Abstract
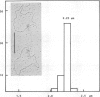
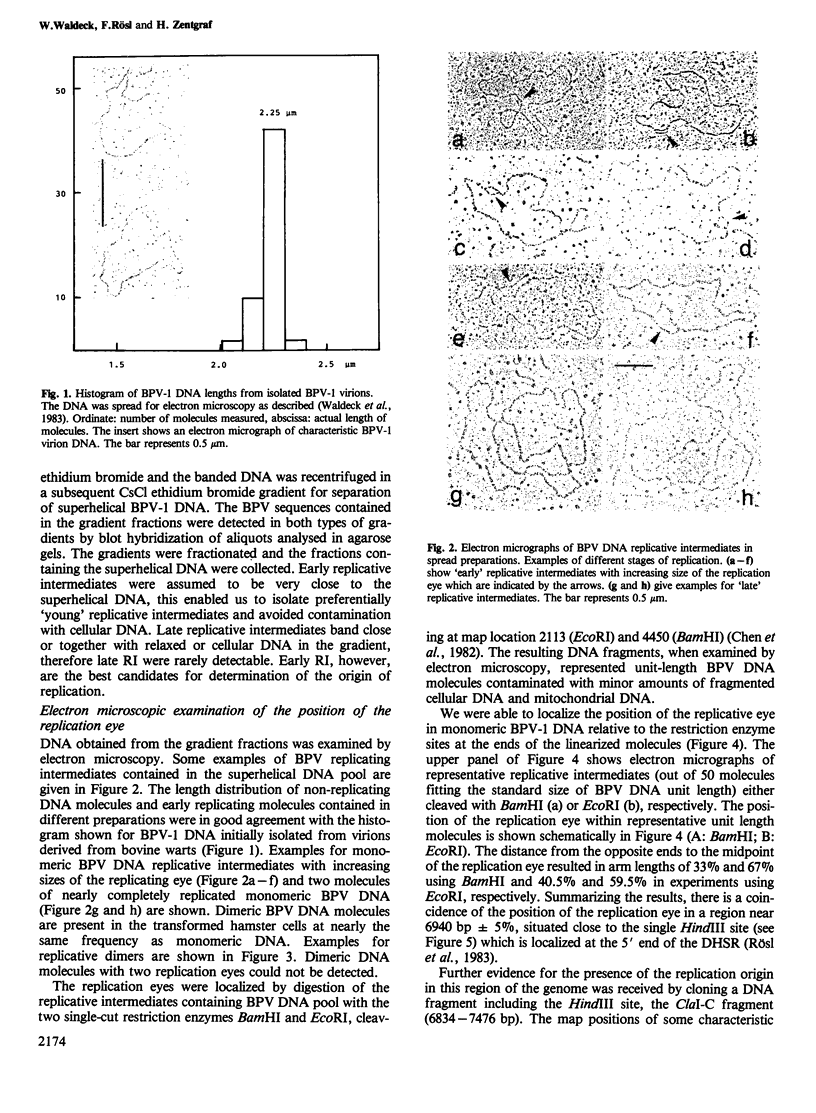
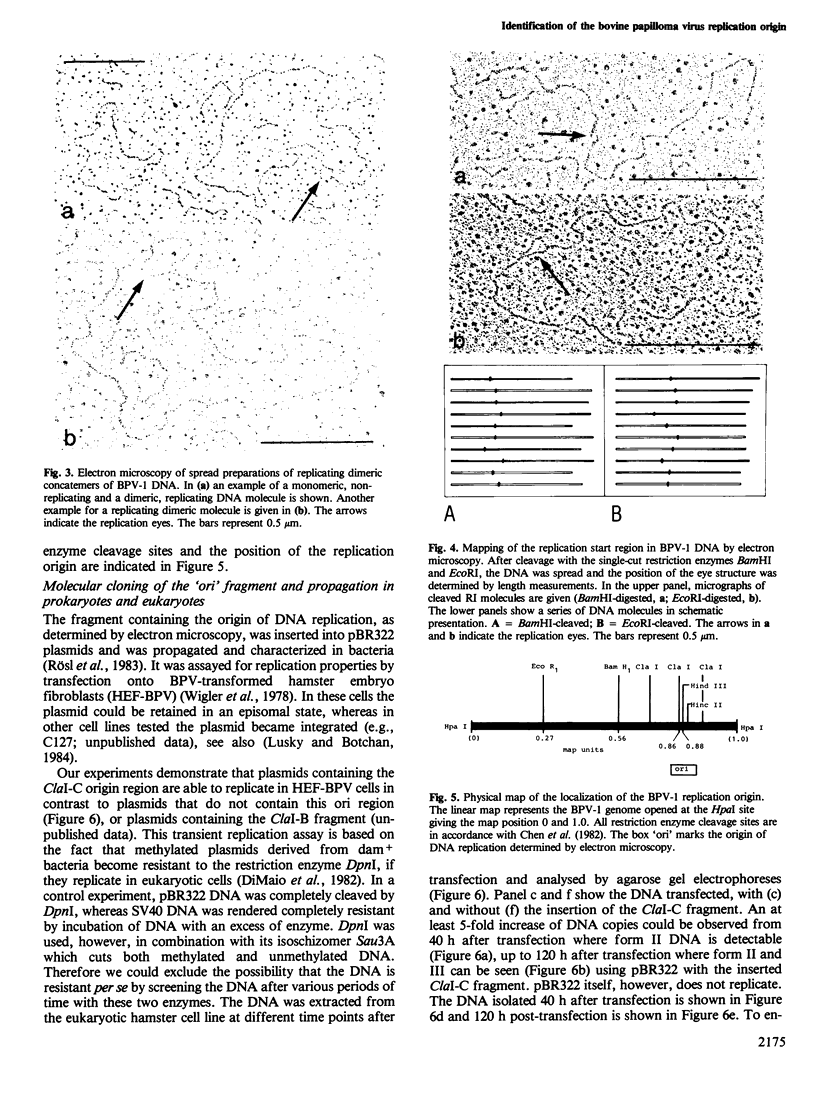
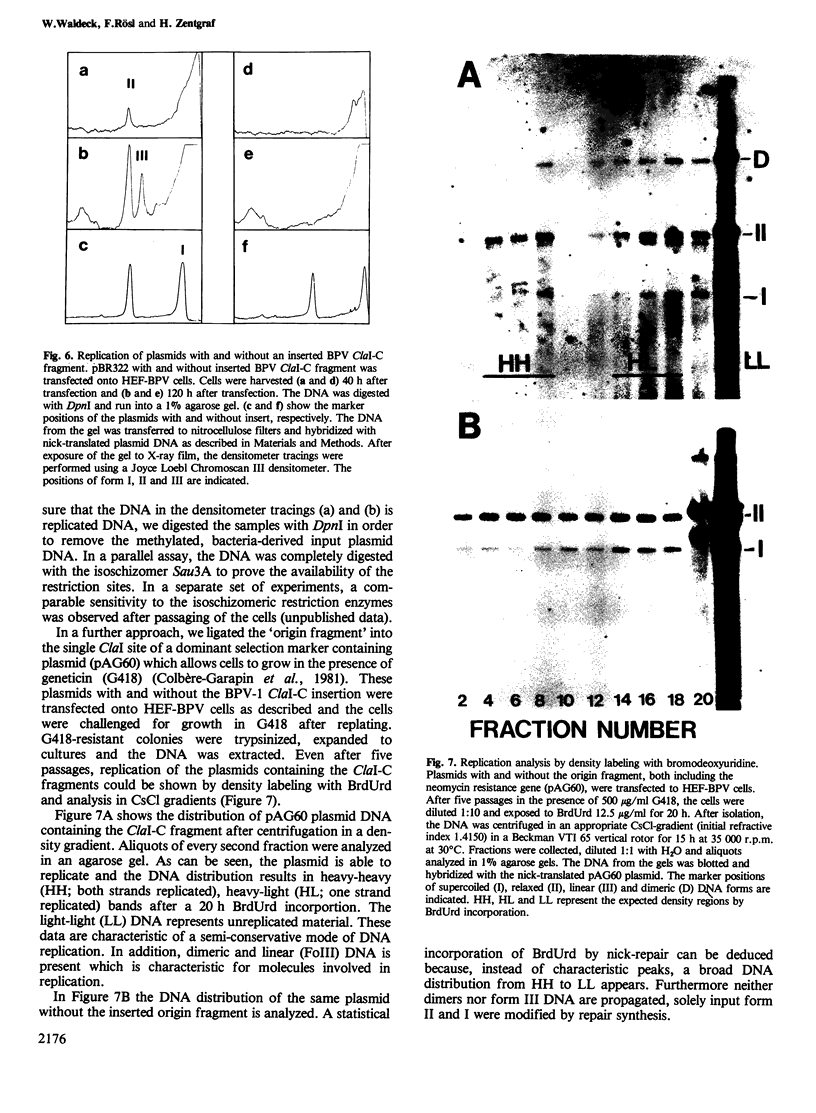
The origin of replication of bovine papilloma virus type 1 (BPV-1) has been determined by isolating replicative intermediates (RI) of BPV-transformed hamster embryo fibroblasts (HEF-BPV). These RI were treated with single cut restriction enzymes to determine the start-position (origin) of the extending replication eyes using electron microscopic techniques. 'Cairns'-type RI molecules were shown to contain one replication eye in monomeric as well as in dimeric molecules. The position of this eye was localized at 6940 +/- 5% bp in the physical map. In a second set of experiments BPV-1 DNA fragments cloned in pBR322 were tested for transient episomal replication. Transfected cells were harvested after increasing periods of time and screened for replication with isoschizomeric restriction enzymes to differentiate between input and replicated DNA. The part of the BPV genome harboring the replication origin spans the BPV ClaI-C restriction fragment corresponding to the non-coding region of the BPV genome and coincides with the DNase I-hypersensitive control region in the chromatin, isolated from transformed cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amtmann E., Müller H., Sauer G. Equine connective tissue tumors contain unintegrated bovine papilloma virus DNA. J Virol. 1980 Sep;35(3):962–964. doi: 10.1128/jvi.35.3.962-964.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright I. L., Abmayr S. M., Fleischmann G., Lowenhaupt K., Elgin S. C., Keene M. A., Howard G. C. Chromatin structure and gene activity: the role of nonhistone chromosomal proteins. CRC Crit Rev Biochem. 1982;13(1):1–86. doi: 10.3109/10409238209108709. [DOI] [PubMed] [Google Scholar]
- Chen E. Y., Howley P. M., Levinson A. D., Seeburg P. H. The primary structure and genetic organization of the bovine papillomavirus type 1 genome. Nature. 1982 Oct 7;299(5883):529–534. doi: 10.1038/299529a0. [DOI] [PubMed] [Google Scholar]
- Colbère-Garapin F., Horodniceanu F., Kourilsky P., Garapin A. C. A new dominant hybrid selective marker for higher eukaryotic cells. J Mol Biol. 1981 Jul 25;150(1):1–14. doi: 10.1016/0022-2836(81)90321-1. [DOI] [PubMed] [Google Scholar]
- Conrad S. E., Liu C. P., Botchan M. R. Fragment spanning the SV40 replication origin is the only DNA sequence required in cis for viral excision. Science. 1982 Dec 17;218(4578):1223–1225. doi: 10.1126/science.6293055. [DOI] [PubMed] [Google Scholar]
- Dagert M., Ehrlich S. D. Prolonged incubation in calcium chloride improves the competence of Escherichia coli cells. Gene. 1979 May;6(1):23–28. doi: 10.1016/0378-1119(79)90082-9. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- DiMaio D., Treisman R., Maniatis T. Bovine papillomavirus vector that propagates as a plasmid in both mouse and bacterial cells. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4030–4034. doi: 10.1073/pnas.79.13.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Harland R. M., Laskey R. A. Regulated replication of DNA microinjected into eggs of Xenopus laevis. Cell. 1980 Oct;21(3):761–771. doi: 10.1016/0092-8674(80)90439-0. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Koch M. A., Eggers H. J., Anderer F. A., Schlumberger H. D., Frank H. Structure of simian virus 40. I. Purification and physical characterization of the virus particle. Virology. 1967 Jul;32(3):503–510. doi: 10.1016/0042-6822(67)90302-9. [DOI] [PubMed] [Google Scholar]
- Law M. F., Lowy D. R., Dvoretzky I., Howley P. M. Mouse cells transformed by bovine papillomavirus contain only extrachromosomal viral DNA sequences. Proc Natl Acad Sci U S A. 1981 May;78(5):2727–2731. doi: 10.1073/pnas.78.5.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A. J., Kang H. S., Billheimer F. E. DNA replication in SV40 infected cells. I. Analysis of replicating SV40 DNA. J Mol Biol. 1970 Jun 14;50(2):549–568. doi: 10.1016/0022-2836(70)90211-1. [DOI] [PubMed] [Google Scholar]
- Lusky M., Botchan M. R. Characterization of the bovine papilloma virus plasmid maintenance sequences. Cell. 1984 Feb;36(2):391–401. doi: 10.1016/0092-8674(84)90232-0. [DOI] [PubMed] [Google Scholar]
- Nordheim A., Rich A. Negatively supercoiled simian virus 40 DNA contains Z-DNA segments within transcriptional enhancer sequences. Nature. 1983 Jun 23;303(5919):674–679. doi: 10.1038/303674a0. [DOI] [PubMed] [Google Scholar]
- Pomerantz B. J., Naujokas M., Hassell J. A. Homologous recombination between transfected DNAs. Mol Cell Biol. 1983 Sep;3(9):1680–1685. doi: 10.1128/mcb.3.9.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rösl F., Waldeck W., Sauer G. Isolation of episomal bovine papillomavirus chromatin and identification of a DNase I-hypersensitive region. J Virol. 1983 May;46(2):567–574. doi: 10.1128/jvi.46.2.567-574.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott W. A., Wigmore D. J. Sites in simian virus 40 chromatin which are preferentially cleaved by endonucleases. Cell. 1978 Dec;15(4):1511–1518. doi: 10.1016/0092-8674(78)90073-9. [DOI] [PubMed] [Google Scholar]
- Steffen M., Krieg P., Pernfuss M., Sauer E., Eisinger V., Sauer G. Growth dynamics of a latent primate papovavirus. J Virol. 1980 Sep;35(3):865–875. doi: 10.1128/jvi.35.3.865-875.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P., Macasaet F. Simian virus 40 deoxyribonucleic acid synthesis: analysis by gel electrophoresis. J Virol. 1972 Oct;10(4):599–604. doi: 10.1128/jvi.10.4.599-604.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshavsky A. J., Sundin O., Bohn M. A stretch of "late" SV40 viral DNA about 400 bp long which includes the origin of replication is specifically exposed in SV40 minichromosomes. Cell. 1979 Feb;16(2):453–466. doi: 10.1016/0092-8674(79)90021-7. [DOI] [PubMed] [Google Scholar]
- Waldeck W., Föhring B., Chowdhury K., Gruss P., Sauer G. Origin of DNA replication in papovavirus chromatin is recognized by endogenous endonuclease. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5964–5968. doi: 10.1073/pnas.75.12.5964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldeck W., Theobald M., Zentgraf H. Catenation of DNA by eucaryotic topoisomerase II associated with simian virus 40 minichromosomes. EMBO J. 1983;2(8):1255–1261. doi: 10.1002/j.1460-2075.1983.tb01578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R. Biochemical transfer of single-copy eucaryotic genes using total cellular DNA as donor. Cell. 1978 Jul;14(3):725–731. doi: 10.1016/0092-8674(78)90254-4. [DOI] [PubMed] [Google Scholar]