Abstract
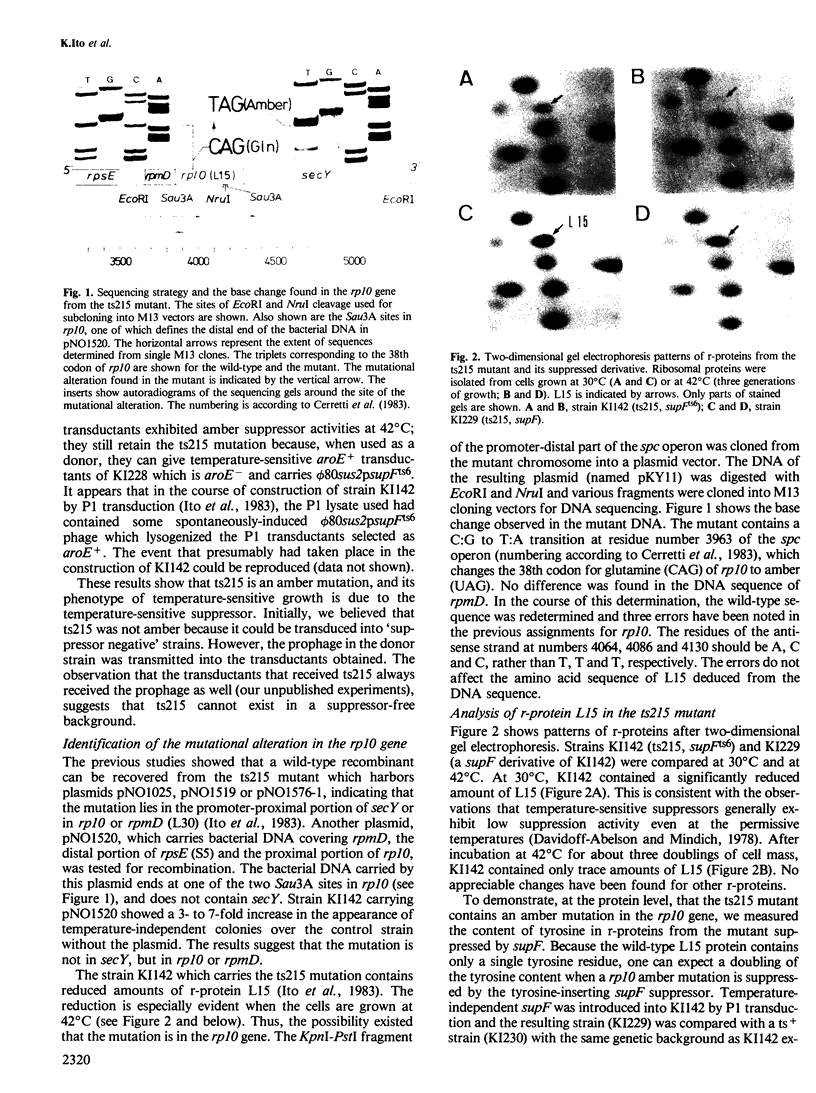
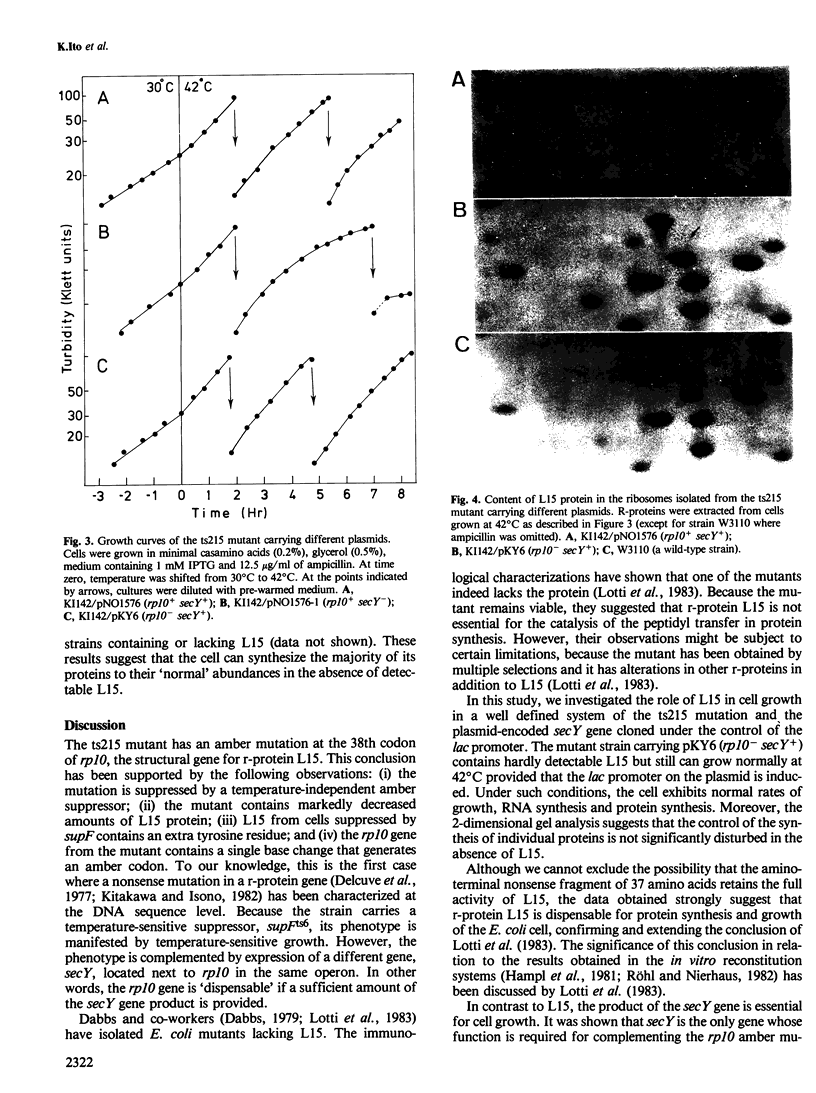
We have previously described a temperature-sensitive mutant, ts215, which is defective in protein secretion. Complementation studies indicated that the mutation was located at the distal part of the spc ribosomal protein operon and the gene secY is required for efficient protein secretion. We now report a more complete genetic and biochemical analysis of the ts215 mutant. These studies revealed that the ts215 mutant has an amber mutation in the gene rp10 for ribosomal protein L15, which is located upstream and adjacent to secY. The amber mutation exerts a polar effect on secY causing a defect in protein secretion. These conclusions were supported by the following observations. The mutant strain carries a phi 80 prophage containing a temperature-sensitive suppressor, supFts6. The strain contains decreased amounts of L15 and is suppressible by a temperature-independent nonsense suppressor. In addition, L15 contains an extra tyrosine residue when suppressed by supF. DNA sequence analysis revealed the presence of a single base change in rp10 resulting in an amber codon at the 38th codon of L15. The mutant phenotype is complemented by a plasmid carrying only the secY gene under lac promoter control. The mutant cells complemented by secY can grow and synthesize proteins at normal rates and abundances at 42 degrees C, despite the fact that their ribosomes contain barely detectable levels of L15. These results indicate that ribosomal protein L15 is dispensable for protein synthesis and cell growth. In contrast, the decreased level of expression of the secY gene leads to defective protein secretion and defective cell growth.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Casadaban M. J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976 Jul 5;104(3):541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- Cerretti D. P., Dean D., Davis G. R., Bedwell D. M., Nomura M. The spc ribosomal protein operon of Escherichia coli: sequence and cotranscription of the ribosomal protein genes and a protein export gene. Nucleic Acids Res. 1983 May 11;11(9):2599–2616. doi: 10.1093/nar/11.9.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabbs E. R. Selection for Escherichia coli mutants with proteins missing from the ribosome. J Bacteriol. 1979 Nov;140(2):734–737. doi: 10.1128/jb.140.2.734-737.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidoff-Abelson R., Mindich L. A mutation that increases the activity of nonsense suppressors in Escherichia coli. Mol Gen Genet. 1978 Feb 16;159(2):161–169. doi: 10.1007/BF00270889. [DOI] [PubMed] [Google Scholar]
- Delcuve G., Cabezón T., Ghysen A., Herzog A., Bollen A. Amber mutations in Escherichia coli essential genes: isolation of mutants affected in the ribosomes. Mol Gen Genet. 1977 Nov 29;157(2):149–153. doi: 10.1007/BF00267392. [DOI] [PubMed] [Google Scholar]
- Geyl D., Böck A., Isono K. An improved method for two-dimensional gel-electrophoresis: analysis of mutationally altered ribosomal proteins of Escherichia coli. Mol Gen Genet. 1981;181(3):309–312. doi: 10.1007/BF00425603. [DOI] [PubMed] [Google Scholar]
- Hampl H., Schulze H., Nierhaus K. H. Ribosomal components from Escherichia coli 50 S subunits involved in the reconstitution of peptidyltransferase activity. J Biol Chem. 1981 Mar 10;256(5):2284–2288. [PubMed] [Google Scholar]
- Isono S., Isono K. Mutations affecting the structural genes and the genes coding for modifying enzymes for ribosomal proteins in Escherichia coli. Mol Gen Genet. 1978 Sep 20;165(1):15–20. doi: 10.1007/BF00270371. [DOI] [PubMed] [Google Scholar]
- Ito K., Wittekind M., Nomura M., Shiba K., Yura T., Miura A., Nashimoto H. A temperature-sensitive mutant of E. coli exhibiting slow processing of exported proteins. Cell. 1983 Mar;32(3):789–797. doi: 10.1016/0092-8674(83)90065-x. [DOI] [PubMed] [Google Scholar]
- Kitakawa M., Isono K. An amber mutation in the gene rpsA for ribosomal protein S1 in Escherichia coli. Mol Gen Genet. 1982;185(3):445–447. doi: 10.1007/BF00334137. [DOI] [PubMed] [Google Scholar]
- Lotti M., Dabbs E. R., Hasenbank R., Stöffler-Meilicke M., Stöffler G. Characterisation of a mutant from Escherichia coli lacking protein L15 and localisation of protein L15 by immuno-electron microscopy. Mol Gen Genet. 1983;192(3):295–300. doi: 10.1007/BF00392165. [DOI] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Oliver D. B., Beckwith J. E. coli mutant pleiotropically defective in the export of secreted proteins. Cell. 1981 Sep;25(3):765–772. doi: 10.1016/0092-8674(81)90184-7. [DOI] [PubMed] [Google Scholar]
- Oliver D. B., Beckwith J. Regulation of a membrane component required for protein secretion in Escherichia coli. Cell. 1982 Aug;30(1):311–319. doi: 10.1016/0092-8674(82)90037-x. [DOI] [PubMed] [Google Scholar]
- Pittard J., Wallace B. J. Distribution and function of genes concerned with aromatic biosynthesis in Escherichia coli. J Bacteriol. 1966 Apr;91(4):1494–1508. doi: 10.1128/jb.91.4.1494-1508.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron E. Z., Kohler R. E., Davis B. D. Polysomes extracted from Escherichia coli by freeze-thaw-lysozyme lysis. Science. 1966 Sep 2;153(3740):1119–1120. doi: 10.1126/science.153.3740.1119. [DOI] [PubMed] [Google Scholar]
- Röhl R., Nierhaus K. H. Assembly map of the large subunit (50S) of Escherichia coli ribosomes. Proc Natl Acad Sci U S A. 1982 Feb;79(3):729–733. doi: 10.1073/pnas.79.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiba K., Ito K., Yura T., Cerretti D. P. A defined mutation in the protein export gene within the spc ribosomal protein operon of Escherichia coli: isolation and characterization of a new temperature-sensitive secY mutant. EMBO J. 1984 Mar;3(3):631–635. doi: 10.1002/j.1460-2075.1984.tb01859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimura Y., Ozeki H. Genetic study on transfer RNA. Adv Biophys. 1973;4:191–226. [PubMed] [Google Scholar]
- Shultz J., Silhavy T. J., Berman M. L., Fiil N., Emr S. D. A previously unidentified gene in the spc operon of Escherichia coli K12 specifies a component of the protein export machinery. Cell. 1982 Nov;31(1):227–235. doi: 10.1016/0092-8674(82)90422-6. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]





