Abstract
Background
Gastric Helicobacter pylori colonization leads to iron deficiency anemia (IDA), especially in children and adolescents. However the pathogenesis is poorly understood.
Objective
We sought to identify specific H. pylori genes involved in IDA development, by comparing bacterial genome-wide expression profiling in patients affected or not.
Methods
H. pylori were isolated from four children with IDA and four from matched controls without IDA. Based on these isolates, cDNA microarrays under iron-replete or depleted conditions were systematically performed to compare gene expression profiles at the whole genome level. Real-time reverse-transcription (RT-) PCR and protein assays were performed for further assessing the profile differentiation of the identified H. pylori IDA-associated genes.
Results
We identified 29 and 11 genes with significantly higher or lower expression in the IDA isolates compared to non-IDA isolates, respectively. Especially notable were higher expression of sabA gene encoding sialic acid-binding adhesin in the IDA isolates, which was confirmed by real-time RT-PCR study. Moreover, iron-depletion in vitro led to up-regulation of fecA1 and frpB1 genes and down-regulation of pfr, as predicted. Known iron-regulated genes such as fur, pfr, fecA, and feoB did not significantly differ between both groups. The IDA isolates had significantly higher expression of vacuolating cytotoxin gene vacA than non-IDA isolates, consistent with the results of VacA protein assays. There were no significant differences in bacterial growth value between IDA and non-IDA isolates.
Conclusions
It is likely that H. pylori carrying high expression of sabA causes IDA, especially in children and adolescents who have increased daily iron demand. In addition, it is possible that several host-interactive genes, including vacA, may play a synergistic role for sabA in IDA development.
Introduction
Helicobacter pylori is closely associated with the development of peptic ulcer disease [1]. Long-term H. pylori colonization also leads to atrophy and intestinal metaplasia, pre-disposing to adenocarcinoma in adults [2]. Many H. pylori characteristics have been related to disease risk, including the cag island encoding both Type IV secretion system and cagA, particular vacA genotypes and genes encoding outer membrane proteins (OMPs), including blood group antigen-binding adhesin BabA, sialic acid-binding adhesin SabA, and outer membrane inflammatory protein OipA [3–5].
H. pylori also induces iron deficiency and iron deficiency anemia (IDA), especially in children and adolescents [6]. Three principal hypotheses have been advanced to explain these observations. First, IDA may be caused by blood loss from H. pylori-induced gastroduodenal lesions. However, most H. pylori-infected children do not have hemorrhagic mucosal lesions [1]. A second hypothesis is based on H. pylori-induced gastric atrophy, with resulting impaired gastric acidity in adults [7]. Non-heme iron accounts for >80% of dietary iron in developed countries and reduction from the ferric (Fe+++) to ferrous (Fe++) form is essential for intestinal absorption; both gastric acid and ascorbic acid play roles in this process [8]. In H. pylori-infected children, however, significant gastric atrophy is rare [9] and gastric acid secretion is not impaired [10].
Third, competition between H. pylori and humans for iron availability could lead to IDA [6]. Many bacteria secrete high-affinity ferric chelators (siderophores), and take up ferric iron-siderophore complexes via specific OMPs, mediated by the energy-transducing TonB-ExbB-ExbD system [11]. Although H. pylori does not have an identified siderophore or its specific receptor, the microorganism expresses proteins associated with iron metabolism, including the ferric uptake regulator (Fur), high-affinity transporters of ferrous iron (FeoB) and ferric dicitrate (FecA), and non-heme iron-containing ferritin (Pfr) [12]. Although pfr or feoB variation has not been implicated [13, 14], comparative proteomic analysis suggests that particular H. pylori polymorphisms could promote IDA [15]. Several iron-responsive OMPs may play roles in H. pylori heme uptake [16, 17].
Knowledge of iron-uptake mechanism in H. pylori is limited. Using a whole-genome DNA microarray, we sought to identify specific H. pylori IDA-associated genes, by comparing bacterial gene expression profiling in patients with or without IDA.
Materials and methods
H. pylori strains
H. pylori strains were isolated from four children aged 13–16 years (two male/two female) with IDA (strains TH1, TH2, TH4, and TH6) and from four age- and sex-matched controls without IDA (control strains TH3, TH5, TH7, and TH8). All four IDA patients had hypochromic microcytic anemia (range of serum hemoglobin values, 6.1–7.4 g/dL) with low serum iron (range, 6–12 μg/dL) and ferritin values (range, 1.1–4.9 ng/mL). In control subjects, ranges of serum hemoglobin, iron, and ferritin values were 13.9–15.1 g/dL, 80–132 μg/dL and 17.5–34.4 ng/mL, respectively. Fecal occult blood tests using anti-hemoglobin antibodies were negative in all eight subjects. Routine biochemical examinations, including liver function tests and total serum protein and albumin, were normal. The patients with and without IDA were referred to Tohoku University Hospital because of moderate or severe IDA or with gastrointestinal symptoms, such as epigastric pain or persistent nausea. All subjects underwent upper gastrointestinal endoscopy and gastric biopsies were obtained. For the IDA patients, upper gastrointestinal endoscopy was performed to examine for gastrointestinal bleeding. Endoscopy showed chronic gastritis without mucosal bleeding in all eight patients; the biopsy specimens were histologically studied and used for diagnosis of H. pylori infection including culture. After the presence of H. pylori was confirmed, the four patients without IDA were regarded as H. pylori-positive non-IDA controls. The IDA patients received a 7-day course of eradication therapy with lansoprazole, amoxicillin and clarithromycin [18]. The patients also received iron supplementation for two months. In all 4 IDA patients, success of H. pylori eradication was confirmed by 13C-urea breath test [19] performed 4 weeks after the completion of eradication therapy. In each case, IDA was substantially improved and no recurrence was observed in any patients at 6 to 12-month follow-up. H. pylori culture from the gastric biopsy specimens was performed under microaerobic conditions (5% O2, 15% CO2, and 80% N2) at 37°C for 72 hour on Mueller-Hinton agar (Eiken, Tokyo, Japan) supplemented with 5% defibrinated sheep blood (Sigma Chemical Co., St Louis MO).
Ethics statement
This study was reviewed and approved by the Ethics Committee of Tohoku University School of Medicine. Written informed consent was obtained from parents of the child participants on their behalf.
Gastric histology
Biopsy specimens obtained from the gastric body and antrum were examined in both IDA and control patients according to the updated Sydney system [20]. The degrees of H. pylori density, neutrophil (activity) and mononuclear cell infiltration (inflammation), atrophy, and intestinal metaplasia were analyzed.
cDNA microarray
For liquid cultures, H. pylori cells were inoculated to OD595 of 0.05–0.10 and cultured in Brucella broth containing 7% horse serum with shaking under microaerobic conditions. Total RNA was purified from each culture using ChargeSwitch® Total RNA Cell Kits (Invitrogen Corporation, Carlsbad CA). The integrity of the purified RNA was verified using 1% agarose gel electrophoresis. The Helicobacter pylori 4-plex gene expression microarray (Roche NimbleGen Madison WI) was used, which represents 1,576 protein-coding genes in the H. pylori 26695 genome (accession number NC_000915, 72,000 features/array). A total of 10 μg of bacterial RNA was processed and labeled as per the standard NimbleChip protocol. Briefly, RNA was converted into cDNA using the SuperScript II cDNA Conversion Kit (Invitrogen). Double-stranded cDNA was random-prime labeled with Cy3-nonamers and hybridized to the microarray for 16 hour at 42°C. The arrays were scanned at 5 μm resolution using a GenePix 4000B microarray scanner (Molecular Devices, Sunnyvale CA). Data were extracted from scanned images using NimbleScanTM software. Quantile normalization was performed across replicate arrays, and RMA (Robust Multiple Average) analysis was performed to generate gene expression values, which were compared between IDA and control strains.
Study of expression of iron-regulated genes
To examine gene regulation by ferric iron, cDNA microarray analysis also was performed for liquid cultures of H. pylori under iron-restricted conditions. Two IDA strains (TH4 and TH6) and two control strains (TH3 and TH7) were cultured in Brucella broth containing 5% horse serum with and without 50 μM of the iron chelating reagent deferoxamine mesylate (DFM) under microaerobic conditions as above. Total RNA was purified from exponential-phase (OD600 = 0.5–0.9) broth cultures and cDNA microarray was performed as indicated above. For each isolate, gene expression values were compared for RNA samples from liquid culture with and without 50 μM DFM.
Assessing gene expression by real-time RT-PCR
Expression of sabA, sabB, coaX, and vacA in the eight H. pylori strains was determined by real-time RT-PCR using the SYBR Green method (Table 1). Absolute quantitation analysis was performed with the ABI real time system 7500 using a standard curve for each gene. The primer sets which were previously reported were used for vacA [21].
Table 1. Primers used for PCR in this study.
| Gene | Primer | Sequence | Reference |
|---|---|---|---|
| sabB | HP0722-F | CCCAACTGGCTTCGTTAAAA | This study |
| HP0722-R | TGGGTATCATCGCCTTAATGT | This study | |
| sabA | HP0725-F | CCAACAACATTGAGCTGGTC | This study |
| HP0725-R | TTGCAARATRGGTATCATCG | This study | |
| coaX | HP0682-F | AAGTGGGGGCGATGTATGCT | This study |
| HP0682-R | CACGCGCAATAAATGCTCTTTG | This study | |
| HP0682-F2 | CAAGGGGTGAATTAGGCAAA | This study | |
| HP0682-R2 | CCAAGCATGCCCAAAACTAT | This study | |
| vacA | VA1-F | ATGGAAATACAACAAACACAC | Ref. 21 |
| VA1-R | CTGCTTGAATGCGCCAAAC | Ref. 21 | |
| VA3-F | GGTCAAAATGCGGTCATGG | Ref. 21 | |
| VA3-R | CCATTGGTACCTGTAGAAAC | Ref. 21 | |
| VA4-F | GGAGCCCCAGGAAACATTG | Ref. 21 | |
| VA4-R | CATAACTAGCGCCTTGCAC | Ref. 21 |
Assessing vacA genotype, expression, and VacA synthesis
vacA genotyping
To evaluate the vacA genotype of each H. pylori isolate, the vacA signal region (s1 or s2 alleles) with the products differentiated on the basis of molecular size (259bp or 286bp) and the middle regions (m1 or m2 alleles) were amplified (Table 1), as described [21]. Genomic DNA was extracted from each strain using the MagExtractor kit (Toyobo, Osaka).
Immunoblot analysis
A 72-hour culture of each isolate was centrifuged at 8,500 g and passaged through a 0.45 μm pore size membrane filter (Millipore) to remove bacteria, and supernatants analyzed for the VacA protein. In brief, 15 μL of sample was mixed with an equal volume of loading buffer (EZ apply, ATTO) and boiled for 3 min. The sample was electrophoresed through a 12.5% sodium dodecyl sulfate (SDS)-polyacrylamide gel. Migrated proteins then were electrophoretically transferred (0.14 mA for 1 hour) onto a PVDF membrane. After incubation in blocking buffer (5% skim milk in PBS), the PVDF membrane was treated with anti-VacA primary antibody (b-300, sc-25790, Santa Cruz Biotechnology Inc.) at room temperature for 1 hour with gentle shaking. Then membranes were washed with PBS containing 0.1% Tween 20 (T-PBS) and then incubated with horseradish-peroxidase-conjugated goat anti-rabbit IgG (Santa Cruz Biotechnology Inc.) for 1 hour at room temperature. After incubation, the membrane was washed 5 times with T-PBS, and specific bands were detected using the Amersham ECL Plus Western blotting system (GE Healthcare), according to the manufacturer’s protocol.
Cytotoxic assay
Assay for VacA activity was essentially as described [22]. In brief, the 72-hour-culture filtrate obtained from each H. pylori isolate was diluted with RPMI-1640 (Sigma) containing 10% heat-inactivated fetal bovine serum (RPMI-FBS) in serial two-fold dilutions. AGS cells (5 x 104) were seeded into 96-well plates in RPMI-FBS and incubated at 37°C in 5% CO2. After 24-hour incubation, cell culture medium was removed from each well, and replaced with 200 μL of each sample. After a further 24-hour incubation, the extent of cytoplasmic vacuolation in AGS cells was observed microscopically. The titer of the vacuolating toxin was determined as the highest dilution of the culture filtrate showing vacuolation of >10% of cells and the reciprocal number of the dilution (toxin titer) was used for statistical analysis.
Statistical analyses
In cDNA microarray, a ≥3.5-fold difference in gene expression values was defined as significant. Student’s t–test with Bonferroni correction were also performed and p <0.05 was considered statistically significant. In the real-time RT-PCR study, the mean of those relative values was used for statistical analysis by the Wilcoxon rank sum test.
Results
Histological study
In the gastric body and antrum, there were no differences in the histologic features between the IDA and control groups (S1 Table). Intestinal metaplasia was not observed in any biopsy site in either group. Thus, as expected, differences in gastric histology did not explain the cause of the IDA.
Bacterial growth of IDA and control isolates
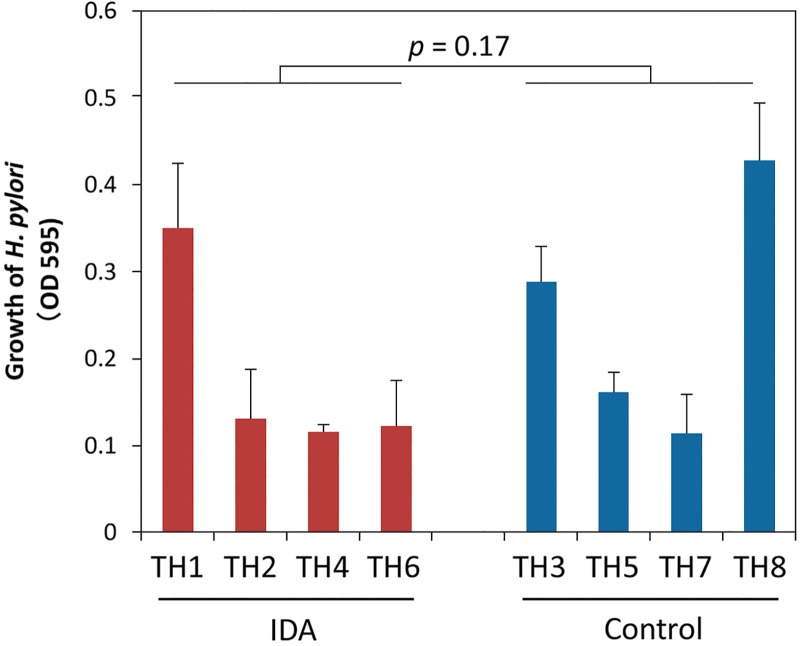
Each strain was inoculated into Brucella broth containing 5% horse serum at an initial OD595 = 0.05. There were no significant differences in bacterial growth value (three independent determinations) measured by microplate photometer (OD595) after 24-hour incubation between IDA (Mean ± SD, 0.18±0.11) and control groups (0.25±0.13) (p = 0.17) (Fig 1).
Fig 1. Growth of H. pylori strains isolated from IDA or control patients.
Each strain was inoculated into Brucella broth containing 5% horse serum at an initial OD595 0.05. The growth of H. pylori was measured (OD595) by microplate photometer after 24-hour incubation. The Mean±SD 24-hour growth value was measured based on three independent determinations for each strain. Bacterial growth was not significantly different between the IDA and control strains (p = 0.17; Wilcoxon rank sum test).
Reliability of microarray data
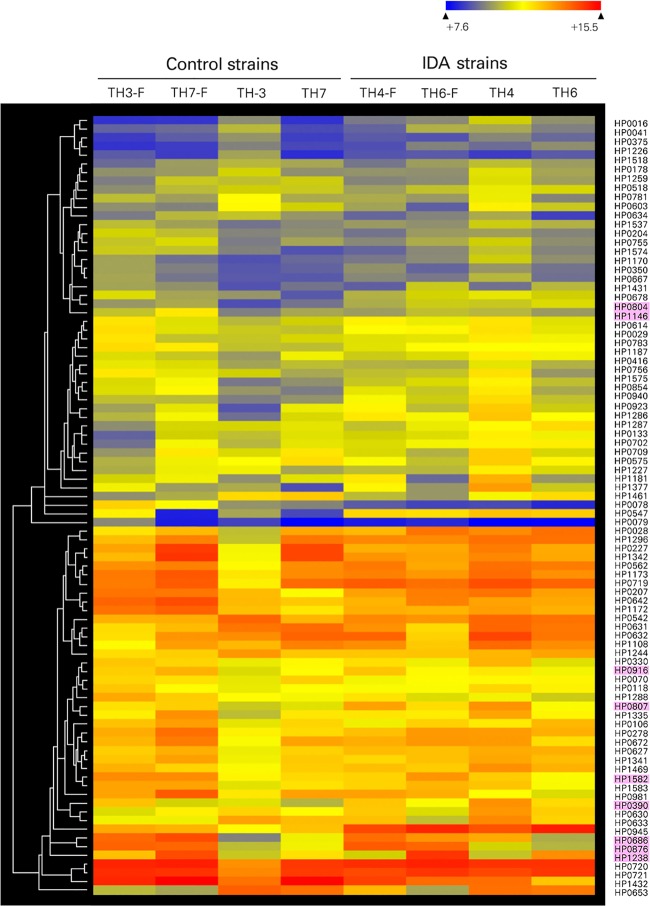
Comparing the gene expression profiles of H. pylori isolates with microarray data clearly indicated differential expression of several genes known to be Fur-regulated under iron-limited conditions (Fig 2 and S2 Table). Under iron-restricted conditions, pfr was down-regulated while fecA1 (HP0686) encoding iron (III) dicitrate transport protein and frpB1 genes (HP0876) encoding iron-regulated OMP were up-regulated in both the IDA and control strains (Table 2), consistent with prior observations of iron-regulated expression [23]. Expression of fur and feoB did not significantly differ between iron-replete and -restricted conditions in either group. The expression of amiF was up-regulated 4.3-fold by iron starvation, confirming a prior report [24]. In total, results of iron-regulated gene expression under iron-restricted conditions confirm the microarray data in the present study.
Fig 2. Hierarchical cluster analysis of gene expression alteration of control H. pylori strains under iron-replete and limited conditions.
Each strain was cultured with DFM (iron-limited condition, indicated as TH3-F, TH7-F, TH4-F, and TH6-F) or without DFM (iron-replete condition, indicated as TH3, TH7, TH4, and TH6). The genes with significant differences in expression were included in hierarchical clustering analysis using average linkage and Euclidean dissimilarity methods. Genes with a Fur-binding site consensus sequence are indicated in pink.
Table 2. Expression of H. pylori genes known to be related to iron metabolism.
| Iron replete | Iron restricted/replete | ||||
|---|---|---|---|---|---|
| condition: | ratio in: | ||||
| Gene designation and category | Gene name | IDA/control ratio a | p value | Control | IDA |
| Non-heme iron ferritin: | |||||
| HP0653 | pfr | 1 | 1 | 0.1 b | 0.3 b |
| Ferric uptake regulator: | |||||
| HP1027 | fur | 0.9 | 1 | 1 | 1.9 |
| Iron (Fe++) transport protein: | |||||
| HP0687 | feoB | 1.3 | 1 | 1.3 | 0.8 |
| Iron (Fe+++) transport protein: | |||||
| HP0807 | fecA1 | 0.9 | 1 | 11.2b | 4.0 b |
| HP0686 | fecA2 | 0.9 | 1 | 1.8 | 1.2 |
| HP1400 | fecA3 | 0.8 | 0.52 | 0.6 | 0.8 |
| Iron-regulated OMP: | |||||
| HP0876 | frpB1 | 0.2 | 0.004 | 8.4 b | 17.3 b |
| HP0915 | frpB2 | 0.6 | 0.61 | 1 | 1.3 |
| HP0916 | frpB3 | 0.9 | 0.61 | 1 | 0.9 |
| HP1512 | frpB4 | 1.1 | 1 | 1 | 1 |
a Values shown are mean of triplicate determinations.
b p values in the control versus IDA group are <0.001.
Possible IDA-related genes in microarray analysis
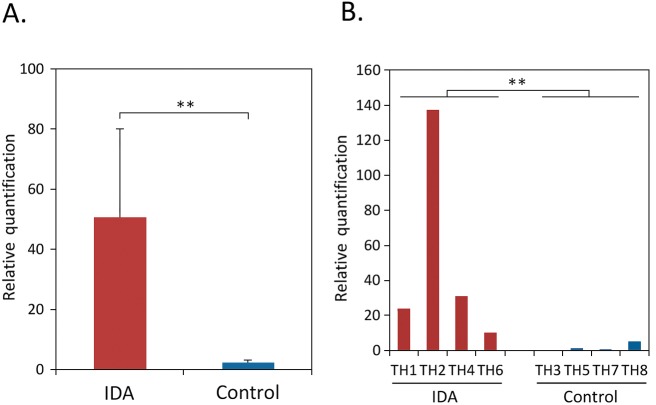
By microarray analysis, 29 genes had significantly higher expression in the IDA group than in the controls (Table 3). These included the genes encoding two OMPs, SabA and its homolog SabB. The real-time RT-qPCR analysis indicated that sabA expression was detected in all four IDA and three control strains; relative expression in the IDA group was significantly higher than in the controls (p = 0.029) (Fig 3). In contrast, expression of sabB was detected by RT-qPCR only in one control (TH8) and two IDA strains (TH2 and TH6). Although coaX showed 84-fold higher expression in the IDA group (Table 3), the expression in the real-time RT-qPCR analysis was not demonstrated in one IDA (TH4) and two control strains (TH3 and TH7); there was no significant difference between both groups (data not shown).
Table 3. H. pylori genes with higher expressiona in IDA strains.
| Gene designation | Gene name | Expression ratio | |||
|---|---|---|---|---|---|
| and category | Predicted function | (IDA / control) | p value | ||
| Cell envelope: | |||||
| HP0722 | sabB (HopO) | adhesin | 10.9 | < 0.001 | |
| HP0725 | sabA (HopP) | adhesin | 3.7 | < 0.001 | |
| HP0217 | cgtA | LPS synthesis | 3.6 | 0.002 | |
| DNA metabolism: | |||||
| HP0142 | mutY | DNA repair | 5.4 | < 0.001 | |
| HP0462 | hsdS | restriction system | 7.3 | < 0.001 | |
| HP0463 | hsdM | restriction system | 10.6 | < 0.001 | |
| HP0592 | res | restriction system | 3.6 | 0.003 | |
| Cellular processes: | |||||
| HP0682 | coaX | CoA synthesis | 84.0 | 0.003 | |
| HP1510 | folB | folic acid synthesis | 3.7 | < 0.001 | |
| HP1511 | predicted frpB-like protein | 3.6 | < 0.001 | ||
| Other categories: | |||||
| HP0428 | terY | phage/colicin | 3.5 | 0.02 | |
| HP0414 | transposase A | 4.3 | 0.018 | ||
| HP0731 | leoA | toxin production | 3.9 | < 0.001 | |
| HP1000 | partition protein A | 6.0 | < 0.001 | ||
| Hypothetical proteins: | |||||
| HP0016 | unknown | 5.2 | < 0.001 | ||
| HP0059 | unknown | 3.9 | 0.001 | ||
| HP0120 | unknown | 6.0 | < 0.001 | ||
| HP0199 | unknown | 4.1 | 0.001 | ||
| HP0435 | COG1112 superfamily DNA and RNA-helicase | unknown | 3.7 | 0.022 | |
| HP0436 | COG3843, virD2 | unknown | 5.9 | 0.015 | |
| HP0489 | unknown | 3.6 | 0.03 | ||
| HP0583 | unknown | 4.5 | < 0.001 | ||
| HP0999 | unknown | 4.6 | 0.021 | ||
| HP1116 | unknown | 3.5 | 0.047 | ||
| HP1165 | tetA | unknown | 4.3 | < 0.001 | |
| HP1334 | COG1432 | unknown | 3.8 | < 0.001 | |
| HP1371 | type III restriction enzyme R protein | unknown | 4.1 | 0.001 | |
| HP1388 | unknown | 4.2 | 0.036 | ||
| HP1589 | COG4735 | unknown | 3.8 | 0.004 | |
a As determined by microarray analysis (see Methods).
Fig 3. Transcription of sabA gene in IDA or control strains by real-time RT-qPCR.
Panel A: overall expression (relative quantitation) of sabA in the four IDA and four control strains, respectively. Panel B: expression (relative quantitation) of sabA in the eight strains studied each. Expression of sabA was normalized in relation to house-keeping gene gyrA. For relative quantitation, H. pylori TH5 (control) strain was used as control, because expression value of the strain was the least (defined as 1.0). Panel A: results are shown as the Mean ± SE for triplicate determinations. ** p = 0.029, using Wilcoxon rank sum test.
On the other hand, 11 genes showed significantly lower expression in the IDA group, including ceuE1, encoding an iron ABC transporter, and frpB1, encoding a heme and hemoglobin-binding membrane protein (Table 4). Five of the 29 genes with significantly higher expression in the IDA strains are related to DNA-processing (Table 3), as are two of the 11 genes with lower IDA-strain expression (Table 4).
Table 4. H. pylori genes with lower expression in IDA strains.
| Gene designation | Gene name | Expression ratio | ||||||
|---|---|---|---|---|---|---|---|---|
| and category | Predicted function | (IDA / control) | p value | |||||
| Iron transport: | ||||||||
| HP0876 | frpB1 | Iron-regulated outer membrane protein | 0.22 | 0.022 | ||||
| HP1561 | ceuE1 | Iron(III) ABC transporter | 0.02 | < 0.001 | ||||
| Cell envelope: | ||||||||
| HP1125 | palA (omp18) | Peptidoglycan associated lipoprotein precursor | 0.03 | < 0.001 | ||||
| DNA metabolism: | ||||||||
| HP1209 | iceA | Ulcer-associated gene, restriction endonuclease | 0.15 | 0.003 | ||||
| HP1352 | Type II methylase | 0.20 | < 0.001 | |||||
| Hypothetical proteins: | ||||||||
| HP0168 | Unknown | 0.17 | < 0.001 | |||||
| HP 0187 | Unknown | 0.16 | < 0.001 | |||||
| HP0556 | Unknown | 0.21 | < 0.001 | |||||
| HP0641 | Unknown | 0.06 | < 0.001 | |||||
| HP1289 | Unknown | 0.09 | 0.008 | |||||
| HP1324 | Unknown | 0.07 | < 0.001 | |||||
Expression of H. pylori genes related to host interaction
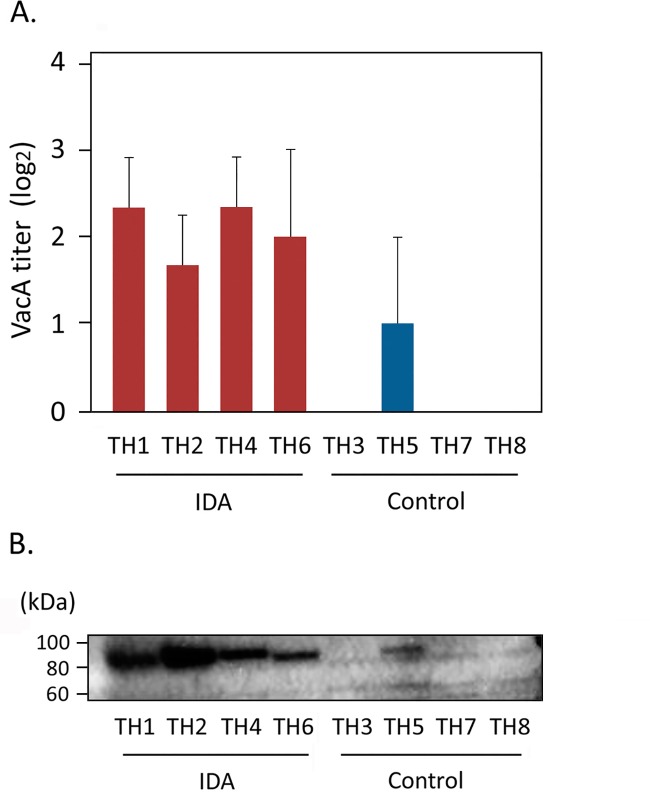
In the microarray analysis, vacA in the IDA strains was highly expressed (3.2-fold difference) compared to the controls. There were no differences in vacA expression of the IDA group between iron-replete and -restricted conditions. By RT-qPCR, there were no significant differences in vacA expression between IDA and control strains (p = 0.77). However, in both cytotoxic assays and immunoblot analysis, all four IDA and one control isolate secreted detectable amounts of the VacA protein (Fig 4). In the vacA genotype analysis, three IDA and one control strains carried the s1/m1 alleles. One IDA and two control strains carried the s1 allele but the mid region allele was not identified. One control strain had s1/m2 alleles. Real-time RT-qPCR showed that mRNA of vacA (signal region) was detected in all four IDA strains but only one control strain carrying the s1/m1 alleles in the vacA genotype analysis. In the microarray analysis, all eight isolates studied carried the cag pathogenicity island (data not shown).
Fig 4. VacA activity in IDA and control strains.
Panel A: H. pylori cytotoxic activity. Filtrate obtained from 72-hour cultures of each H. pylori strain was serially diluted and added to AGS cell cultures. After 24-hour incubation, the final dilution with visible vacuoles was determined microscopically and reciprocal number of the dilution used for calculation. Panel B: Immunoblot analysis of culture filtrate of the eight H. pylori strains.
The expression of cagN and babB were significantly lower in the IDA group than in the control (Table 5). For the IDA strains, there was no difference in the expression of these genes between iron-replete and restricted culture conditions, nor were there differences for sabA and sabB. In total, these results indicated that IDA strains differ from the control strains in expression of several genes related to host interaction, including vacA.
Table 5. Expression of H. pylori OMPs and genes associated with host interaction.
| Iron replete | Iron restricted/replete | ||||
|---|---|---|---|---|---|
| Gene designation | Gene | Ratio in: | Ratio in: | ||
| and category | name | IDA/Control | p value a | Control | IDA |
| Major OMP family: | |||||
| HP1243 | babA | 1.1 | 1 | 1 | 1.1 |
| HP0896 | babB | 0.4 | <0.001 | 0.7 | 0.8 |
| HP0638 | oipA | 1.3 | 1 | 0.8 | 1.1 |
| HP0725 | sabA | 3.7 | <0.001 | 0.9 | 1 |
| HP0722 | sabB | 10.9 | <0.001 | 0.9 | 1.1 |
| Host interaction: | |||||
| HP0243 | napA | 1.4 | 0.85 | 1.2 | 0.7 |
| HP0887 | vacA | 3.2 | <0.001 | 0.7 | 0.7 |
| HP0547 | cagA | 0.2 | 0.18 | 1.6 | 0.9 |
| HP0538 | cagN | 0.5 | <0.001 | 0.9 | 0.9 |
a Student’s t–test with Bonferroni correction.
Discussion
By isolating H. pylori strains from children with IDA and from age- and sex-matched control without IDA, and then systemically comparing gene expression profiles under iron-replete or depleted conditions, we identified a group of H. pylori genes with significantly different expression between the IDA and control groups. IDA was associated with sabA, encoding the sialic acid-binding adhesin (SabA), which mediates H. pylori binding to human gastric epithelial cells during colonization [25]. Binding to glycosylated epithelial cells is essential for H. pylori to persistently colonize and adherence to gastric mucosa is dependent on SabA [25]. SabA also has been associated with gastroduodenal disease [26], playing a role in non-opsonic activation of human neutrophils [27], and contributing to bacterial persistence [28]. H. pylori sabA expression is phase-variable via both a homopolymeric T tract in its promoter region [29], and multiple CT tracts in the coding region [26]. Its product, SabA, can interact with multiple sialylated host receptors on neutrophils, sialyl-Lewisx [28], sialyl-Lewisa [25], laminin, and zinc-alpha 2-glycoprotein [25, 27, 28]. Our identification of higher expression in IDA suggests a link between H. pylori-signaling to host cells and iron deficiency.
The IDA strains had significantly higher vacA expression than in controls, consistent with the results of VacA protein assays (Fig 4). H. pylori isolates carrying vacA s1/m1genotypes show higher vacuolating activity than those carrying other genotypes, and are more frequently associated with gastric pathologies [3]. One hypothesis is that strains with particular vacA genotypes may more effectively obtain iron under iron-depleted conditions through their interactions with host cells. The VacA protein also interferes with the T cell/interleukin-2 signaling pathway and efficiently blocks T cell proliferation [30]. A prior study using mRNA expression profiling suggested that VacA escapes host immune defenses by differentially regulating the expression of host genes related to immune evasion [31]. It is possible that host-interactive genes including vacA and sabA, may play synergistic roles in H. pylori-associated IDA development by enhancing chronic inflammation. The VacA protein induces cytotoxicity that can damage host cells [21, 32]. This may lead to increased iron concentrations in tissue. We hypothesize that H. pylori cells that highly express sabA take up iron via SabA, an outer membrane protein; however, examining this hypothesis will require further study.
It is possible that reduced iron in tissue results in overexpression of sabA. The IDA and control patients, who are age- and sex-matched, have histological chronic gastritis to a comparable extent. There are no other obvious differences between the groups except for the presence or absence of IDA. The microarray study showed that in both IDA and control strains, several known iron-regulated genes were expressed, as reported [23, 24]. Moreover, our study showed that IDA strains consistently expressed sabA to a high degree in both replete and restricted iron conditions (Table 5). Significantly higher expression of sabA was also demonstrated by real-time RT-PCR. These data are consistent with the hypothesis that high-level expression of SabA is involved in the causation of IDA, rather than the consequence. However, this hypothesis must be tested directly in future studies.
One limitation of the present study is a small sample size, which may not be sufficient to fully examine the findings. In the present study, we compared the strains from H. pylori-positive children with and without IDA, and we restricted the analysis to moderate or severe IDA but not mild IDA. We believe that mild IDA is not suitable for narrowing down the candidate genes, because mild IDA can be induced by causes other than H. pylori. It is not common for children to present for medical attention with moderate or severe H. pylori-related IDA, in whom both intestinal bleeding and insufficient dairy iron intake have been excluded. Therefore, we studied strains from only four of these highly targeted patients. Even with such a small group, we did demonstrate that there is a significant difference in sabA expression between the strains from the IDA and control groups.
The endoscopic and histological diagnosis in all of the patients with and without IDA is chronic gastritis induced by H. pylori, and the only consistent difference between cases and controls was the presence or absence of IDA. Therefore, we believe that the strains from non-IDA patients are suitable as controls for IDA strains. In addition, we used a 3.5-fold difference in gene expression as the threshold for considering significant differences between the groups of strains. The choice was empirical because at the threshold of 3.5-fold difference, there were 40 implicated genes, but at 3.0, there were 71 genes. To minimize false discovery, we used the higher threshold for increased stringency.
H. pylori frpB1 and ceuE1, both Fur-regulated [33], are involved in iron uptake. That H. pylori isolates from IDA patients express frpB1 and ceuE1 at lower levels than control strains may reflect that H. pylori has ample iron in the IDA milieu. Another gene with lower expression in the IDA strains, palA (HP1125), encodes a peptidoglycan-associated lipoprotein that enhances Th1 responses by activating dendritic cell maturation [34]. Whether the low palA expression contributes to IDA or is a consequence is uncertain.
Few studies have focused upon iron-associated H. pylori genes in the context of IDA pathogenesis. Protein sequences encoded by pfr do not differ in isolates from patients with or without IDA [13], nor has feoB been implicated [14]. In addition to these genes, the present microarray data do not show involvement of the major iron-regulated genes, including fur and fecA, in the pathogenesis of H. pylori-induced IDA, nor is there an association between nikR, encoding a nickel-dependent regulator protein, and IDA (data not shown).
In iron-replete environments, H. pylori expresses a single fecA3 (HP1400) and frpB4 (HP1512) OMP [12]. Under iron-restriction, however, all putative iron transport proteins are expressed [35], consistent with our finding of greater transcription of fecA1 (HP0807) and frpB1 (HP0876) under iron-restriction than repletion. That IDA strains express less frpB1 (HP0876) than control strains (Table 4), may reflect more iron availability in IDA patients.
Under iron-replete culture conditions, H. pylori isolates from IDA patients have been reported to show more rapid growth and enhanced uptake of both ferrous and ferric ions compared to those from non-IDA patients [36]. However, the similar growth of IDA and control strains under iron-replete conditions that we observed suggests that other hypotheses should be considered.
In childhood and adolescence, it is well known that daily iron demand increases especially for catch-up growth. In addition, more enhanced iron supply is necessary for athletes or pubescent females. Choe et al. reported that adolescent female athletes may have H. pylori-related IDA, suggesting that increased daily iron demand is important in childhood [37].
Supporting information
(DOCX)
(DOCX)
Acknowledgments
A part of the present study was presented at Digestive Disease Week 2016, San Diego, CA, U.S.A in 2016.
Data Availability
All of the microarray analysis results are available to the public through the GEO repository (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE101807).
Funding Statement
The work of SeK is supported by a Grant-in-Aid for Scientific Research (C) from the Japan Society for the Promotion of Science (https://www.jsps.go.jp/j-grantsinaid/index.html) to SeK (Grant No. JP19590709). The work of TO and ShK is supported by a Grant-in-Aid for Scientific Research (C) from the Japan Society for the Promotion of Science (https://www.jsps.go.jp/j-grantsinaid/index.html) to TO (Grant No. JP21590492). The work of XSZ and MJB is supported by the National Institutes of Health (R01GM63270) (https://grants.nih.gov/grants/oer.htm) and the Knapp Family Fund (https://www.theknappfamilyfoundation.org/) to MJB. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kato S, Nishino Y, Ozawa K, Konno M, Maisawa S, Toyoda S, et al. The prevalence of Helicobacter pylori in Japanese children with gastritis or peptic ulcer disease. J Gastroenterol. 2004; 39: 734–738. doi: 10.1007/s00535-004-1381-2 [DOI] [PubMed] [Google Scholar]
- 2.Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001; 345: 784–789. doi: 10.1056/NEJMoa001999 [DOI] [PubMed] [Google Scholar]
- 3.Kusters JG, van Vliet AH, Kuipers EJ. Pathogenesis of Helicobacter pylori infection. Clin Microbiol Rev. 2006; 19: 449–490. doi: 10.1128/CMR.00054-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naz A, Awan FM, Obaid A, Muhammad SA, Paracha RZ, Ahmad J, et al. Identification of putative vaccine candidates against Helicobacter pylori exploiting exoproeome and secretome: a reverse vaccinology based approach. Infect Genet Evol. 2015; 32: 280–291. doi: 10.1016/j.meegid.2015.03.027 [DOI] [PubMed] [Google Scholar]
- 5.de Klerk N, Maudsdotter L, Gebreegziabher H, Saroj SD, Eriksson B, Eriksson OS, et al. Lactobacilli reduce Helicobacter pylori attachment to host gastric epithelial cells by inhibiting adhesion gene expression. Infect Immun. 2016; 84: 1526–1535. doi: 10.1128/IAI.00163-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barabino A. Helicobacter pylori-related iron deficiency anemia: a review. Helicobacter. 2002; 7: 71–75. [DOI] [PubMed] [Google Scholar]
- 7.Annibale B, Capurso G, Lahner E, Passi S, Ricci R, Maggio F, et al. Concomitant alterations in intragastric pH and ascorbic acid concentration in patients with Helicobacter pylori gastritis and associated iron deficiency anaemia. Gut. 2003; 52: 496–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lombard M, Chua E, O'Toole P. Regulation of intestinal non-haem iron absorption. Gut. 1997; 40: 435–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kato S, Nakajima S, Nishino Y, Ozawa K, Minoura T, Konno M, et al. Association between gastric atrophy and Helicobacter pylori infection in Japanese children: a retrospective multicenter study. Dig Dis Sci. 2006; 51: 99–104. doi: 10.1007/s10620-006-3091-5 [DOI] [PubMed] [Google Scholar]
- 10.Kato S, Ozawa K, Koike T, Sekine H, Ohara S, Minoura T, et al. Effect of Helicobacter pylori infection on gastric acid secretion and meal-stimulated serum gastrin in children. Helicobacter. 2004; 9: 100–105. doi: 10.1111/j.1083-4389.2004.00205.x [DOI] [PubMed] [Google Scholar]
- 11.Andrews SC, Robinson AK, Rodriguez-Quinones F. Bacterial iron homeostasis. FEMS Microbiol Rev. 2003; 27: 215–237. [DOI] [PubMed] [Google Scholar]
- 12.van Vliet AH, Stoof J, Vlasblom R, Wainwright SA, Hughes NJ, Kelly DJ, et al. The role of the Ferric Uptake Regulator (Fur) in regulation of Helicobacter pylori iron uptake. Helicobacter. 2002; 7: 237–244. [DOI] [PubMed] [Google Scholar]
- 13.Choe YH, Hwang TS, Kim HJ, Shin SH, Song SU, Choi MS. A possible relation of the Helicobacter pylori pfr gene to iron deficiency anemia? Helicobacter. 2001; 6: 55–59. [DOI] [PubMed] [Google Scholar]
- 14.Jeon BH, Oh YJ, Lee NG, Choe YH. Polymorphism of the Helicobacter pylori feoB gene in Korea: a possible relation with iron-deficiency anemia? Helicobacter. 2004; 9: 330–334. doi: 10.1111/j.1083-4389.2004.00239.x [DOI] [PubMed] [Google Scholar]
- 15.Park SA, Lee HW, Hong MH, Choi YW, Choe YH, Ahn BY, et al. Comparative proteomic analysis of Helicobacter pylori strains associated with iron deficiency anemia. Proteomics. 2006; 6: 1319–1328. doi: 10.1002/pmic.200500293 [DOI] [PubMed] [Google Scholar]
- 16.Worst DJ, Otto BR, de Graaff J. Iron-repressible outer membrane proteins of Helicobacter pylori involved in heme uptake. Infect Immun. 1995; 63: 4161–4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dhaenens L, Szczebara F, Husson MO. Identification, characterization, and immunogenicity of the lactoferrin-binding protein from Helicobacter pylori. Infect Immun. 1997; 65: 514–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kato S, Ritsuno H, Ohnuma K, Iinuma K, Sugiyama T, Asaka M. Safety and efficacy of one-week triple therapy for eradicating Helicobacter pylori in children. Helicobacter. 1998; 3: 278–282. [DOI] [PubMed] [Google Scholar]
- 19.Kato S, Ozawa K, Konno M, Tajiri H, Yoshimura N, Shimizu T, et al. Diagnostic accuracy of the 13C-urea breath test for childhood Helicobacter pylori infection: A multicenter Japanese study. Am J Gastroenterol. 2002; 97: 1668–1673. doi: 10.1111/j.1572-0241.2002.05825.x [DOI] [PubMed] [Google Scholar]
- 20.Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996; 20: 1161–1181. [DOI] [PubMed] [Google Scholar]
- 21.Atherton JC, Cao P, Peek RM Jr., Tummuru MK, Blaser MJ, Cover TL. Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori. Association of specific vacA types with cytotoxin production and peptic ulceration. J Biol Chem. 1995; 270: 17771–17777. [DOI] [PubMed] [Google Scholar]
- 22.Yamaguchi H, Osaki T, Taguchi H, Kamiya S. Production of monoclonal antibodies neutralizing vacuolation of cultured cells by Helicobacter pylori cytotoxin. FEMS Microbiol Lett. 1998; 168: 277–282. [DOI] [PubMed] [Google Scholar]
- 23.Ernst FD, Bereswill S, Waidner B, Stoof J, Mäder U, Kusters JG, et al. Transcriptional profiling of Helicobacter pylori Fur- and iron-regulated gene expression. Microbiology. 2005; 151: 533–46. doi: 10.1099/mic.0.27404-0 [DOI] [PubMed] [Google Scholar]
- 24.Merrell DS, Thompson LJ, Kim CC, Mitchell H, Tompkins LS, Lee A, et al. Growth phase-dependent response of Helicobacter pylori to iron starvation. Infect Immun. 2003; 71: 6510–6525. doi: 10.1128/IAI.71.11.6510-6525.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aspholm M, Olfat FO, Norden J, Sonden B, Lundberg C, Sjöström R, et al. SabA is the H. pylori hemagglutinin and is polymorphic in binding to sialylated glycans. PLoS Pathog. 2006; 2: e110 doi: 10.1371/journal.ppat.0020110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamaoka Y. Increasing evidence of the role of Helicobacter pylori SabA in the pathogenesis of gastroduodenal disease. J Infect Dev Ctries. 2008; 2: 174–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Unemo M, Aspholm-Hurtig M, Ilver D, Bergström J, Boren T, Danielsson D, et al. The sialic acid binding SabA adhesin of Helicobacter pylori is essential for nonopsonic activation of human neutrophils. J Biol Chem. 2005; 280: 15390–15397. doi: 10.1074/jbc.M412725200 [DOI] [PubMed] [Google Scholar]
- 28.Mahdavi J, Sonden B, Hurtig M, Olfat FO, Forsburg L, Roche N, et al. Helicobacter pylori SabA adhesin in persistent infection and chronic inflammation. Science. 2002; 297: 573–578. doi: 10.1126/science.1069076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kao CY, Sheu BS, Sheu SM, Yang HB, Chang WL, Cheng HC, et al. Higher motility enhances bacterial density and inflammatory response in dyspeptic patients infected with Helicobacter pylori. Helicobacter. 2012; 17: 411–416. doi: 10.1111/j.1523-5378.2012.00974.x [DOI] [PubMed] [Google Scholar]
- 30.Gebert B, Fischer W, Weiss E, Hoffmann R, Haas R. Helicobacter pylori vacuolating cytotoxin inhibits T lymphocyte activation. Science. 2003; 301: 1099–1102. doi: 10.1126/science.1086871 [DOI] [PubMed] [Google Scholar]
- 31.Yuan JP, Li T, Li ZH, Yang GZ, Hu BY, Shi XD, et al. mRNA expression profiling reveals a role of Helicobacter pylori vacuolating toxin in escaping host defense. World J Gastroenterol. 2004; 10: 1528–1532. doi: 10.3748/wjg.v10.i10.1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Basso D, Zambon CF, Letley DP, Stranges A, Marchet A, Rhead JL, et al. Clinical relevance of Helicobacter pylori cagA and vacA gene polymorphism. Gastroenterology. 2008; 135: 91–99. doi: 10.1053/j.gastro.2008.03.041 [DOI] [PubMed] [Google Scholar]
- 33.Delany I, Pacheco AB, Spohn G, Rappuoli R, Scarlato V. Iron-dependent transcription of the frpB gene of Helicobacter pylori is controlled by the Fur repressor protein. J Bacteriol. 2001; 183: 4932–4937. doi: 10.1128/JB.183.16.4932-4937.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rathinavelu S, Kao JY, Zavros Y, Merchant JL. Helicobacter pylori outer membrane protein 18 (Hp1125) induces dendritic cell maturation and function. Helicobacter. 2005; 10: 424–432. doi: 10.1111/j.1523-5378.2005.00350.x [DOI] [PubMed] [Google Scholar]
- 35.Velayudhan J, Hughes NJ, McColm AA, Bagshaw J, Clayton CL, Andrews SC, Kelly DJ. Iron acquisition and virulence in Helicobacter pylori: a major role for FeoB, a high-affinity ferrous iron transporter. Mol Microbiol. 2000; 37: 274–286. [DOI] [PubMed] [Google Scholar]
- 36.Yokota S, Konno M, Mino E, Sato K, Takahashi M, Fujii N. Enhanced Fe ion-uptake activity in Helicobacter pylori strains isolated from patients with iron-deficiency anemia. Clin Infect Dis. 2008; 46: e31–33. doi: 10.1086/526784 [DOI] [PubMed] [Google Scholar]
- 37.Choe YH, Kwon YS, Jung MK, Kang SK, Hwang TS, Hong YC. Helicobacter pylori-associated iron deficiency anemia in adolescent female athletes. J Pediatr. 2001; 139: 100–104. doi: 10.1067/mpd.2001.114700 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
Data Availability Statement
All of the microarray analysis results are available to the public through the GEO repository (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE101807).