahydrocannabinol on spasticity: a pilot study with 2 patients. Int J Clin Pharmacol Ther. 1996;34:446–452 [PubMed] [Google Scholar]
- 48.Gronewold A, Skopp G. A preliminary investigation on the distribution of cannabinoids in man. Forensic Sci Int. 2011;210:e7–e11 [DOI] [PubMed] [Google Scholar]
- 49.Fabritius M, Staub C, Mangin P, et al. . Distribution of free and conjugated cannabinoids in human bile samples. Forensic Sci Int. 2012;223:114–118 [DOI] [PubMed] [Google Scholar]
- 50.Siemens AJ, Walczak D, Buckley FE. Characterization of blood disappearance and tissue distribution of [3H]cannabidiol. Biochem Pharmacol. 1980;29:462–464 [DOI] [PubMed] [Google Scholar]
- 51.Alozie SO, Martin BR, Harris LS, et al. . 3H-Δ9-Tetrahydrocannabinol, 3H-cannabinol and 3H-cannabidiol: penetration and regional distribution on rat brain. Pharmacol Biochem Behav. 1980;12:217–221 [DOI] [PubMed] [Google Scholar]
- 52.Deiana S, Watanabe A, Yamasaki Y, et al. . Plasma and brain pharmacokinetic profile of cannabidiol (CBD), cannabidivarin (CBDV), Δ9-tetrahydrocannabivarin (THCV) and cannabigerol (CBG) in rats and mice following oral and intraperitoneal administration and CBD action on obsessive-compulsive beaviour. Psychopharmacology. 2012;219:859–873 [DOI] [PubMed] [Google Scholar]
- 53.Buggins TR, Dickinson PA, Taylor G. The effects of pharmaceutical excipients on drug disposition. Adv Drug Deliv Rev. 2007;59:1482–1503 [DOI] [PubMed] [Google Scholar]
- 54.Engel A, Oswald S, Siegmund W, et al. . Pharmaceutical excipients influence the function of human uptake transporting proteins. Mol Pharm. 2012;9:2577–2581 [DOI] [PubMed] [Google Scholar]
- 55.Karler R, Sangdee P, Turkanis SA, et al. . The pharmacokinetic fate of cannabidiol and its relationship to barbiturate sleep time. Biochem Pharmacol. 1979;28:777–784 [DOI] [PubMed] [Google Scholar]
- 56.Christiansen J, Rafaelsen OJ. Cannabis metabolites in urine after oral administration. Psychopharmacologia. 1969;15:60–63 [DOI] [PubMed] [Google Scholar]
- 57.Nilsson I, Agurell S, Nilsson JLG, et al. . Two cannabidiol metabolites formed by rat liver. J Pharm Pharmacol. 1973;25:486–487 [DOI] [PubMed] [Google Scholar]
- 57a.Nilsson IM, Agurell S, Leander K, et al. . Cannabidiol: structure of three metabolites formed in rat liver. Acta Pharm Suec. 1971;8:70–1. [Google Scholar]
- 58.Harvey DJ, Samara E, Mechoulam R. Urinary metabolites of cannabidiol in dog, rat and man and their identification by gas chromatography–mass spectrometry. J Chromatogr. 1991;562:299–322 [DOI] [PubMed] [Google Scholar]
- 59.Harvey DJ, Mechoulam R. Metabolites of cannabidiol identified in human urine. Xenobiotica. 1990;20:303–320 [DOI] [PubMed] [Google Scholar]
- 60.Jiang R, Yamaori S, Takeda S, et al. . Identification of cytochrome P450 enzymes responsible for metabolism of cannabidiol by human liver microsomes. Life Sci. 2011;89:165–170 [DOI] [PubMed] [Google Scholar]
- 61.Düsing RH. Single-dose tolerance and pharmacokinetics of 2-n-propyl-2(E)-pentenoate (Δ2(E)-valproate) in healthy male volunteers. Pharm Weekbl Sci. 1992;14:152–158 [DOI] [PubMed] [Google Scholar]
- 62.Mazur A, Lichti CF, Prather PL, et al. . Characterization of human hepatic and extrahepatic UDP-glucuronosyltransferase enzymes involved in the metabolism of classic cannabinoids. Drug Metab Dispos. 2009;37:1496–1504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bergamaschi MM, Barnes A, Queiroz RHC, et al. . Impact of enzymatic and alkaline hydrolysis on CBD concentration in urine. Anal Bioanal Chem. 2013;405:4679–4689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sachse-Seeboth C, Pfeil J, Sehrt D, et al. . Interindividual variation in the pharmacokinetics of Δ9-tetrahydrocannabinol as related to genetic polymorphisms in CYP2C9. Clin Pharmacol Ther. 2009;85:273–276 [DOI] [PubMed] [Google Scholar]
- 65.Shani A, Mechoulam R. Photochemical reactions of cannabidiol. Cyclization to Δ1-tetrahydrocannabinol and other transformations. Tetrahedron. 1971;27:601–606 [Google Scholar]
- 66.Yamamoto I, Gohda H, Narimatsu S, et al. . Identification of cannabielsoin, a new metabolite of cannabidiol formed by guinea-pig hepatic microsomal enzymes, and its pharmacological activity in mice. J Pharmacobiodyn. 1988;11:833–838 [DOI] [PubMed] [Google Scholar]
- 67.Yamamoto I, Gohda H, Narimatsu S, et al. . Cannabielsoin as a new metabolite of cannabidiol in mammals. Pharmacol Biochem Behav. 1991;40:541–546 [DOI] [PubMed] [Google Scholar]
- 68.Mechoulam R, Ben-Zvi Z, Gaoni Y. Hashish–XIII: on the nature of the Beam test. Tetrahedron. 1968;24:5615–5624 [DOI] [PubMed] [Google Scholar]
- 69.Haj CG, Sumariwalla PF, Hanuš L, et al. . HU-444, a novel, potent anti-inflammatory, nonpsychotropic cannabinoid. J Pharmacol Exp Ther. 2015;355:66–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kozela E, Haj C, Hanuš L, et al. . HU-446 and HU-465, derivatives of the non-psychoactive cannabinoid cannabidiol, decrease the activation of encephalitogenic T cells. Chem Biol Drug Des. 2016;87:143–153 [DOI] [PubMed] [Google Scholar]
- 71.Nye JS, Seltzman HH, Pitt CG, et al. . High-affinity cannabinoid binding sites in brain membranes labeled with [3H]-5'-trimethylammonium Δ8-tetrahydrocannabinol. J Pharmacol Exp Ther. 1985;234:784–791 [PubMed] [Google Scholar]
- 72.Bisogno T, Hanuš L, de Petrocellis L, et al. . Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br J Pharmacol. 2001;134:845–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fride E, Feigin C, Ponde DE, et al. . (+)-Cannabidiol analogues which bind cannabinoid receptors but exert peripheral activity only. Eur J Pharmacol. 2004;506:179–188 [DOI] [PubMed] [Google Scholar]
- 74.Hanuš LO, Tchilibon S, Ponde DE, et al. . Enantiomeric cannabidiol derivatives: synthesis and binding to cannabinoid receptors. Org Biomol Chem. 2005;3:1116–1123 [DOI] [PubMed] [Google Scholar]
- 75.Bornheim LM, Everhart ET, Li J, et al. . Characterization of cannabidiol-mediated cytochrome P450 inactivation. Biochem Pharmacol. 1993;45:1323–1331 [DOI] [PubMed] [Google Scholar]
- 76.Jiang R, Yamaori S, Okamoto Y, et al. . Cannabidiol is a potent inhibitor of the catalytic acitivity of cytochrome P450 2C19. Drug Metab Pharmacokinet. 2013;28:332–338 [DOI] [PubMed] [Google Scholar]
- 77.Mechoulam R, Tchilibon S, Fride E, et al. . Pharmaceutical compositions comprising cannabidiol derivatives. US Patent 7759526, 2010
- 78.Watanabe K, Usami N, Yamamoto I. Inhibitory effect of cannabidiol hydroxy-quinone, an oxidative product of cannabidiol, on the hepatic microsomal drug-metabolizing enzymes of mice. J Pharmacobiodyn. 1991;14:421–427 [DOI] [PubMed] [Google Scholar]
- 79.Bornheim LM, Grillo MP. Characterization of cytochrome P450 3A inactivation by cannabidiol: possible involvement of cannabidiol-hydroxyquinone as a P450 inactivator. Chem Res Toxicol. 1998;11:1209–1216 [DOI] [PubMed] [Google Scholar]
- 80.Wu H-Y, Han T-R. Cannabidiol hydroxyquinone-induced apoptosis of splenocytes is mediated predominantly by thiol depletion. Toxicol Lett. 2010;195:68–74 [DOI] [PubMed] [Google Scholar]
- 81.Kogan NM, Schlesinger M, Priel E, et al. . HU-331, a novel cannabinoid-based anticancer topoisomerase II inhibitor. Mol Cancer Ther. 2007;6:173–183 [DOI] [PubMed] [Google Scholar]
- 82.Regal KM, Mercer SL, Deweese JE. HU-331 is a catalytic inhibitor of topoisomerase IIα. Chem Res Toxicol. 2014;27:2044–2051 [DOI] [PubMed] [Google Scholar]
- 83.Peters M, Kogan NM. HU-331: a cannabinoid quinone, with uncommon cytotoxic properties and low toxicity. Expert Opin Investig Drugs. 2007;16:1405–1413 [DOI] [PubMed] [Google Scholar]
- 84.Fride E, Ponde D, Breuer A, et al. . Peripheral, but not central effects of cannabidiol derivatives: mediation by CB1 and unidentifed receptors. Neuropsychopharmacology. 2005;48:1117–1129 [DOI] [PubMed] [Google Scholar]
- 85.Green K, Symonds CM, Oliver NW, et al. . Intraocular pressure following systemic administration of cannabinoids. Curr Eye Res. 1982–1983;2:247–253 [DOI] [PubMed] [Google Scholar]
- 86.Uliss DB, Razdan RK, Daizell HC. Stereospecific intramolecular epoxide cleavage by phenolate anion. Synthesis of novel and biologically active cannabinoids. J Am Chem Soc. 1974;96:7372–7374 [DOI] [PubMed] [Google Scholar]
- 87.ElSohly MA, Harland EC, Benigni DA, et al. . Cannabinoids in glaucoma II: the effect of different cannabinoids in intraocular pressure of the rabbit. Curr Eye Res. 1984;3:841–850 [DOI] [PubMed] [Google Scholar]
- 88.Carlini EA, Mechoulam R, Lander N. Anticonvulsant activity of four oxygenated cannabidiol derivatives. Res Commun Chem Pathol Pharmacol. 1975;12:1–15 [PubMed] [Google Scholar]
- 89.Samara E, Bialer M, Harvey DJ. Identification of glucose conjugates as major urinary metabolites of cannabidiol in the dog. Xenobiotica. 1990;20:177–183 [DOI] [PubMed] [Google Scholar]
- 90.Pertwee RG. The pharmacology and therapeutic potential of cannabidiol. In: Cannabinoids (Di Marzo V, ed.). Landes Bioscience: Georgetown, TX, 2004, pp. 32–83 [Google Scholar]
- 91.Adams R. Marijuana. Bull N Y Acad Med. 1942;18:705–730 [PMC free article] [PubMed] [Google Scholar]
- 92.Paton WDM, Pertwee RG. Effect of cannabis and certain of its constituents on pentobarbital sleeping time and phenazone metabolism. Br J Pharmacol. 1972;44:250–261 [PMC free article] [PubMed] [Google Scholar]
- 93.Dalton WS, Martz R, Rodda BE, et al. . Influence of cannabidiol on secobarbital effects and plasma kinetics. Clin Pharmacol Ther. 1976;20:695–700 [DOI] [PubMed] [Google Scholar]
- 94.Benowitz NL, Nguyen T-L, Jones RT, et al. . Metabolic and psychophysiologic studies of cannabidiol-hexobarbital interaction. Clin Pharmacol Ther. 1980;28:115–120 [DOI] [PubMed] [Google Scholar]
- 95.Stout SM, Cimino NM. Exogenous cannabinoids as substrates, inhibitors and inducers of human drug metabolizing enzymes: a systematic review. Drug Metab Rev. 2014;46:86–95 [DOI] [PubMed] [Google Scholar]
- 96.Bornheim LM, Everhart ET, Li J, et al. . Induction and genetic regulation of mouse hepatic cytochrome P450 by cannabidiol. Biochem Pharmacol. 1994;48:161–171 [DOI] [PubMed] [Google Scholar]
- 97.Yamaori S, Kinugasa Y, Jiang R, et al. . Cannabidiol induces expression of human cytochrome P450 1A1 that is possibly mediated through aryl hydrocarbon receptor signaling in HepG2 cells. Life Sci. 2015;136:87–93 [DOI] [PubMed] [Google Scholar]
- 98.Bornheim LM, Kim KY, Chen B, et al. . The effect of cannabidiol on mouse hepatic microsomal cytochrome P450-dependent anandamide metabolism. Biochem Biophys Res Commun. 1993;197:740–746 [DOI] [PubMed] [Google Scholar]
- 99.Stott C, White L, Wright S, et al. . A Phase I, open-label, randomized, crossover study in three parallel groups to evaluate the effect of Rifampicin, Ketoconazole, and Omeprazole on the pharmacokinetics of THC/CBD oromucosal spray in healthy volunteers. Springerplus. 2013;2:23–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Geffrey AL, Pollack SF, Bruno PL, et al. . Drug–drug interaction between clobazepam and cannabidiol in children with refractory epilepsy. Epilepsia. 2015;56:1246–1251 [DOI] [PubMed] [Google Scholar]
- 101.Zhu H-J, Wang J-S, Markowitz JS, et al. . Characterization of P-glycoprotein inhibition by major cannabinoids from marijuana. J Pharmacol Exp Ther. 2006;317:850–857 [DOI] [PubMed] [Google Scholar]
- 102.Holland ML, Allen JD, Arnold JC. Interaction of plant cannabinoids with the multidrug transporter ABCC1(MRP1). Eur J Pharmacol. 2008;591:128–131 [DOI] [PubMed] [Google Scholar]
- 103.Arnold JC, Hone P, Holland ML, et al. . CB2 and TRPV1 receptors mediate cannabinoid actions on MDR1 expression in multidrug resistant cells. Pharmacol Rep. 2012;64:751–757 [DOI] [PubMed] [Google Scholar]
- 104.Feinshtein V, Erez O, Ben-Zvi Z, et al. . Cannabidiol changes P-gp and BCRP expression in trophoblast cell lines. PeerJ. 2013;1:e15–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Harvey DJ. Mass spectrometry of the cannabinoids and their metabolites. Mass Spectrom Rev. 2013;6:135–229 [Google Scholar]
- 106.Agurell S, Dahmén J, Gustafsson B, et al. . Metabolic fate of tetrahydrocannabinol. In: Cannabis and its derivatives: pharmacology and experimental psychology (Paton WDM, Crown J, eds.). Oxford University Press: London, 1972, pp. 16–38 [Google Scholar]
- 107.Binder M, Agurell S, Leander K, et al. . Zur Identifikation potentieller Metabolite von Cannabis-Inhaltstoffen: Kernresonanz- und massen-spektroskopische Untersuchungen an seitenkettenhydroxylierte Cannabinoiden. Helv Chim Acta. 1974;57:1626–1641 [DOI] [PubMed] [Google Scholar]
- 108.Lander N, Ben-Zvi Z, Mechoulam R, et al. . Total syntheses of cannabidiol and Δ1-tetrahydrocannabinol metabolites. J Chem Soc Perkin 1 1976;(1):8–16 [PubMed] [Google Scholar]
- 109.Kobayashi Y, Takeuchi A, Wang Y-G. Synthesis of cannabidiols via alkenylation of cyclohexenyl monoacetate. Org Lett. 2006;8:2699–2702 [DOI] [PubMed] [Google Scholar]
- 110.Tchilibon S, Mechoulam R. Synthesis of a primary metabolite of cannabidiol. Org Lett. 2000;2:3301–3303 [DOI] [PubMed] [Google Scholar]
- 111.Jorapur VS, Duffley RP, Razdan RK. A procedure for the conversion of cannabidiol into 12β-substituted tetrahydrocannabinols (THC's): synthesis of 12β-hydroxy-Δ8-THC. Synth Commun. 1984;14:655–660 [Google Scholar]
- 112.Yamamoto I, Gohda H, Narimatsu S, et al. . Mechanism of biological formation of cannabielsoin from cannabidiol in the guinea-pig, mouse, rat and rabbit. J Pharmacobiodyn. 1989;12:488–494 [DOI] [PubMed] [Google Scholar]
- 113.Yamamoto I, Nagai K, Watanabe K, et al. . A novel metabolite, an oxepin formed from cannabidiol with guinea-pig hepatic microsomes. J Pharm Pharmacol. 1995;47:683–686 [DOI] [PubMed] [Google Scholar]
- 114.Robertson LW, Koh S-W, Huff SR, et al. . Microbiological oxidation of the pentyl side chain of cannabinoids. Experientia. 1978;34:1020–1022 [DOI] [PubMed] [Google Scholar]
- 115.Mechoulam R, Fride E. Pharmaceutical compositions containing (+) cannabidiol and derivatives thereof and some such novel derivatives. US Patent 7884133, 2011
- 116.Martin BR, Harvey DJ, Paton WDM. Biotransformation of cannabidiol in mice: identification of new acid metabolites. Drug Metab Dispos. 1977;5:259–267 [PubMed] [Google Scholar]
- 117.Harvey DJ, Martin BR, Paton WDM. Comparative in vivo metabolism of Δ1-tetrahydrocannabinol (Δ1-THC), cannabidiol (CBD) and cannabinol (CBN) by several species. In: Recent developments in mass spectrometry in biochemistry and medicine, Vol. 2 (Frigerio A, ed.). Plenum Press: New York, 1978, pp. 161–184 [Google Scholar]
- 118.Harvey DJ, Brown NK. In vitro metabolism of cannabidiol in the rabbit: identification of seventeen new metabolites including thirteen dihydroxylated in the isopropenyl side chain. Biomed Environ Mass Spectrom. 1990;19:559–567 [DOI] [PubMed] [Google Scholar]
- 119.Samara E, Bialer M, Harvey DJ. Identification of urinary metabolites of cannabidiol in the dog. Drug Metab Dispos. 1978;18:571–579 [PubMed] [Google Scholar]
- 120.Crombie L, Crombie WML, Tuchinda P. Synthesis of cannabinoids carrying ω-carboxy substituents: the cannabidiols, cannabinol and Δ1- and Δ6-tetrahydrocannabinols of this series. J Chem Soc Perkin 1. 1988;1255–1262 [Google Scholar]
- 121.Makriyannis A, Nikas SP, Alapafuja SO. Angiogenic resorcinol derivatives. WO 2011/006099, 2011
- 122.Lyle MA, Pallante S, Head K, et al. . Synthesis and characterization of glucuronides of cannabinol, cannabidiol, Δ9-tetrahydrocannabinol and Δ8-tetrahydrocannabinol. Biomed Mass Spectrom. 1977;4:190–196 [DOI] [PubMed] [Google Scholar]
- 123.Küppers FJEM, Lousberg RJJC, Bercht CAL, et al. . Cannabis-VIII. Pyrolysis of cannabidiol. Structure elucidation of the main pyrolytic product. Tetrahedron. 1973;29:2797–2802 [Google Scholar]
- 124.Shani A, Mechoulam R. Cannabielsoic acids. Isolation and synthesis by a novel oxidative cyclization. Tetrahedron. 1974;30:2437–2446 [Google Scholar]
- 125.Hartsel SC, Loh WH-T, Robertson LW. Biotransformation of cannabidiol to cannabielsoin by suspension cultures of Cannabis sativa and Saccharum officinarum. Planta Med. 1983;48:17–19 [DOI] [PubMed] [Google Scholar]
- 126.Obach RS. Pharmacologically active drug metabolites: impact on drug discovery and pharmacotherapy. Pharmacol Rev. 2013;65:578–640 [DOI] [PubMed] [Google Scholar]
- 127.Zuardi AW, Hallak JEC, Dursun SM, et al. . Cannabidiol monotherapy for treatment-resistance schizophrenia. J Psychopharmacol. 2006;20:683–686 [DOI] [PubMed] [Google Scholar]
- 128.Watanabe K, Itokawa Y, Yamaori S, et al. . Conversion of cannabidiol to Δ9-tetrahydrocannabinol and related cannabinoids in artifical gastric juice, and their pharmacological effects in mice. Forensic Toxicol. 2007;25:16–21 [Google Scholar]
References
Cite this article as: Ujváry I, Hanuš L (2016) Human metabolites of cannabidiol: a review on their formation, biological activity, and relevance in therapy, Cannabis and Cannabinoid Research 1:1, 90–101, DOI: 10.1089/can.2015.0012.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.