Abstract
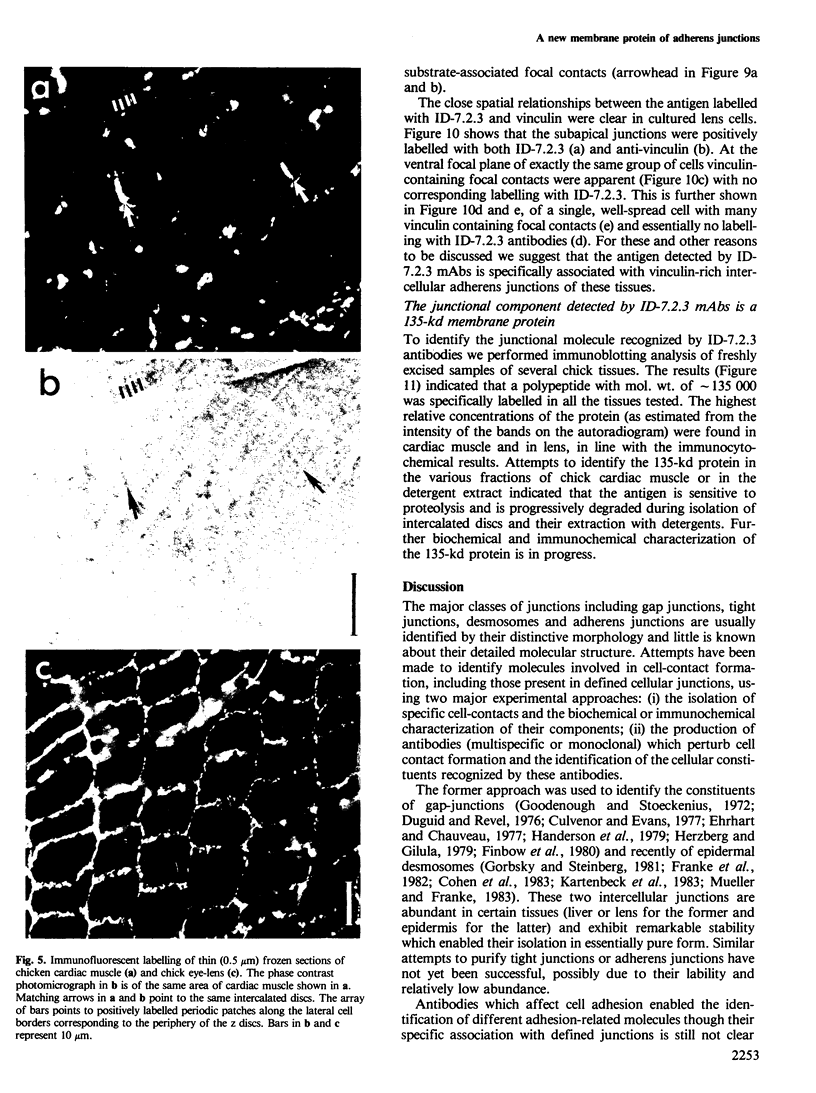
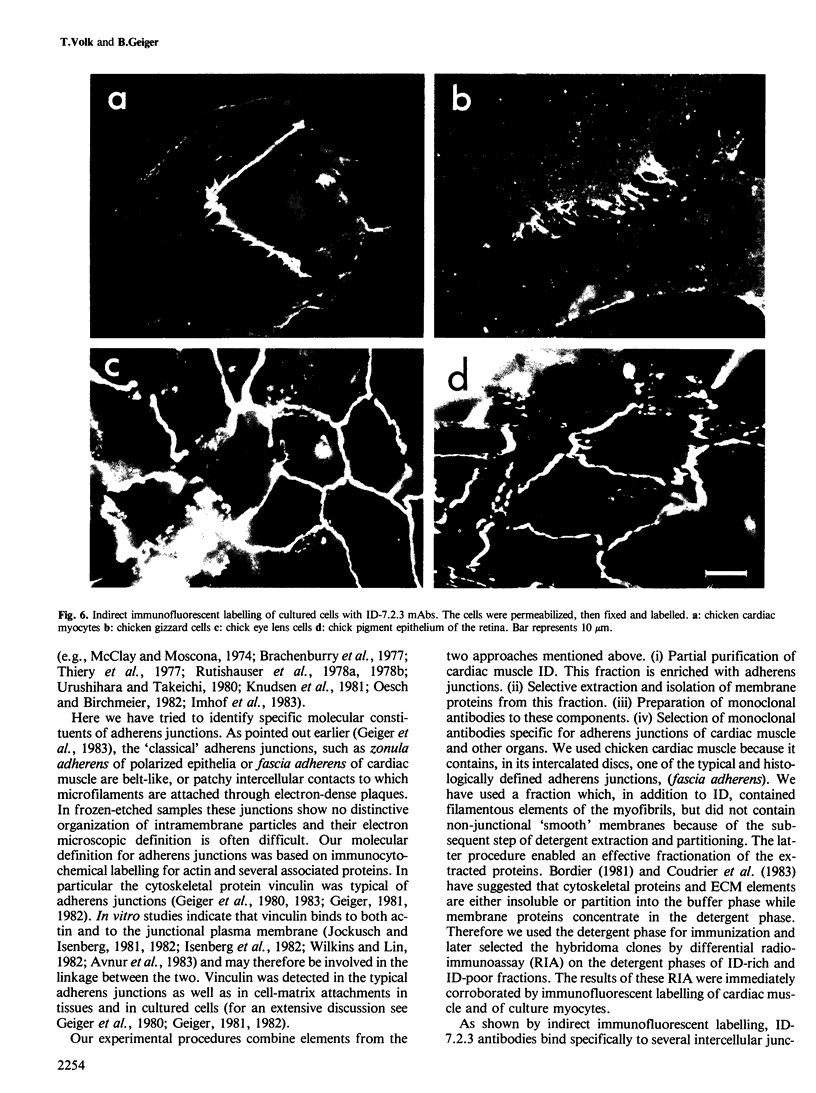
We report here on a new 135-kd membrane protein which is specifically associated with intercellular adherens-type junctions. This surface component was identified by a monoclonal antibody, ID-7.2.3, raised against detergent-extracted components of membranes of chicken cardiac muscle rich in intercalated discs. The antibodies stain extensively adherens junctions in intact cardiac muscle and in lens, as well as in cultured cells derived from these tissues. In living cultured cells only very little immunolabelling was obtained with ID-7.2.3 antibodies, probably due to the limited accessibility of the antibodies to the intercellular gap. However, upon the removal of extracellular Ca2+ ions a dissociation of the junction occurred, leading to the rapid exposure of the 135-kd protein. Immunoelectron microscopic labelling of EGTA-treated, or detergent-permeabilized cells indicated that the antigen is found along the plasma membrane and highly enriched in contact areas. Double immunolabelling for both the 135-kd protein and vinculin pointed to the close association of the two in intercellular junctions and to the apparent absence of the former protein from the vinculin-rich focal contacts of cultured cells and from dense plaque of smooth muscle. Immunoblotting indicated that the 135-kd protein is present in many tissues but is particularly enriched in heart, lens and brain.
Full text
PDF





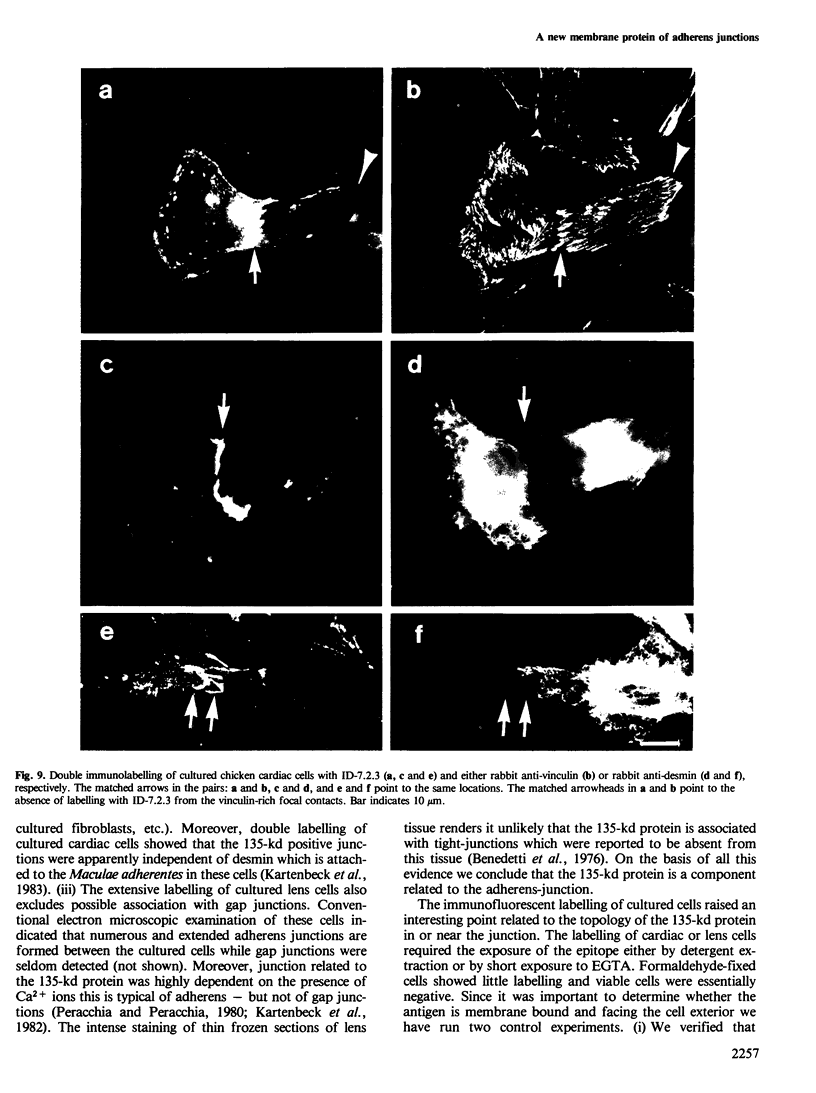
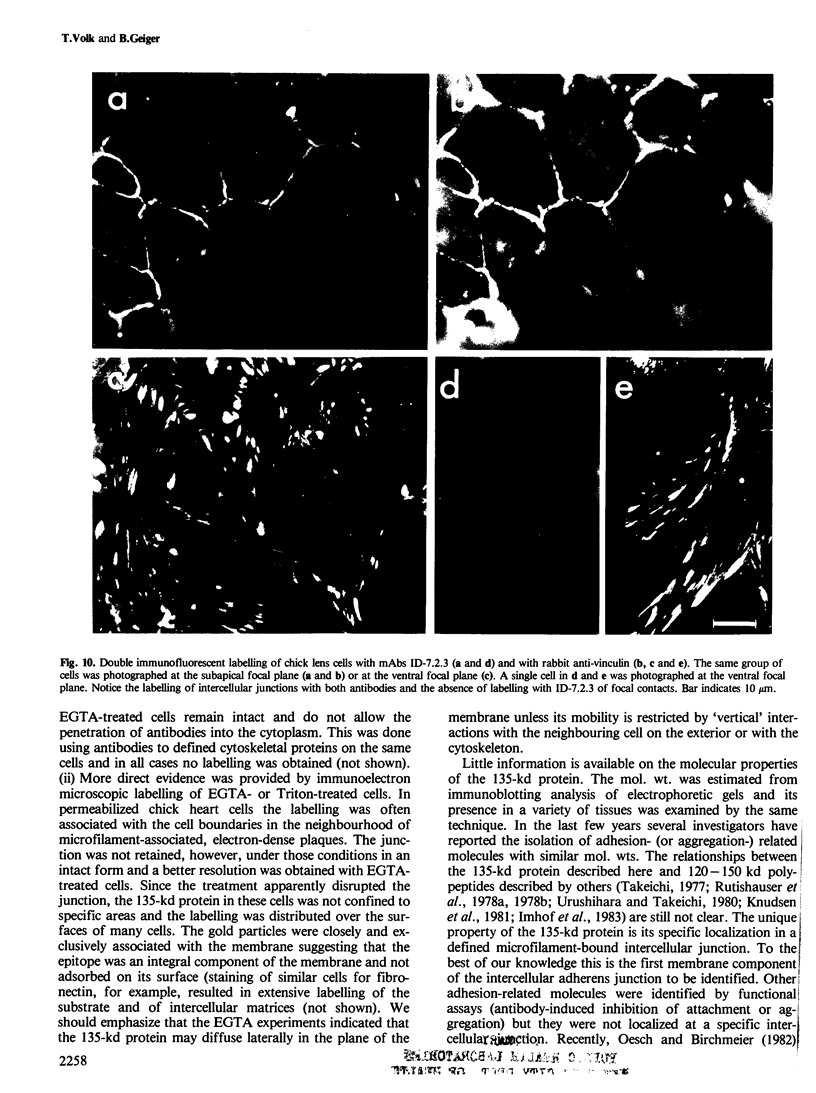


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aplin J. D., Hughes R. C. Complex carbohydrates of the extracellular matrix structures, interactions and biological roles. Biochim Biophys Acta. 1982 Dec;694(4):375–418. doi: 10.1016/0304-4157(82)90003-x. [DOI] [PubMed] [Google Scholar]
- Avnur Z., Geiger B. The removal of extracellular fibronectin from areas of cell-substrate contact. Cell. 1981 Jul;25(1):121–132. doi: 10.1016/0092-8674(81)90236-1. [DOI] [PubMed] [Google Scholar]
- Avnur Z., Small J. V., Geiger B. Actin-independent association of vinculin with the cytoplasmic aspect of the plasma membrane in cell-contact areas. J Cell Biol. 1983 Jun;96(6):1622–1630. doi: 10.1083/jcb.96.6.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti E. L., Dunia I., Bentzel C. J., Vermorken A. J., Kibbelaar M., Bloemendal H. A portrait of plasma membrane specializations in eye lens epithelium and fibers. Biochim Biophys Acta. 1976 Dec 14;457(3-4):353–384. doi: 10.1016/0304-4157(76)90004-6. [DOI] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Brackenbury R., Thiery J. P., Rutishauser U., Edelman G. M. Adhesion among neural cells of the chick embryo. I. An immunological assay for molecules involved in cell-cell binding. J Biol Chem. 1977 Oct 10;252(19):6835–6840. [PubMed] [Google Scholar]
- Brandtzaeg P. Conjugates of immunoglobulin G with different fluorochromes. I. Characterization by anionic-exchange chromatography. Scand J Immunol. 1973;2(3):273–290. doi: 10.1111/j.1365-3083.1973.tb02037.x. [DOI] [PubMed] [Google Scholar]
- Burridge K. Direct identification of specific glycoproteins and antigens in sodium dodecyl sulfate gels. Methods Enzymol. 1978;50:54–64. doi: 10.1016/0076-6879(78)50007-4. [DOI] [PubMed] [Google Scholar]
- Cohen S. M., Gorbsky G., Steinberg M. S. Immunochemical characterization of related families of glycoproteins in desmosomes. J Biol Chem. 1983 Feb 25;258(4):2621–2627. [PubMed] [Google Scholar]
- Colaco C. A., Evans W. H. A biochemical dissection of the cardiac intercalated disk: isolation of subcellular fractions containing fascia adherentes and gap junctions. J Cell Sci. 1981 Dec;52:313–325. doi: 10.1242/jcs.52.1.313. [DOI] [PubMed] [Google Scholar]
- Colaco C. A., Evans W. H. Partial purification of an intercalated disc-containing cardiac plasma membrane fraction. Biochim Biophys Acta. 1982 Jan 4;684(1):40–46. doi: 10.1016/0005-2736(82)90046-3. [DOI] [PubMed] [Google Scholar]
- Coudrier E., Reggio H., Louvard D. Characterization of membrane glycoproteins involved in attachment of microfilaments to the microvillar membrane. Ciba Found Symp. 1983;95:216–232. doi: 10.1002/9780470720769.ch13. [DOI] [PubMed] [Google Scholar]
- Culvenor J. G., Evans W. H. Preparation of hepatic gap (communicating) junctions. Identification of the constituent polypeptide subunits. Biochem J. 1977 Dec 15;168(3):475–481. doi: 10.1042/bj1680475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duguid J. R., Revel J. P. The protein components of the gap junction. Cold Spring Harb Symp Quant Biol. 1976;40:45–47. doi: 10.1101/sqb.1976.040.01.007. [DOI] [PubMed] [Google Scholar]
- Ehrhart J. C., Chauveau J. The protein component of mouse hepatocyte gap junctions. FEBS Lett. 1977 Jun 15;78(2):295–299. doi: 10.1016/0014-5793(77)80327-x. [DOI] [PubMed] [Google Scholar]
- Eshhar Z., Blatt C., Bergman Y., Heimovich J. Induction of secretion of IgM from cells of the B cell line 38c-13 by somatic cell hybridization. J Immunol. 1979 Jun;122(6):2430–2434. [PubMed] [Google Scholar]
- FARQUHAR M. G., PALADE G. E. Junctional complexes in various epithelia. J Cell Biol. 1963 May;17:375–412. doi: 10.1083/jcb.17.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finbow M., Yancey S. B., Johnson R., Revel J. P. Independent lines of evidence suggesting a major gap junctional protein with a molecular weight of 26,000. Proc Natl Acad Sci U S A. 1980 Feb;77(2):970–974. doi: 10.1073/pnas.77.2.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W., Moll R., Schiller D. L., Schmid E., Kartenbeck J., Mueller H. Desmoplakins of epithelial and myocardial desmosomes are immunologically and biochemically related. Differentiation. 1982;23(2):115–127. doi: 10.1111/j.1432-0436.1982.tb01274.x. [DOI] [PubMed] [Google Scholar]
- Galfrè G., Milstein C. Preparation of monoclonal antibodies: strategies and procedures. Methods Enzymol. 1981;73(Pt B):3–46. doi: 10.1016/0076-6879(81)73054-4. [DOI] [PubMed] [Google Scholar]
- Geiger B. A 130K protein from chicken gizzard: its localization at the termini of microfilament bundles in cultured chicken cells. Cell. 1979 Sep;18(1):193–205. doi: 10.1016/0092-8674(79)90368-4. [DOI] [PubMed] [Google Scholar]
- Geiger B. Involvement of vinculin in contact-induced cytoskeletal interactions. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 2):671–682. doi: 10.1101/sqb.1982.046.01.063. [DOI] [PubMed] [Google Scholar]
- Geiger B., Schmid E., Franke W. W. Spatial distribution of proteins specific for desmosomes and adhaerens junctions in epithelial cells demonstrated by double immunofluorescence microscopy. Differentiation. 1983;23(3):189–205. doi: 10.1111/j.1432-0436.1982.tb01283.x. [DOI] [PubMed] [Google Scholar]
- Geiger B., Singer S. J. Association of microtubules and intermediate filaments in chicken gizzard cells as detected by double immunofluorescence. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4769–4773. doi: 10.1073/pnas.77.8.4769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B., Singer S. J. The participation of alpha-actinin in the capping of cell membrane components. Cell. 1979 Jan;16(1):213–222. doi: 10.1016/0092-8674(79)90202-2. [DOI] [PubMed] [Google Scholar]
- Geiger B., Tokuyasu K. T., Dutton A. H., Singer S. J. Vinculin, an intracellular protein localized at specialized sites where microfilament bundles terminate at cell membranes. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4127–4131. doi: 10.1073/pnas.77.7.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B., Tokuyasu K. T., Singer S. J. Immunocytochemical localization of alpha-actinin in intestinal epithelial cells. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2833–2837. doi: 10.1073/pnas.76.6.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenough D. A., Stoeckenius W. The isolation of mouse hepatocyte gap junctions. Preliminary chemical characterization and x-ray diffraction. J Cell Biol. 1972 Sep;54(3):646–656. doi: 10.1083/jcb.54.3.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbsky G., Steinberg M. S. Isolation of the intercellular glycoproteins of desmosomes. J Cell Biol. 1981 Jul;90(1):243–248. doi: 10.1083/jcb.90.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell F. Cellular adhesiveness and extracellular substrata. Int Rev Cytol. 1978;53:65–144. doi: 10.1016/s0074-7696(08)62241-x. [DOI] [PubMed] [Google Scholar]
- Henderson D., Eibl H., Weber K. Structure and biochemistry of mouse hepatic gap junctions. J Mol Biol. 1979 Aug 5;132(2):193–218. doi: 10.1016/0022-2836(79)90391-7. [DOI] [PubMed] [Google Scholar]
- Hertzberg E. L., Gilula N. B. Isolation and characterization of gap junctions from rat liver. J Biol Chem. 1979 Mar 25;254(6):2138–2147. [PubMed] [Google Scholar]
- Imhof B. A., Vollmers H. P., Goodman S. L., Birchmeier W. Cell-cell interaction and polarity of epithelial cells: specific perturbation using a monoclonal antibody. Cell. 1983 Dec;35(3 Pt 2):667–675. doi: 10.1016/0092-8674(83)90099-5. [DOI] [PubMed] [Google Scholar]
- Isenberg G., Leonard K., Jockusch B. M. Structural aspects of vinculin-actin interactions. J Mol Biol. 1982 Jun 25;158(2):231–249. doi: 10.1016/0022-2836(82)90431-4. [DOI] [PubMed] [Google Scholar]
- Jockusch B. M., Isenberg G. Interaction of alpha-actinin and vinculin with actin: opposite effects on filament network formation. Proc Natl Acad Sci U S A. 1981 May;78(5):3005–3009. doi: 10.1073/pnas.78.5.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jockusch B. M., Isenberg G. Vinculin and alpha-actinin: interaction with actin and effect on microfilament network formation. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 2):613–623. doi: 10.1101/sqb.1982.046.01.057. [DOI] [PubMed] [Google Scholar]
- Kartenbeck J., Franke W. W., Moser J. G., Stoffels U. Specific attachment of desmin filaments to desmosomal plaques in cardiac myocytes. EMBO J. 1983;2(5):735–742. doi: 10.1002/j.1460-2075.1983.tb01493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kartenbeck J., Schmid E., Franke W. W., Geiger B. Different modes of internalization of proteins associated with adhaerens junctions and desmosomes: experimental separation of lateral contacts induces endocytosis of desmosomal plaque material. EMBO J. 1982;1(6):725–732. doi: 10.1002/j.1460-2075.1982.tb01237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly D. E. Fine structure of desmosomes. , hemidesmosomes, and an adepidermal globular layer in developing newt epidermis. J Cell Biol. 1966 Jan;28(1):51–72. doi: 10.1083/jcb.28.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen K. A., Rao P. E., Damsky C. H., Buck C. A. Membrane glycoproteins involved in cell--substratum adhesion. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6071–6075. doi: 10.1073/pnas.78.10.6071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McClay D. R., Moscona A. A. Purification of the specific cell-aggregating factor from embryonic neural retina cells. Exp Cell Res. 1974 Aug;87(2):438–443. doi: 10.1016/0014-4827(74)90514-x. [DOI] [PubMed] [Google Scholar]
- Mueller H., Franke W. W. Biochemical and immunological characterization of desmoplakins I and II, the major polypeptides of the desmosomal plaque. J Mol Biol. 1983 Feb 5;163(4):647–671. doi: 10.1016/0022-2836(83)90116-x. [DOI] [PubMed] [Google Scholar]
- Oesch B., Birchmeier W. New surface component of fibroblast's focal contacts identified by a monoclonal antibody. Cell. 1982 Dec;31(3 Pt 2):671–679. doi: 10.1016/0092-8674(82)90322-1. [DOI] [PubMed] [Google Scholar]
- Peracchia C., Peracchia L. L. Gap junction dynamics: reversible effects of divalent cations. J Cell Biol. 1980 Dec;87(3 Pt 1):708–718. doi: 10.1083/jcb.87.3.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutishauser U., Gall W. E., Edelman G. M. Adhesion among neural cells of the chick embryo. IV. Role of the cell surface molecule CAM in the formation of neurite bundles in cultures of spinal ganglia. J Cell Biol. 1978 Nov;79(2 Pt 1):382–393. doi: 10.1083/jcb.79.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutishauser U., Thiery J. P., Brackenbury R., Edelman G. M. Adhesion among neural cells of the chick embryo. III. Relationship of the surface molecule CAM to cell adhesion and the development of histotypic patterns. J Cell Biol. 1978 Nov;79(2 Pt 1):371–381. doi: 10.1083/jcb.79.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staehelin L. A. Structure and function of intercellular junctions. Int Rev Cytol. 1974;39:191–283. doi: 10.1016/s0074-7696(08)60940-7. [DOI] [PubMed] [Google Scholar]
- Takeichi M. Functional correlation between cell adhesive properties and some cell surface proteins. J Cell Biol. 1977 Nov;75(2 Pt 1):464–474. doi: 10.1083/jcb.75.2.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiery J. P., Brackenbury R., Rutishauser U., Edelman G. M. Adhesion among neural cells of the chick embryo. II. Purification and characterization of a cell adhesion molecule from neural retina. J Biol Chem. 1977 Oct 10;252(19):6841–6845. [PubMed] [Google Scholar]
- Tokuyasu K. T. Immunochemistry on ultrathin frozen sections. Histochem J. 1980 Jul;12(4):381–403. doi: 10.1007/BF01011956. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urushihara H., Takeichi M. Cell-cell adhesion molecule: identification of a glycoprotein relevant to the Ca2+-independent aggregation of Chinese hamster fibroblasts. Cell. 1980 Jun;20(2):363–371. doi: 10.1016/0092-8674(80)90622-4. [DOI] [PubMed] [Google Scholar]
- Wilkins J. A., Lin S. High-affinity interaction of vinculin with actin filaments in vitro. Cell. 1982 Jan;28(1):83–90. doi: 10.1016/0092-8674(82)90377-4. [DOI] [PubMed] [Google Scholar]