Abstract
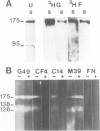
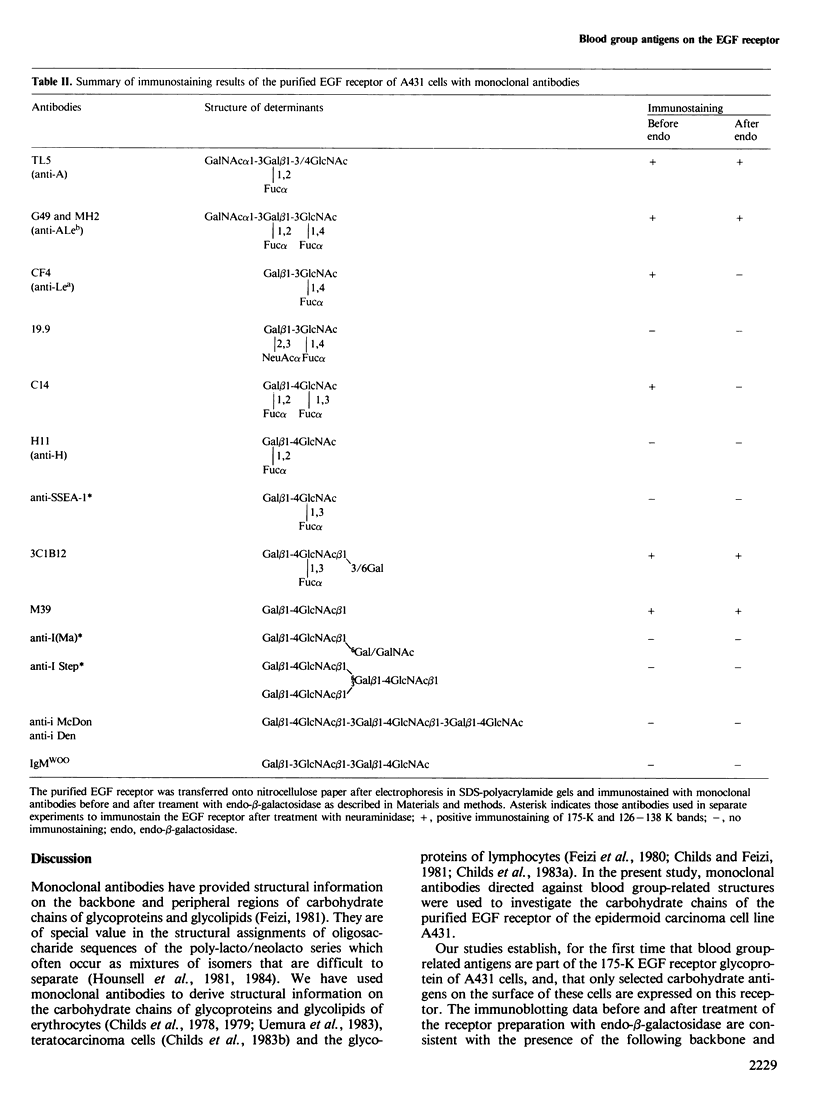
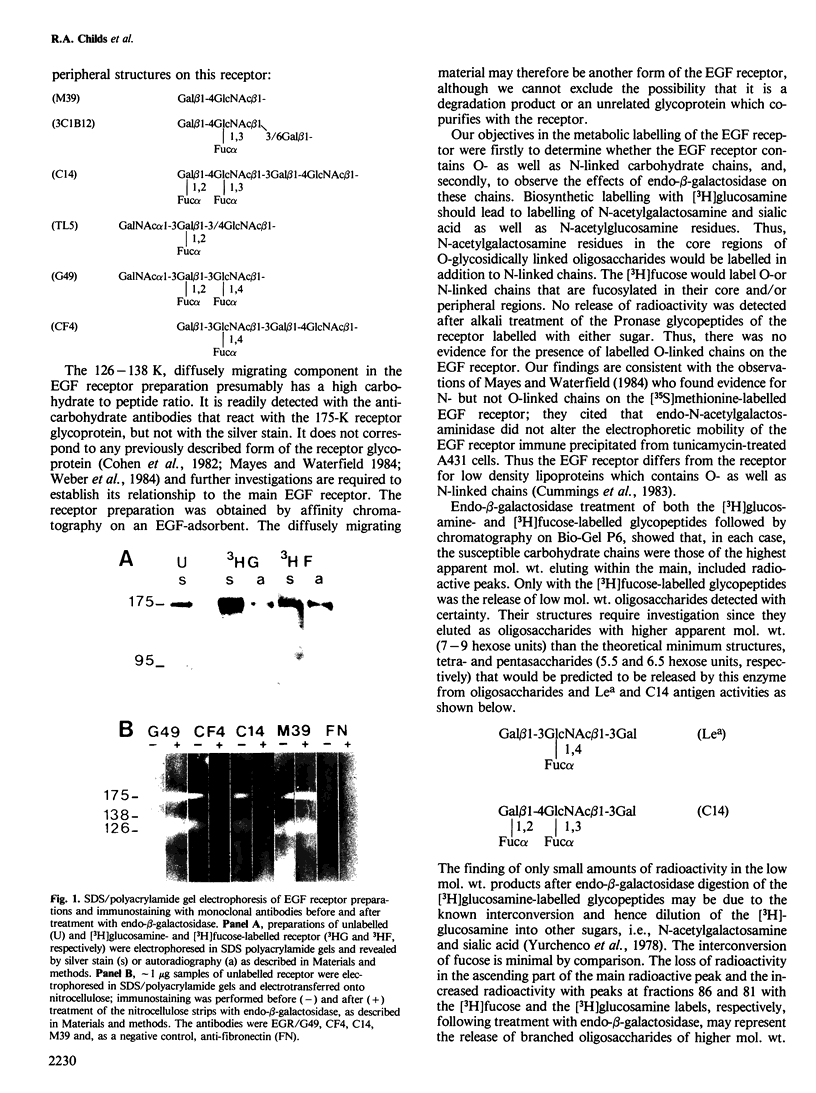
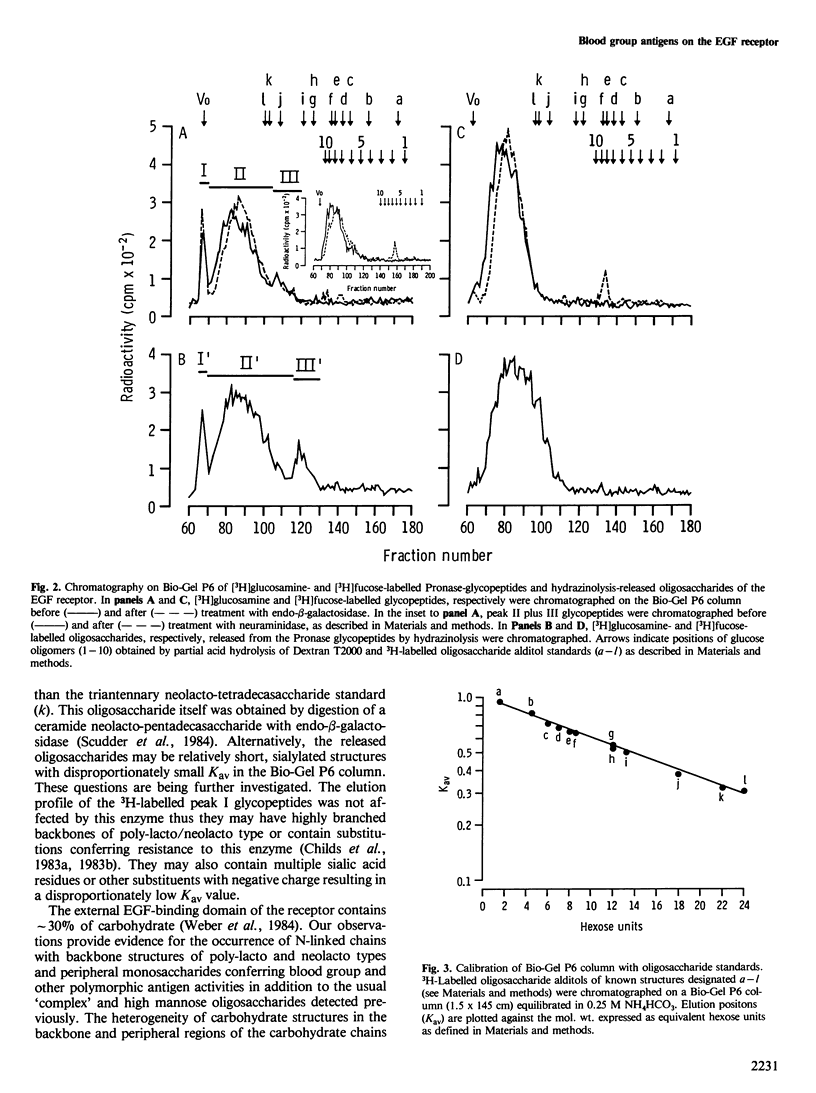
The antigens expressed on the carbohydrate chains of the receptor for epidermal growth factor of A431 cells were studied by immunoblotting with monoclonal antibodies. Blood group A and the Type 1 based blood group ALeb and Lea antigens were detected as well as antigens associated with unsubstituted, monofucosylated and difucosylated Type 2 blood group chains. The Lea and the difucosylated Type 2 antigen activities were abolished by treating the blotted receptor with endo-beta-galactosidase, indicating that they are expressed on backbone structures of poly-lacto/neolacto type. (The term 'poly-lacto/neolacto' is used here to describe oligosaccharide backbone structures consisting of repeating Type 1, Gal beta 1-3GlcNAc (lacto) or Type 2, Gal beta 1-4GlcNAc (neolacto) sequences.) The glycosidic linkage of oligosaccharides to protein was investigated using Pronase digests of the receptor biosynthetically labelled with [3H]glucosamine or [3H]fucose. The oligosaccharides were alkali-resistant, consistent with N- rather than O-glycosidically linked chains. A proportion of [3H]fucose-labelled glycopeptides was susceptible to endo-beta-galactosidase, confirming the immunoblotting experiment using antibodies against the Lea and the difucosylated Type 2 antigenic determinants. Oligosaccharides were released from the [3H]fucose- and [3H]-glucosamine-labelled glycopeptides by hydrazinolysis. Chromatography of the oligosaccharides on Bio-Gel P6 and Concanavalin A columns indicated a spectrum of oligosaccharides which include those of high mannose type labelled with [3H]glucosamine, and a mixture of oligosaccharides labelled with [3H]fucose and [3H]glucosamine of bi- and multiantennary complex types of which a subpopulation is susceptible to digestion with endo-beta-galactosidase.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown A., Feizi T., Gooi H. C., Embleton M. J., Picard J. K., Baldwin R. W. A monoclonal antibody against human colonic adenoma recognizes difucosylated Type-2-blood-group chains. Biosci Rep. 1983 Feb;3(2):163–170. doi: 10.1007/BF01121947. [DOI] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. Influence of lectins on the binding of 125I-labeled EGF to human fibroblasts. Biochem Biophys Res Commun. 1977 Nov 21;79(2):545–552. doi: 10.1016/0006-291x(77)90192-9. [DOI] [PubMed] [Google Scholar]
- Childs R. A., Dalchau R., Scudder P., Hounsell E. F., Fabre J. W., Feizi T. Evidence for the occurrence of O-glycosidically linked oligosaccharides of poly-N-acetyllactosamine type on the human leucocyte common antigen. Biochem Biophys Res Commun. 1983 Jan 27;110(2):424–431. doi: 10.1016/0006-291x(83)91166-x. [DOI] [PubMed] [Google Scholar]
- Childs R. A., Feizi T. Differences in carbohydrate moieties of high molecular weight glycoproteins of human lymphocytes of T and B origins revealed by monoclonal autoantibodies with anti-I and anti-i specificities. Biochem Biophys Res Commun. 1981 Oct 30;102(4):1158–1164. doi: 10.1016/s0006-291x(81)80133-7. [DOI] [PubMed] [Google Scholar]
- Childs R. A., Feizi T., Fukuda M., Hakomori S. I. Blood-group-I activity associated with band 3, the major intrinsic membrane protein of human erythrocytes. Biochem J. 1978 Jul 1;173(1):333–336. doi: 10.1042/bj1730333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs R. A., Feizi T., Tonegawa Y. Multiplicity of molecules carrying blood-group-I antigen on erythrocyte membranes. Biochem J. 1979 Sep 1;181(3):533–538. doi: 10.1042/bj1810533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs R. A., Pennington J., Uemura K., Scudder P., Goodfellow P. N., Evans M. J., Feizi T. High-molecular-weight glycoproteins are the major carriers of the carbohydrate differentiation antigens I, i and SSEA-1 of mouse teratocarcinoma cells. Biochem J. 1983 Dec 1;215(3):491–503. doi: 10.1042/bj2150491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Carpenter G., King L., Jr Epidermal growth factor-receptor-protein kinase interactions. Co-purification of receptor and epidermal growth factor-enhanced phosphorylation activity. J Biol Chem. 1980 May 25;255(10):4834–4842. [PubMed] [Google Scholar]
- Cohen S. The epidermal growth factor (EGF). Cancer. 1983 May 15;51(10):1787–1791. doi: 10.1002/1097-0142(19830515)51:10<1787::aid-cncr2820511004>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Cohen S., Ushiro H., Stoscheck C., Chinkers M. A native 170,000 epidermal growth factor receptor-kinase complex from shed plasma membrane vesicles. J Biol Chem. 1982 Feb 10;257(3):1523–1531. [PubMed] [Google Scholar]
- Cummings R. D., Kornfeld S. Fractionation of asparagine-linked oligosaccharides by serial lectin-Agarose affinity chromatography. A rapid, sensitive, and specific technique. J Biol Chem. 1982 Oct 10;257(19):11235–11240. [PubMed] [Google Scholar]
- Cummings R. D., Kornfeld S., Schneider W. J., Hobgood K. K., Tolleshaug H., Brown M. S., Goldstein J. L. Biosynthesis of N- and O-linked oligosaccharides of the low density lipoprotein receptor. J Biol Chem. 1983 Dec 25;258(24):15261–15273. [PubMed] [Google Scholar]
- Feizi T., Kapadia A., Yount W. J. I and i antigens of human peripheral blood lymphocytes cocap with receptors for concanavalin A. Proc Natl Acad Sci U S A. 1980 Jan;77(1):376–380. doi: 10.1073/pnas.77.1.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feizi T. The blood group Ii system: a carbohydrate antigen system defined by naturally monoclonal or oligoclonal autoantibodies of man. Immunol Commun. 1981;10(2):127–156. doi: 10.3109/08820138109050693. [DOI] [PubMed] [Google Scholar]
- Foster C. S., Dinsdale E. A., Edwards P. A., Neville A. M. Monoclonal antibodies to the human mammary gland. II. Distribution of determinants in breast carcinomas. Virchows Arch A Pathol Anat Histol. 1982;394(3):295–305. doi: 10.1007/BF00430672. [DOI] [PubMed] [Google Scholar]
- Fredman P., Richert N. D., Magnani J. L., Willingham M. C., Pastan I., Ginsburg V. A monoclonal antibody that precipitates the glycoprotein receptor for epidermal growth factor is directed against the human blood group H type 1 antigen. J Biol Chem. 1983 Sep 25;258(18):11206–11210. [PubMed] [Google Scholar]
- Gooi H. C., Feizi T., Kapadia A., Knowles B. B., Solter D., Evans M. J. Stage-specific embryonic antigen involves alpha 1 goes to 3 fucosylated type 2 blood group chains. Nature. 1981 Jul 9;292(5819):156–158. doi: 10.1038/292156a0. [DOI] [PubMed] [Google Scholar]
- Gooi H. C., Schlessinger J., Lax I., Yarden Y., Libermann T. A., Feizi T. Monoclonal antibody reactive with the human epidermal-growth-factor receptor recognizes the blood-group-A antigen. Biosci Rep. 1983 Nov;3(11):1045–1052. doi: 10.1007/BF01121031. [DOI] [PubMed] [Google Scholar]
- Gooi H. C., Uemura K., Edwards P. A., Foster C. S., Pickering N., Feizi T. Two mouse hybridoma antibodies against human milk-fat globules recognise the I(Ma) antigenic determinant beta-D-Galp-(1 leads to 4)-beta-D-GlcpNAc-(1 leads to 6). Carbohydr Res. 1983 Aug 16;120:293–302. doi: 10.1016/0008-6215(83)88023-9. [DOI] [PubMed] [Google Scholar]
- Gregoriou M., Rees A. R. Studies on the structure and function of the epidermal-growth-factor receptor. Biochem Soc Trans. 1984 Apr;12(2):160–165. doi: 10.1042/bst0120160. [DOI] [PubMed] [Google Scholar]
- Gregorou M., Rees A. R. Properties of a monoclonal antibody to epidermal growth factor receptor with implications for the mechanism of action of EGF. EMBO J. 1984 May;3(5):929–937. doi: 10.1002/j.1460-2075.1984.tb01910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsell E. F., Gooi H. C., Feizi T. The monoclonal antibody anti-SSEA-1 discriminates between fucosylated type 1 and type 2 blood group chains. FEBS Lett. 1981 Aug 31;131(2):279–282. doi: 10.1016/0014-5793(81)80384-5. [DOI] [PubMed] [Google Scholar]
- Kabat E. A., Liao J., Shyong J., Osserman E. F. A monoclonal IgM lambda macroglobulin with specificity for lacto-N-tetraose in a patient with bronchogenic carcinoma. J Immunol. 1982 Feb;128(2):540–544. [PubMed] [Google Scholar]
- Kawamoto T., Sato J. D., Le A., Polikoff J., Sato G. H., Mendelsohn J. Growth stimulation of A431 cells by epidermal growth factor: identification of high-affinity receptors for epidermal growth factor by an anti-receptor monoclonal antibody. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1337–1341. doi: 10.1073/pnas.80.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles R. W., Bai Y., Daniels G. L., Watkins W. Monoclonal anti-type 2 H: an antibody detecting a precursor of the A and B blood group antigens. J Immunogenet. 1982 Apr;9(2):69–76. doi: 10.1111/j.1744-313x.1982.tb00965.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Magnani J. L., Nilsson B., Brockhaus M., Zopf D., Steplewski Z., Koprowski H., Ginsburg V. A monoclonal antibody-defined antigen associated with gastrointestinal cancer is a ganglioside containing sialylated lacto-N-fucopentaose II. J Biol Chem. 1982 Dec 10;257(23):14365–14369. [PubMed] [Google Scholar]
- Mayes E. L., Waterfield M. D. Biosynthesis of the epidermal growth factor receptor in A431 cells. EMBO J. 1984 Mar;3(3):531–537. doi: 10.1002/j.1460-2075.1984.tb01842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richert N. D., Willingham M. C., Pastan I. Epidermal growth factor receptor. Characterization of a monoclonal antibody specific for the receptor of A431 cells. J Biol Chem. 1983 Jul 25;258(14):8902–8907. [PubMed] [Google Scholar]
- Schreiber A. B., Lax I., Yarden Y., Eshhar Z., Schlessinger J. Monoclonal antibodies against receptor for epidermal growth factor induce early and delayed effects of epidermal growth factor. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7535–7539. doi: 10.1073/pnas.78.12.7535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber A. B., Libermann T. A., Lax I., Yarden Y., Schlessinger J. Biological role of epidermal growth factor-receptor clustering. Investigation with monoclonal anti-receptor antibodies. J Biol Chem. 1983 Jan 25;258(2):846–853. [PubMed] [Google Scholar]
- Scudder P., Hanfland P., Uemura K., Feizi T. Endo-beta-D-galactosidases of Bacteroides fragilis and Escherichia freundii hydrolyze linear but not branched oligosaccharide domains of glycolipids of the neolacto series. J Biol Chem. 1984 May 25;259(10):6586–6592. [PubMed] [Google Scholar]
- Scudder P., Uemura K., Dolby J., Fukuda M. N., Feizi T. Isolation and characterization of an endo-beta-galactosidase from Bacteroides fragilis. Biochem J. 1983 Aug 1;213(2):485–494. doi: 10.1042/bj2130485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solter D., Knowles B. B. Monoclonal antibody defining a stage-specific mouse embryonic antigen (SSEA-1). Proc Natl Acad Sci U S A. 1978 Nov;75(11):5565–5569. doi: 10.1073/pnas.75.11.5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura K., Childs R. A., Hanfland P., Feizi T. A multiplicity of erythrocyte glycolipids of the neolacto series revealed by immuno-thin-layer chromatography with monoclonal anti-I and anti-i antibodies. Biosci Rep. 1983 Jun;3(6):577–588. doi: 10.1007/BF01120703. [DOI] [PubMed] [Google Scholar]
- Voak D., Sacks S., Alderson T., Takei F., Lennox E., Jarvis J., Milstein C., Darnborough J. Monoclonal anti-A from a hybrid-myeloma: evaluating as a blood grouping reagent. Vox Sang. 1980 Sep;39(3):134–140. doi: 10.1111/j.1423-0410.1980.tb01848.x. [DOI] [PubMed] [Google Scholar]
- Weber W., Gill G. N., Spiess J. Production of an epidermal growth factor receptor-related protein. Science. 1984 Apr 20;224(4646):294–297. doi: 10.1126/science.6324343. [DOI] [PubMed] [Google Scholar]
- Yamashita K., Tachibana Y., Kobata A. Oligosaccharides of human milk. Structural studies of two new octasaccharides, difucosyl derivatives of para-lacto-N-hexaose and para-lacto-N-neohexaose. J Biol Chem. 1977 Aug 10;252(15):5408–5411. [PubMed] [Google Scholar]
- Yamashita K., Tachibana Y., Nakayama T., Kitamura M., Endo Y., Kobata A. Structural studies of the sugar chains of human parotid alpha-amylase. J Biol Chem. 1980 Jun 25;255(12):5635–5642. [PubMed] [Google Scholar]
- Young W. W., Jr, Johnson H. S., Tamura Y., Karlsson K. A., Larson G., Parker J. M., Khare D. P., Spohr U., Baker D. A., Hindsgaul O. Characterization of monoclonal antibodies specific for the Lewis a human blood group determinant. J Biol Chem. 1983 Apr 25;258(8):4890–4894. [PubMed] [Google Scholar]
- Yurchenco P. D., Ceccarini C., Atkinson P. H. Labeling complex carbohydrates of animal cells with monosaccharides. Methods Enzymol. 1978;50:175–204. doi: 10.1016/0076-6879(78)50019-0. [DOI] [PubMed] [Google Scholar]