Abstract
The Western Diet – characterized by high protein, sugar, fat and low fiber intake – is widely believed to contribute to the incidence and pathogenesis of inflammatory bowel diseases (IBD). However, high sodium chloride salt content, a defining feature of processed foods, has not been considered as a possible environmental factor that might drive IBD. We set out to bridge this gap. We examined murine models of colitis on either a high salt diet (HSD) or a low salt diet (LSD). We demonstrate that a HSD exacerbates inflammatory pathology in the IL-10-deficient murine model of colitis relative to mice fed a LSD. This was correlated with enhanced expression of numerous pro-inflammatory cytokines. Surprisingly, sodium accumulated in the colons of mice on a HSD, suggesting a direct effect of salt within the colon. Similar to the IL-10-deficient model, a HSD also enhanced cytokine expression during infection by Salmonella typhimurium. This occurred in the first three day of infection, suggesting that a HSD potentiates innate immune response. Indeed, in cultured dendritic cells we found that high salt media potentiates cytokine expression downstream of TLR4 activation via p38 MAPK and SGK1. A third common colitis model, administration of dextran sodium sulfate (DSS), was hopelessly confounded by the high sodium content of the DSS. Our results raise the possibility that high dietary salt is an environmental factor that drives increased inflammation in IBD.
Keywords: dietary salt, colitis, IL-10-deficient, Salmonella
Introduction
The Western diet, characterized by foods high in salt, sugar, protein, and fat, but low in fiber from fruits and vegetables, has long been understood to drive atherosclerosis, diabetes, inflammatory bowel disease (IBD), and other diseases of the developed world (1, 2). For example, the incidence of Crohn’s disease – one of the two primary IBDs – is 4 times higher in North America than in the Middle East or Asia, reflecting the fact that environmental stimuli like diet drove enormous surges in incidence in developed countries in the last fifty years (3–5). Further, numerous recent studies have linked elements of the Western diet to poor gastrointestinal health: diets high in sugar and fat lead to a dysbiotic, pro-inflammatory microbiota (6), while diets low in fiber lead to poor immunosuppressive activity of Tregs and dysfunctional immunity (7).
One aspect of the Western diet that has not been studied in the context of IBD, however, is dietary salt (NaCl). Processed foods typical of a Western diet can contain 100 times the NaCl of similar home-cooked meals (8, 9), and many Americans consume more than twice the American Heart Association’s daily recommended NaCl content. High NaCl consumption is associated with increased risk of developing high blood pressure (10), correlates with increased disability and progression in multiple sclerosis (11), interferes with and damages normal kidney function(10, 12–14) and may be involved in atherosclerosis (15). These data are corroborated by studies in human volunteers, where a controlled high salt diet correlated with enhanced blood monocyte counts and pro-inflammatory serum cytokines (including IL-17, IL-6, and IL-23) (14, 16, 17).
Several recent studies have shown that a high salt diet (HSD) in laboratory mice is pro-inflammatory (18). Excess NaCl in vitro was shown to enhance Th17 differentiation and activity via a p38 MAPK→NFAT5→SGK1 pathway, and that a HSD plus 1% NaCl-supplemented water exacerbates experimental autoimmune encephalitis (EAE) in mice (19, 20). Moreover, macrophages cultured in hyperosmotic NaCl produced more IL-1β, and WT but not inflammasome deficient mice developed worse EAE on a HSD plus 1% NaCl-supplemented water (21). In contrast, Tregs cultured in high salt media (HSM) adopted a proinflammatory phenotype (expressing Ifng, Il17a, and Rorc) and were unable to suppress inflammation when transferred into two different disease models exogenously (22). Similarly, M2 macrophages in HSM failed to suppress T cell proliferation, and a HSD plus 1% NaCl water inhibited wound healing in vivo (23). Finally, a HSD with 0.9% NaCl water led to hypernatremia in the skin, which drove anti-microbicidal activity against Leishmania major by macrophages (24). Therefore, a high salt diet has significant pro-inflammatory effects in the brain and skin.
Since dietary salt is first encountered in the gastrointestinal tract, we hypothesized that a HSD would also potentiate intestinal inflammation caused by either immunologic dysfunction or bacterial infection.
Materials and Methods
Western Diet Salt Concentrations
Dietary salt was calculated using data from the SELF Nutrition Database (25). Dry weight was calculated as the mass of a serving size minus the mass of water. Where the water mass was unavailable, the mass of all contents was summed to provide the dry weight. Where sodium mass was zero, we assumed 1 mg as an upper limit for calculations.
Sodium concentration experiments
Animals were placed on either a HSD or a LSD after day 0 sample collection. Weight, food weight, water volume, fecal and urine samples were taken every 12 hours for four days. Upon necropsy, serum, urine, feces, all alimentary contents and tissues from the stomach to the colon were collected. Solid samples were weighed, dried in a fume hood for ≥72 hours, weighed, reconstituted in a known volume of 1M HCl, and disrupted using a bead homogenizer.
Flame Photometry
All liquid samples were separated from debris and diluted 1:100 in 1.5mM CsCl buffer for the flame photometer (Model 943 Flame Photometer, Instrumentation Laboratory Co.). Briefly, the machine measures minute quantities of sodium and potassium by incinerating a laminar flow of the sample solution, creating an ionized gas from which spectra are observed. This is translated into a mM concentration readout of ions in the original sample. The original concentration of sodium and potassium is then determined by back-calculation: concentration is multiplied by the known volume of acid to determine millimoles, and then divided by the volume of sample water.
Animal Diets
Animal diets were obtained from Harlan Teklad in irradiated, sterile packaging. Animals were subjected to either a high salt diet (HSD, TD.92034) of 4% NaCl w/w, or a low salt diet (LSD, TD.96208) of .49% NaCl w/w. Animals were permitted standard cage water ad libitum during all experiments except where noted.
IL-10 Animal experiments
129 SvEv Il10−/− animals and wild-type controls were obtained in germ-free conditions from the National Gnotobiotic Rodent Resource Center and transferred to a specific pathogen free (SPF) housing facility. Animals were swabbed with slurry of feces from wild-type SPF mice on their mouths and anuses to induce colonization, and placed in cages with either their HSD or LSD.
Myeloperoxidase and Lipocalin 2 Assays
MPO and LCN2 were assayed using ELISA kits from R&D Systems (DY3667 and DY1857, respectively). Fecal samples were homogenized in PBS containing 0.1% Tween, and ELISA values were then standardized against protein content as determined by BCA.
Tissue Culture
Bone-marrow derived dendritic cells (BMDCs) from C57BL/6 mice were cultured from bone marrow in ultra-low binding polystyrene plates with 10ng/ml IL-4 and G-CSF. Cells were preconditioned at least 16 hours prior to use either in normal salt media (DMEM+10% FBS +0mM NaCl, NSM) or high salt media (same +40mM NaCl, HSM). Cells were pre-treated for 30 minutes in the indicated doses of inhibitor, then subjected to 1ng/ml LPS (E. coli, Sigma, Cat# L3024). Supernatants were then collected and analyzed by RnD DuoSet ELISAs. SGK1 inhibitor GSK 650394 (Tocris Biotechne); p38 MAPK inhibitor SB203580 (Invivogen)
DSS Animal experiments
SPF C57Bl/6 mice were transferred to either a HSD or a LSD 24 hours prior to DSS exposure. Animals were initially fed 3% w/v DSS for 5 days before harvest. Further experiments used the initial water consumption rates to calculate DSS concentrations that would normalize the dose of DSS/day. Stool samples were taken and tested for fecal occult blood, which was then scored based on the degree of biochemical reaction.
In Vivo Infections
SPF mice were placed on their diets 48 hours prior to infection. The next day, mice were deprived of food and water for four hours prior to gavage with 20mg streptomycin, after which food and water were restored. Salmonella typhimurium SL1344 was grown overnight to an OD between 2–6. Cultures were then diluted in PBS to 105 CFU/ml. After 4 hours without food and water, mice were gavaged with 100ul of this inoculum, followed by reintroduction of water. After 2 hours, food was restored. Feces were collected daily and plated on LB plates containing streptomycin and ampicillin.
Histology
Cecal tissues were cut from the alimentary tract; colons were cut from cecum to (and including) the anus. Tissues were then placed in 10% formalin for at least 24 hours prior to embedding, cutting, and H&E staining by the Cell Services and Histology Core. Slides were then given to a blinded scorer for assessment.
Salmonella typhimurium infection: Histology was assessed using a modified scheme adapted from Barthel et al (26). Slides were blind scored using a 4-point scale of severity for each of four criteria: epithelial hyperplasia and damage; immune infiltrate into the mucosa and lamina propria; goblet cell loss; and submucosal edema.
Il10−/− Model: Blinded histologic scores were assessed using a modified scheme adapted from Kim et al. (27). Slides were blind scored using a 4-point scale of severity for each of three criteria: epithelial hyperplasia and damage; immune (particularly T cell infiltrate) into the mucosa and lamina propria; and goblet cell loss.
mRNA Expression and Real-Time Quantification
Tissues for real-time PCR quantification (qPCR) were snap-frozen in RNAlater (Qiagen, 76106) during harvest and placed at −80C until use. For extraction, tissues were thawed on ice, and homogenized for 10 minutes in a lysis solution containing 1% β-mercaptoethanol and RNaseZap (ThermoFisher, AM9780)-treated beads. Extraction proceeded using a PureLink RNA mini kit (ThermoFisher, 12183018A). RNA quantified by nanodrop and treated with Turbo DNase post elution, with subsequent heat inactivation and reverse transcription. cDNA was run on a Life Quantstudio 6 real-time PCR machine using iTaq Universal Sybr Green Supermix (Bio-rad). Data were analyzed using the ΔΔCT method with HPRT as the control gene and either streptomycin pre-treated mice for Salmonella experiments, or co-transferred gnotobiotic WT 129 mice as the control sample.
Results
Comparison of mouse HSD chow to human foods
The most obvious way a HSD could affect IBD is by elevating NaCl content in the gut, which could directly alter the immune system. Although sodium is quickly and efficiently absorbed in the stomach and upper small intestine, a HSD could increase gastrointestinal tract salinity locally, or portal blood and lymph to drive systemic effects (19, 20).
To begin, we considered whether the mouse chow HSD is similar in NaCl content to commonly available foods in grocery stores, as well as fast food restaurants. We calculated the content by dry weight for these foods using the SELF Nutrition database (25), which uses the Nutritional Facts label in the United States. In contrast, mouse chow containing 4.0% w/w NaCl is a common HSD, while 0.49% NaCl is a common low salt diet (LSD). Comparing these diets revealed that the mouse HSD has comparable salt content to many fast food items (Fig. 1A and Table S1). Conversely, the LSD was similar to fruits and vegetables. In many murine experimental models using a HSD, the water available to the mice also contains 0.9–1.0% w/v NaCl (19, 20, 22–24, 28, 29). However, the NaCl content of commonly consumed beverages was neglible (Fig. 1B and Table S1). Therefore, in the studies below, we provided mice with ad libitum tap water with no added NaCl.
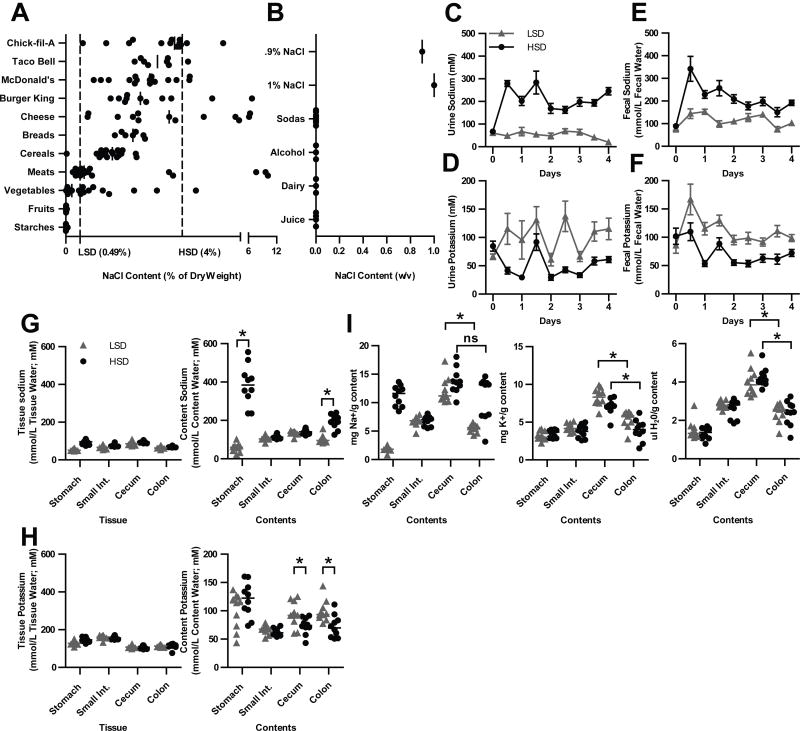
Figure 1. A HSD creates a high sodium concentration in the feces.
(A) Comparison of the NaCl content by dry weight of selected processed and non-processed foods to mouse chow diets, from SELF Nutrition Data. (B) Comparison of the NaCl content by weight per volume of selected drinks and 0.9–1% NaCl water content. (C–I) Flame Photometric analysis of (C–D) urine, (E–F) feces, and (G–H) GI tissues and contents. (E–H) Millimoles of Na+ or K+ are divided by the water content of the sample. (I) Milligrams of Na+ or K+ are divided by the dry weight of the sample. * = p < 0.05 by Mann Whitney nonparametric test. Data are representative of 3 replicate experiments.
A HSD causes colonic contents to have high Na+ concentration
Having established that the mouse HSD is comparable to elements of a Western diet, we sought to determine if the HSD alters sodium concentration in the gut. To assess this, animals were fed a HSD or a LSD for four days, and sodium content was determined in gastrointestinal tissues and contents using a flame photometer. Mice ate equivalent amounts of food and gained equal weight on both diets. We expected that sodium would be largely absorbed in the upper GI tract, then excreted by the kidney into the urine. Indeed, mice on a HSD drank significantly more water and excreted significantly higher concentrations of sodium in their urine starting 12h after administration of the diet, with output stabilizing after two days (Fig. 1C). Concomitantly, we observed lower potassium concentrations in the urine (Fig. 1D).
Surprisingly, mice on a HSD also contained significantly higher concentrations of sodium in their feces (Fig. 1E), again with concomitantly lower potassium (Fig. 1F). However, the idea that high sodium would be present through the GI tract is at odds with the conventional wisdom that sodium should be largely absorbed in the upper GI tract(30).
We therefore measured the sodium concentration of the GI tissue and luminal contents for both sodium and potassium. Tissue content was largely similar between the diets throughout the GI tract (Fig. 1G). As expected, the stomach contents – largely composed of the HSD – contained significantly more sodium. This sodium was normalized within the small intestine, but was then paradoxically elevated in the colonic contents (Fig 1G). As before, GI content potassium levels were inverted (Fig. 1H). Therefore, the colon contents of mice on a HSD experience a local high sodium concentration compared to those on a LSD.
How can we explain the apparent re-appearance of sodium in the colon? The primary function of the colon is to absorb water from the feces by absorbing ions, including sodium. However, mice on a HSD are sodium overloaded. This represses aldosterone production, which in turn represses expression of the sodium absorption channel ENaC. Thus, on a HSD, the colon will absorb potassium rather than sodium(31). Indeed, sodium mass in the cecal and colonic content only dropped in LSD animals, while potassium mass dropped in both groups (Fig. 1I). Then, as water is removed, the concentration of sodium rises (Fig. 1I, G). This gives the illusion of sodium “re-appearing” in the feces. Interestingly, while potassium is reduced in the HSD feces, the magnitude was not equivalent to the sodium elevation. However, since the normal osmolar gradient that exists from the villus tip to the villus base exceeds the osmoles of both sodium and potassium in the content, it is likely other osmolytes contribute to water absorption (32).
Effect of HSD on experimental murine colitis
We sought to determine if the local sodium increase we observed in colon contents might alter colonic immune responses. To test this, we examined the effect of our diets in the Il10−/− model of murine colitis.
Il10−/− mice spontaneously develop inflammatory colitis driven by resident intestinal bacteria (33). While colitis develops asynchronously under normal SPF housing conditions, colonizing adult germ free Il10−/− mice with SPF fecal microbiota synchronizes disease. Mice develop progressive colitis beginning after one week, plateauing after 4–6 weeks in our facility and lasting for the life of the animal (34, 35). The inflammatory response is characterized by transmural mononuclear cell infiltrate in the cecum and colon, as well as epithelial hyperplasia. We therefore colonized germ-free Il10−/− and wild type mice on a 129SvEv background and simultaneously transferred mice to either a HSD or the LSD. After three weeks we examined intestinal inflammation by H&E staining and tissue cytokine expression (Fig. 2–3).
Figure 2. A HSD drives exacerbates histopathologic inflammation in colons of Il10−/− mice.
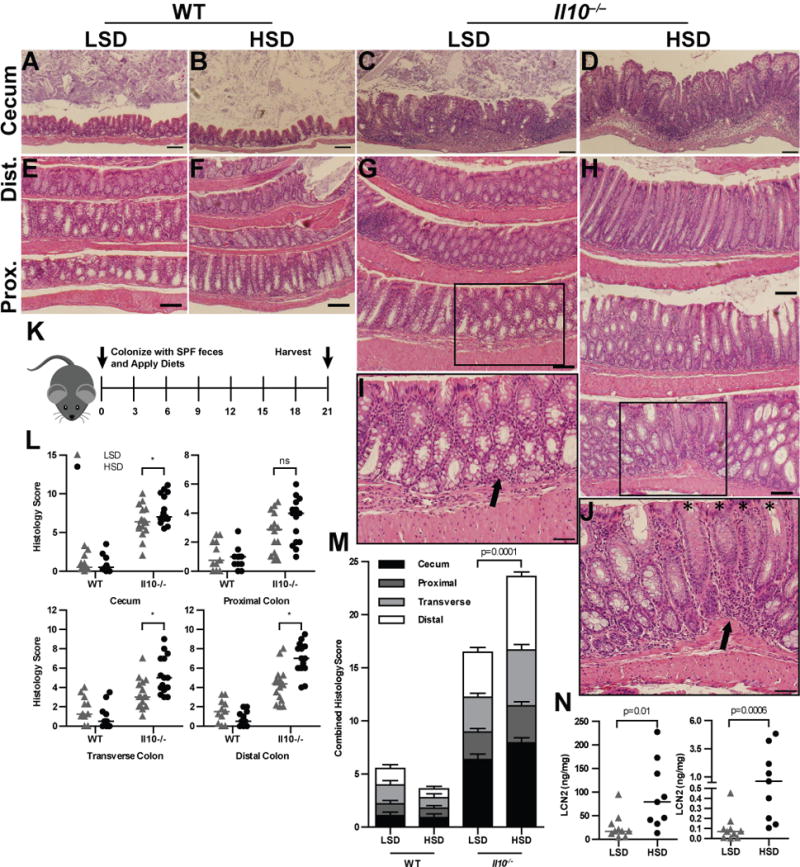
GF mice were colonized with SPF fecal microbiota and harvested 21d later. (A–D) 10× magnification of representative H&E stained sections of cecal tissue from WT (A,B) and Il10−/− (C,D) animals. (E–H) 10× magnification of representative H&E stained sections of colon Swiss rolls, oriented with more proximal tissue on the bottom and distal tissue on the top. Boxes indicate the location of the insert. (I–J) 20× magnifications of boxed inserts in (G,H) respectively. Arrows indicate mononuclear cell infiltration; asterisks indicate crypts where goblet cells have expelled mucus, thus creating an apparent ‘loss’ of goblet cells. Sections shown originate from a representative mouse that had histologic scoring at the average histologic score for the group (in L,M). Data are representative of 3 replicate experiments. (K) GF mice were colonized with SPF feces and fed either a HSD or LSD. Animals were harvested after 3 weeks. (L) Histologic scoring of WT and Il10−/− animals for the cecum, proximal colon, transverse colon, and distal colon. (M) Combined histologic scores, representing the sum of scores from the cecum and colonic regional tissues. (N) LCN2 and MPO ELISA results from feces; results were normalized to protein content as determined by BCA assay. P values determined by Mann Whitney nonparametric tests, or ANOVA for multiple comparisons; * represents p <0.05. Data are combined from 3 (L–M) or 2 (N) replicate experiments.
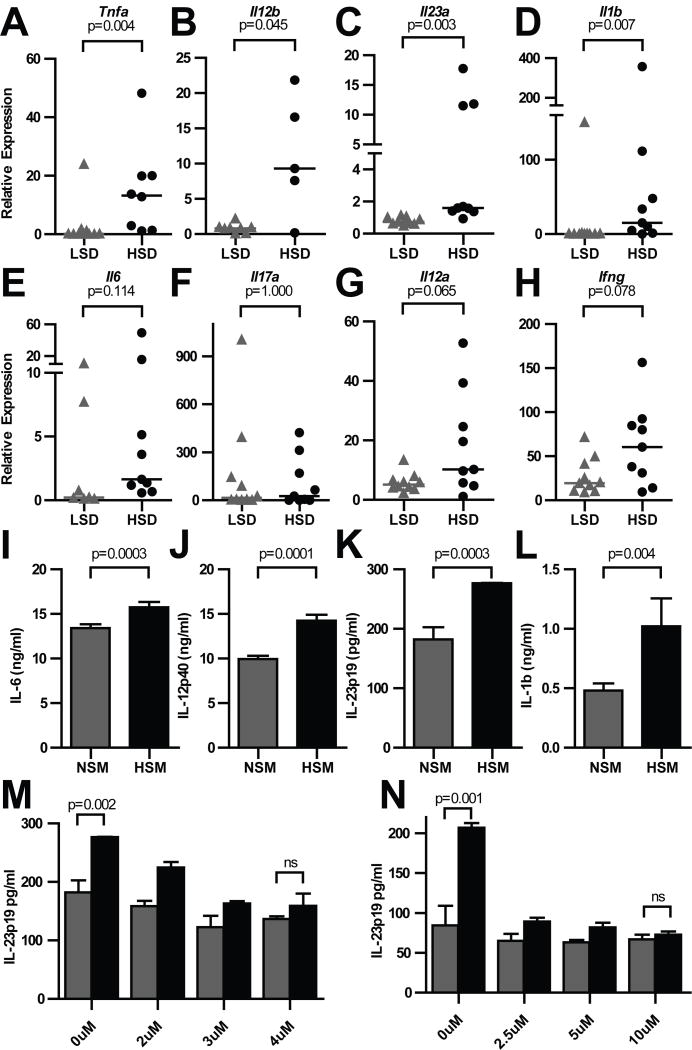
Figure 3. A HSD exacerbates colonic cytokine expression in Il10−/− animals.
(A–H) qPCR analysis of colonic mRNA from mice in Fig. 3. Data were analyzed using the ΔΔCT method against WT controls. (I–N) Bone marrow-derived dendritic cells (BMDCs) in HSM or normal salt media (NSM) were treated with 1ng/ml LPS for 8 hours before indicated cytokines were quantitated by ELISA of supernatants or, for pro-IL-1β, cell lysate (I–L) without, or with different concentrations (M) of the SGK1 inhibitor GSK 650394 or (N) the p38 MAPK inhibitor SB203580. Samples that failed quality control by melt curve analysis are omitted. P values determined by Mann Whitney nonparametric tests. Data are combined from 2 replicate experiments.
Histologic examination showed that a HSD broadly exacerbates colitis in both the ceca (Fig. 2A–D) and colons (Fig. 2E–J). Both the HSD ceca and colons had increased immune infiltration, increased epithelial hyperplasia, and extensive goblet cell loss. In the ceca this was accompanied by edema and immune cell infiltration of the submucosa and lamina propria, which was not observed in LSD mice (Fig. 2C). Qualitatively, the HSD exacerbated blinded histologic inflammatory scores in all segments of the colon. The HSD significantly increased colitis scores in the cecum, trended towards an increase in the proximal colon, which again became significant in the transverse and distal colonic segments (Fig. 2L–N). Germ free WT animals colonized with the same SPF displayed no appreciable inflammation in either diet group (Fig. 2L–M), indicating that a HSD alone is not overtly inflammatory. Consistent with this scoring, Il10−/− mice had higher fecal myeloperoxidase (MPO) and lipocalin-2 (LCN2) levels (Fig. 2N), two luminal biomarkers of inflammation (36). Together, these data suggest a general enhancement of inflammation, rather than a specific effect on epithelial growth or immune cell infiltrate.
Previous reports have noted that a HSD is capable of driving enhanced cytokine production as well as immune cell recruitment and stronger antimicrobial barrier defenses. Therefore, in order to better understand how a HSD exacerbated inflammation in this model, we examined transcription of several key cytokines via real-time quantitative PCR (qPCR) within the colonic tissues of LSD and HSD- fed mice. Indeed, a HSD induced significantly enhanced expression of Tnf and Il12b (Fig. 3A–B), as well as the Th17-axis cytokines Il23a, and Il1b, though Il6 trended but did not reach significance (Fig. 3B–E). Although we observed that a HSD increased Il17a expression in our first replicate, the statistical significance of this difference was not observed after experiments were combined (Fig. 3F). Likewise, the Th1-axis cytokines Il12a and Ifng trended but did not reach significant enhanced expression (Fig. 3G–H).
The strong increase in expression of the IL-12 family genes Il23a and Il12b, as well as the myeloid cytokine Il1b suggested that macrophages and dendritic cells (DCs) might respond to high intestinal sodium. Indeed, macrophages have already been shown to respond to high NaCl media (HSM) in vitro, suggesting that myeloid cells are a key sodium-responsive population (24). In line with our observations in vivo, we found that LPS-stimulated DCs exposed to HSM produced significantly more proIL-1b, IL-12p40, IL-23p19, and to a lesser extent IL-6 (Fig. 3I–L) than cells in normal NaCl media (NSM).
In vitro work has described a p38→NFAT5→SGK1 pathway for enhanced cytokine secretion in response to stimulation and HSM (19–21, 24). We tested whether this pathway was responsible for the enhanced DC cytokine production using the SGK1 inhibitor GSK 650394 and the p38 inhibitor SB203580. As hypothesized, HSM potentiation of IL-23p19 production was eliminated by either inhibitor (Fig. 3M–N), suggesting that DCs may use the same pathway to detect and enhance inflammatory responses in the presence of increased NaCl concentrations.
These data add DCs to the list of salt responsive cell types, which also includes T cells and macrophages(19, 20, 24). Any combination of these cell types could be driving the high salt response in our models.
The DSS colitis model is confounded by a HSD and by DSS itself
The observed increase in expression of Il23a and Il1b suggests an effect on innate immune responses. Therefore, we examined the effect of a HSD in the dextran sodium sulfate (DSS) model of colitis. In this model, animals are fed DSS, which injures intestinal epithelial cells. This drives an inflammatory response to luminal bacteria, the early phase of which is primarily mediated by the innate immune system. Thus, by treating HSD or LSD mice with 3% DSS for five days, we sought to examine whether a HSD would exacerbate innate immune-driven intestinal inflammation.
Although HSD animals did exhibit greater weight loss (Fig. 4A), and elevated fecal hemoccult scores (Fig. 4B), these data are confounded by the fact that mice on a HSD drank nearly twice as much DSS water as LSD animals. HSD mice thus received significantly more DSS mass than LSD animals (Fig. 4C). We then attempted to normalize DSS consumption by reducing the concentration of DSS provided to HSD animals to 1.9% DSS (Fig. 4D–F). This nearly normalized DSS consumption rates (Fig. 4F), but also normalized hemoccult scores between HSD and LSD mice.
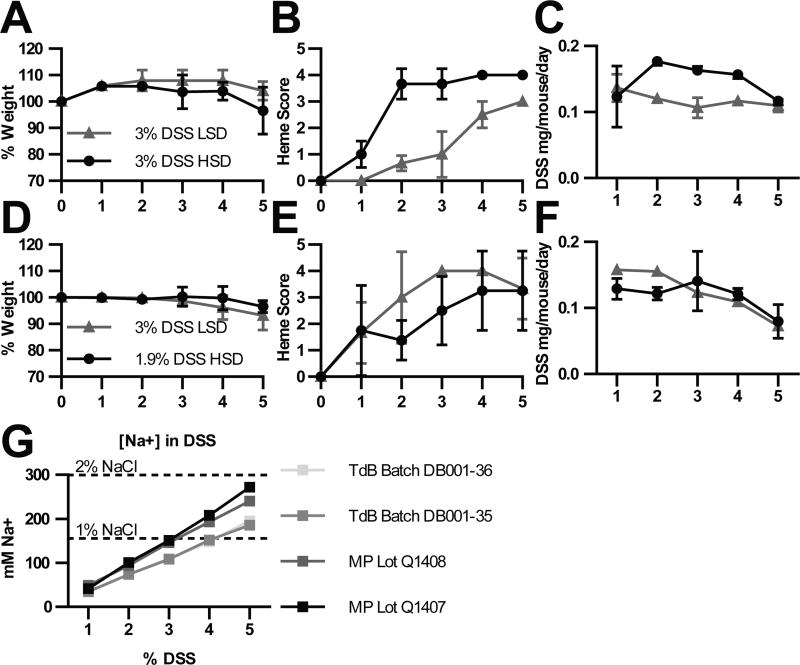
Figure 4. A HSD in the DSS model is confounded by sodium in DSS itself.
Mice were moved to a HSD or LSD and treated with the indicated DSS solutions for 5 days. Mice were monitored daily for (A, D) weight, and (B, E) hemoccult score by fecal hemoccult assay. (C, D) Mass of DSS consumed daily by each mouse as determined by the percent content of DSS in the water and the volume consumed. (G) Flame photometric analysis of 1–5% DSS water. DSS vendors: TdB (TdB Consultancy) and MP (MP Biomedicals). Data are from a single experiment (A–C), or representative of 2 experiments (D–F).
This led us to consider whether DSS itself might be ‘salty.’ Indeed, we found that depending on the manufacturer, a 3–4% solution provides as many millimoles of sodium per ml as a 1% NaCl solution (Fig. 4g). Therefore, the HSD and LSD mice both experience high sodium intake, but originating from the DSS water, which prevents the study of dietary sodium effects in this model. We therefore sought an alternative model to test intestinal innate transcriptional responses to a HSD.
A HSD enhances inflammatory cytokines during intestinal infection
Another model of innate intestinal immune responses is oral infection with Salmonella enterica serovar Typhimurium (Fig. 5A). Because this model rapidly induces extreme inflammation, we speculated that a HSD might generally enhance cytokine responses, but fail to further accelerate histologic inflammation. Indeed, S. typhimurium infected mice had similar bacterial burdens and histopathology on both diets (Fig. S1, 5B). Thus, this model allows us to examine the effect of a HSD on cytokine expression in isolation from these factors.
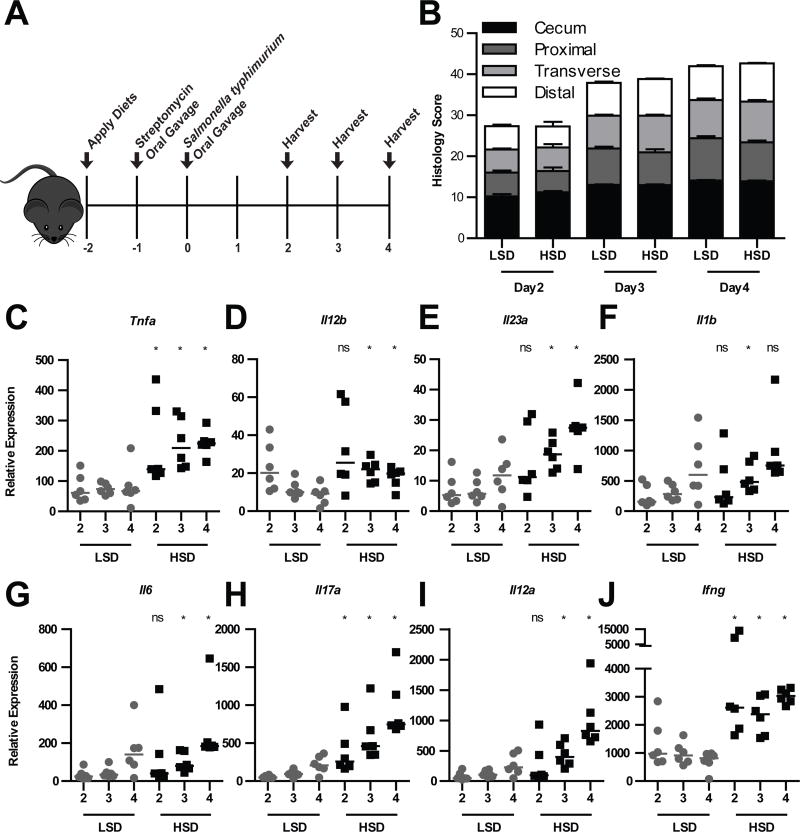
Figure 5. A HSD exacerbates cytokine expression but not histology or bacterial burdens in the S. typhimurium model of colitis.
(A) Mice were placed on a HSD or LSD at day −, treated with streptomycin at day −1, and infected with S. typhimurium on day 0, and harvested on the indicated day post infection. (B) Combined histological scoring of cecum and colonic tissues, as assessed by immune infiltration, goblet cell ‘loss’, epithelial hyperplasia, and edema. (C–J) qPCR analysis of cecal mRNA. Data were analyzed using the ΔΔCT method. * represents p <0.05 vs. LSD comparable value by Mann Whitney nonparametric test, and ANOVA test for multiple comparisons. Data are representative of 3 replicate experiments.
In agreement with the Il10−/− model, HSD mice infected with S. typhimurium also had elevated cytokine responses. HSD mice had significantly increased expression of Tnf and Il12b (Fig. 5C–D), as well as the Th17 axis cytokines Il23a, and Il1b, the latter of which trended towards significance, though only reaching significance on day 3 (Fig. 5E–F). Likewise, Il6 trended towards significance, but in this case was statistically significant on days 3 and 4 (Fig. 5G). Unlike the Il10−/− model, Il17a was significantly increased during S. typhimurium infection (Fig. 5H). The S. typhimurium model may thus have a greater sensitivity to detect cytokine increases, and similarly, the Th1 axis cytokines Il12a and Ifng, which trended in the Il10−/− model, were significantly increased by the HSD during S. typhimurium infection (Fig. 5i–j). These results are consistent with the conclusions from the Il10−/− model that a HSD enhances innate cytokine responses.
Because the NLRP3 inflammasome responds to loss of cellular potassium(37), we hypothesized that caspase-1/11 knockout mice would fail to respond to a HSD in the S. typhimurium infection model. However, despite delayed inflammation from loss of these genes, there were still differences in colonic cytokine expression (Supp. Fig. 2). Interestingly, HSD and LSD mice expressed equivalent levels of Ifng and Il17a, which is likely due to the loss of IL-18 and IL-1β processing, respectively.
Discussion
It is clear that components of the Western diet have significant, negative impacts on human health. Evidence suggests the Western diet is linked to the incidence of several autoimmune diseases, including multiple sclerosis and diabetes (38). Animal studies have shown that diets high in protein, fat, and sugar, and low in fiber can alter immune cell function (7), and promote metabolic syndrome (39). Further, the Western diet is associated with an increasing incidence of IBD (1, 2). However, the high salt content of the Western diet has not been studied as a component that drives IBD.
Although the effect of dietary NaCl on immune function has been described outside the GI track, the mechanism by which dietary NaCl exerts systemic effects is difficult to explain. Dogma suggests that sodium is quickly stripped from food in the proximal intestine(30), and is then stored in the skin (24, 29) or re-concentrated in the kidney prior to excretion. Therefore, one would expect HSD effects to be limited to the upper GI tract, the urinary tract, and the skin.
Our data surprisingly argue that a HSD actually creates a high local concentration of sodium in the colon. Even though sodium levels from food are rapidly normalized in the small intestine, presumably by a combination of ion absorption and intestinal and pancreatic secretion, we observed an apparent re-enrichment of sodium in the feces. Nevertheless, this observation can be explained by comparison with the reciprocal absorption of potassium.
Like sodium, potassium levels are normalized in the small intestine. As the contents pass through the cecum and colon, both groups of mice absorb potassium, decreasing its mass. However, while LSD mice also absorb sodium, HSD mice do not, leaving it behind. This leads to an increase in concentration of sodium in the colons of HSD mice relative to the LSD mice as water is removed. Therefore, we speculate that humans consuming a Western diet may also experience high sodium concentrations in their colons.
How does luminal salt concentration cause an immunologic response in the seeming absence of unchanged tissue salt concentrations? We can imagine numerous possible mechanisms. For example, the high sodium concentration in the colonic lumen caused by the HSD likely creates a sodium microgradient across the epithelium that is experienced primarily by the epithelial cells. These epithelial cells could experience this as a static, elevated concentration gradient, or as increased sodium flux across their cytosol and membranes. Both the concentration and flux changes could alter epithelial signaling cascades to promote inflammation. However, because the blood rapidly washes this sodium away, it is undetected in our examination of total tissue sodium content.
Additionally, immune cells in the lamina propria may also be affected by altered microgradients or flux of sodium from the colon. In particular, DCs that extend pseudopodia into the colonic lumen to sample its contents might also directly experience the new high sodium environment. These effects would be exacerbated during inflammation when the epithelial barrier becomes more permeable to luminal contents or is effaced.
How exactly does a HSD exacerbate colitis, or the other immune phenotypes previously observed? The first reports demonstrated a role for sodium on the T cell compartment, but subsequent studies have also implicated macrophages. Our data now show that DCs respond to HSM by expressing additional proIL-1β, IL-6, IL-12, and IL-23, which would be expected to drive effector T cell responses in IBD. Indeed, colitis was exacerbated three weeks after colonization in the Th1/Th17-driven Il10−/− model, although we did not observe consistent elevation in Il17a. This was in contrast to the effect of a HSD upon EAE, which is predominantly Th17 driven (19–21, 28). Nevertheless, we examined a time point where clinical severity has not yet reached a plateau, so it may be that a HSD will also potentiate Th17 responses later. Enhanced expression of the myeloid cytokines Il1b as well as Il23a also suggests additional effects on innate immune cells. The S. typhimurium model, by comparison, displayed enhanced Il17a expression, perhaps originating from ILC3s, along with enhancement of the Il1b and Il23a myeloid cytokines. Thus, the data we have presented highlights a novel role for a HSD in the DC compartment.
Placing our data in context with published reports leads one to believe that a HSD initiates numerous pro-inflammatory effects on the immune system, which may create a series of positive feedback loops. While the high sodium concentration in the colons may have direct effects on T cells via the SGK1 mechanism, they may also be driven by the enhanced cytokine production and costimulatory molecule expression of myeloid cells. Simultaneous HSD-mediated impairment of regulatory T cell function could fuel a pro-inflammatory environment in the colon. Future studies could also look at indirect effects of a HSD, which have not currently been ruled-out. A HSD could have important effects on the composition of the colonic microbiota, or alter the function of certain microbes, as has already been described with the cag gene island of Helicobacter pylori (40). Finally, a HSD strongly affects the renin-angiotensin-aldosterone system (RAAS), inhibition of which has been shown ameliorate murine EAE.(41)
In conclusion, a HSD generates a high sodium concentration in the colon, which exacerbates a murine model of chronic, immune- mediated colitis. Our data may inspire clinical studies in humans to determine if dietary salt has detrimental effects on IBD.
Supplementary Material
Acknowledgments
We thank M. Goy and R. Fellner for assistance with the flame photometer and discussions, C. Suitt for sectioning and staining, and B. Grubb for early discussions.
This work was supported by NIH grants AI097518 (E.A.M.), AI119073 (E.A.M.), and DK105784 (A.L.T.), the UNC CGIBD Pilot Program DK34987 (E.A.M.), and the Yang Biomedical Scholars Award (E.A.M.), as well as NIH grant DK065988 (T.D.R.). Gnotobiotic experiments through the NGRRC were supported by 5-P30-DK034987 and 5-P40-OD010995.
Footnotes
Contributions: A.L.T., E.A.M. and R.B.S. conceived the project. A.L.T., B.L., and T.D.R. performed experiments. A.L.T. and E.A.M wrote the manuscript.
Literature Cited
- 1.Hou JK, Abraham B, El-Serag H. Dietary intake and risk of developing inflammatory bowel disease: a systematic review of the literature. The American journal of gastroenterology. 2011;106:563–73. doi: 10.1038/ajg.2011.44. [DOI] [PubMed] [Google Scholar]
- 2.Chapman-Kiddell CA, Davies PSW, Gillen L, Radford-Smith GL. Role of diet in the development of inflammatory bowel disease. Inflammatory bowel diseases. 2010;16:137–51. doi: 10.1002/ibd.20968. [DOI] [PubMed] [Google Scholar]
- 3.Loftus EV., Jr Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504–17. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 4.Ng SC, Bernstein CN, Vatn MH, Lakatos PL, Loftus EV, Tysk C, O'Morain C, Moum B, Colombel J-F. Geographical variability and environmental risk factors in inflammatory bowel disease. Gut. 2013;62:630–649. doi: 10.1136/gutjnl-2012-303661. [DOI] [PubMed] [Google Scholar]
- 5.Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW, Kaplan GG. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2011;142:46. doi: 10.1053/j.gastro.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Carmody RN, Gerber GK, Luevano JM, Gatti DM, Somes L, Svenson KL, Turnbaugh PJ. Diet dominates host genotype in shaping the murine gut microbiota. Cell Host Microbe. 2014;17:72–84. doi: 10.1016/j.chom.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M, Glickman JN, Garrett WS. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science (New York, NY) 2013;341:569–73. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Appel LJ, Frohlich ED, Hall JE, Pearson TA, Sacco RL, Seals DR, Sacks FM, Smith SC, Jr, Vafiadis DK, van Horn LV. The importance of population-wide sodium reduction as a means to prevent cardiovascular disease and stroke: a call to action from the American Heart Association. Circulation. 2011;123:1138–43. doi: 10.1161/CIR.0b013e31820d0793. [DOI] [PubMed] [Google Scholar]
- 9.Irarrazabal CE, Williams CK, Ely MA, Birrer MJ, Garcia-Perez A, Burg MB, Ferraris JD. Activator protein-1 contributes to high NaCl-induced increase in tonicity-responsive enhancer/osmotic response element-binding protein transactivating activity. J Biol Chem. 2007;283:2554–2563. doi: 10.1074/jbc.M703490200. [DOI] [PubMed] [Google Scholar]
- 10.Fellner RC, Cook AK, O'Connor PM, Zhang S, Pollock DM, Inscho EW. High-salt diet blunts renal autoregulation by a reactive oxygen species-dependent mechanism. Am J Physiol Renal Physiol. 2014;307:F33–40. doi: 10.1152/ajprenal.00040.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paling D, Solanky BS, Riemer F, Tozer DJ, Wheeler-Kingshott CAM, Kapoor R, Golay X, Miller DH. Sodium accumulation is associated with disability and a progressive course in multiple sclerosis. Brain. 2013;136:2305–2317. doi: 10.1093/brain/awt149. [DOI] [PubMed] [Google Scholar]
- 12.Abu-Amarah I, Bidani AK, Hacioglu R, Williamson GA, Griffin KA. Differential effects of salt on renal hemodynamics and potential pressure transmission in stroke-prone and stroke-resistant spontaneously hypertensive rats. Am J Physiol Renal Physiol. 2005;289:F305–13. doi: 10.1152/ajprenal.00349.2004. [DOI] [PubMed] [Google Scholar]
- 13.Fellner RC, Guan Z, Cook AK, Pollock DM, Inscho EW. Endothelin contributes to blunted renal autoregulation observed with a high-salt diet. Am J Physiol Renal Physiol. 2015;309:F687–96. doi: 10.1152/ajprenal.00641.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou X, Zhang L, Ji W-J, Yuan F, Guo Z-Z, Pang B, Luo T, Liu X, Zhang W-C, Jiang T-M, Zhang Z, Li Y-M. Variation in Dietary Salt Intake Induces Coordinated Dynamics of Monocyte Subsets and Monocyte-Platelet Aggregates in Humans: Implications in End Organ Inflammation. PLoS ONE. 2013;8:e60332. doi: 10.1371/journal.pone.0060332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amador CA, Barrientos V, Peña J, Herrada AA, González M, Valdés S, Carrasco L, Alzamora R, Figueroa F, Kalergis AM, Michea L. Spironolactone decreases DOCA-salt-induced organ damage by blocking the activation of T helper 17 and the downregulation of regulatory T lymphocytes. Hypertension. 2014;63:797–803. doi: 10.1161/HYPERTENSIONAHA.113.02883. [DOI] [PubMed] [Google Scholar]
- 16.Wen W, Wan Z, Ren K, Zhou D, Gao Q, Wu Y, Wang L, Yuan Z, Zhou J. Potassium supplementation inhibits IL-17A production induced by salt loading in human T lymphocytes via p38/MAPK-SGK1 pathway. Exp Mol Pathol. 2016;100:370–377. doi: 10.1016/j.yexmp.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 17.Yi B, Titze J, Rykova M, Feuerecker M, Vassilieva G, Nichiporuk I, Schelling G, Morukov B, Choukèr A. Effects of dietary salt levels on monocytic cells and immune responses in healthy human subjects: a longitudinal study. Transl Res. 2014;166:103–110. doi: 10.1016/j.trsl.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schatz V, Neubert P, Schröder A, Binger K, Gebhard M, Luft FC, Titze J, Jantsch J. Elementary immunology: Na(+) as a regulator of immunity. Pediatr Nephrol. 2016 doi: 10.1007/s00467-016-3349-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kleinewietfeld M, Manzel A, Titze J, Kvakan H, Yosef N, Linker RA, Muller DN, Hafler DA. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature. 2013;496:518–22. doi: 10.1038/nature11868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu C, Yosef N, Thalhamer T, Zhu C, Xiao S, Kishi Y, Regev A, Kuchroo VK. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature. 2013;496:513–7. doi: 10.1038/nature11984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ip WKE, Medzhitov R. Macrophages monitor tissue osmolarity and induce inflammatory response through NLRP3 and NLRC4 inflammasome activation. Nat Commun. 2015;6:6931. doi: 10.1038/ncomms7931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hernandez AL, Kitz A, Wu C, Lowther DE, Rodriguez DM, Vudattu N, Deng S, Herold KC, Kuchroo VK, Kleinewietfeld M, Hafler DA. Sodium chloride inhibits the suppressive function of FOXP3+ regulatory T cells. J Clin Invest. 2015;125:4212–4222. doi: 10.1172/JCI81151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Binger KJ, Gebhardt M, Heinig M, Rintisch C, Schroeder A, Neuhofer W, Hilgers K, Manzel A, Schwartz C, Kleinewietfeld M, Voelkl J, Schatz V, Linker RA, Lang F, Voehringer D, Wright MD, Hubner N, Dechend R, Jantsch J, Titze J. High salt reduces the activation of IL-4- and IL-13-stimulated macrophages. J Clin Invest. 2015;125:4223–4238. doi: 10.1172/JCI80919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jantsch J, Schatz V, Friedrich D, Schröder A, Kopp C, Siegert I, Maronna A, Wendelborn D, Linz P, Binger KJ, Gebhardt M, Heinig M, Neubert P, Fischer F, Teufel S, David J-P, Neufert C, Cavallaro A, Rakova N, Küper C, Beck F-X, Neuhofer W, Muller DN, Schuler G, Uder M, Bogdan C, Luft FC, Titze J. Cutaneous Na+ storage strengthens the antimicrobial barrier function of the skin and boosts macrophage-driven host defense. Cell Metab. 2015;21:493–501. doi: 10.1016/j.cmet.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nast C. SELF Nutrition Data. 2014 http://nutritiondataselfcom/tools/nutrient-search.
- 26.Barthel M, Hapfelmeier S, Quintanilla-Martínez L, Kremer M, Rohde M, Hogardt M, Pfeffer K, Rüssmann H, Hardt W-D. Pretreatment of mice with streptomycin provides a Salmonella enterica serovar Typhimurium colitis model that allows analysis of both pathogen and host. Infection and immunity. 2003;71:2839–58. doi: 10.1128/IAI.71.5.2839-2858.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim SC, Tonkonogy SL, Albright CA, Tsang J, Balish EJ, Braun J, Huycke MM, Sartor RB. Variable phenotypes of enterocolitis in interleukin 10-deficient mice monoassociated with two different commensal bacteria. Gastroenterology. 2005;128:891–906. doi: 10.1053/j.gastro.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 28.Hucke S, Eschborn M, Liebmann M, Herold M, Freise N, Engbers A, Ehling P, Meuth SG, Roth J, Kuhlmann T, Wiendl H, Klotz L. Sodium chloride promotes pro-inflammatory macrophage polarization thereby aggravating CNS autoimmunity. J Autoimmun. 2015;67:90–101. doi: 10.1016/j.jaut.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 29.Machnik A, Neuhofer W, Jantsch J, Dahlmann A, Tammela T, Machura K, Park J-K, Beck F-X, Derer W, Goss J, Ziomber A, Dietsch P, Wagner H, van Rooijen N, Kurtz A, Hilgers KF, Alitalo K, Eckardt K-U, Luft FC, Kerjaschki D, Titze J. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat Med. 2009;15:545–552. doi: 10.1038/nm.1960. [DOI] [PubMed] [Google Scholar]
- 30.Rao MC, Sarathy J, Ao M. Intestinal Water and Electrolyte Transport in Health and Disease. Colloquium Series on Integrated …. 2012;4:1. [Google Scholar]
- 31.Rechkemmer G, Halm DR. Aldosterone stimulates K secretion across mammalian colon independent of Na absorption. Proceedings of the National Academy of Sciences. 1989;86:397–401. doi: 10.1073/pnas.86.1.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jodal M, Hallbäck DA, Lundgren O. Tissue osmolality in intestinal villi during luminal perfusion with isotonic electrolyte solutions. Acta Physiol Scand. 1978;102:94–107. doi: 10.1111/j.1748-1716.1978.tb06049.x. [DOI] [PubMed] [Google Scholar]
- 33.Sellon RK, Tonkonogy S, Schultz M, Dieleman LA, Grenther W, Balish E, Rennick DM, Sartor RB. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infection and immunity. 1998;66:5224–31. doi: 10.1128/iai.66.11.5224-5231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kühn R, Löhler J, Rennick D, Rajewsky K, Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–74. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 35.Spencer DM, Veldman GM, Banerjee S, Willis J, Levine AD. Distinct inflammatory mechanisms mediate early versus late colitis in mice. Gastroenterology. 2002;122:94–105. doi: 10.1053/gast.2002.30308. [DOI] [PubMed] [Google Scholar]
- 36.Chassaing B, Srinivasan G, Delgado MA, Young AN, Gewirtz AT, Vijay-Kumar M. Fecal lipocalin 2, a sensitive and broadly dynamic non-invasive biomarker for intestinal inflammation. PloS one. 2012;7:e44328. doi: 10.1371/journal.pone.0044328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muñoz-Planillo R, Kuffa P, Martínez-Colón G, Smith BL, Rajendiran TM, Núñez G. K+ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity. 2013;38:1142–53. doi: 10.1016/j.immuni.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manzel A, Muller DN, Hafler DA, Erdman SE, Linker RA, Kleinewietfeld M. Role of “Western diet” in inflammatory autoimmune diseases. Curr Allergy Asthma Rep. 2013;14:404. doi: 10.1007/s11882-013-0404-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lutsey PL, Steffen LM, Stevens J. Dietary intake and the development of the metabolic syndrome: the Atherosclerosis Risk in Communities study. Circulation. 2008;117:754–761. doi: 10.1161/CIRCULATIONAHA.107.716159. [DOI] [PubMed] [Google Scholar]
- 40.Gaddy JA, Radin JN, Loh JT, Zhang F, Washington MK, Peek RM, Algood HMS, Cover TL. High dietary salt intake exacerbates Helicobacter pylori-induced gastric carcinogenesis. Infect Immun. 2013;81:2258–2267. doi: 10.1128/IAI.01271-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blocking angiotensin-converting enzyme induces potent regulatory T cells and modulates TH1- and TH17-mediated autoimmunity. Proceedings of the National Academy of Sciences. 2009;106:14948. doi: 10.1073/pnas.0903958106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.