Abstract
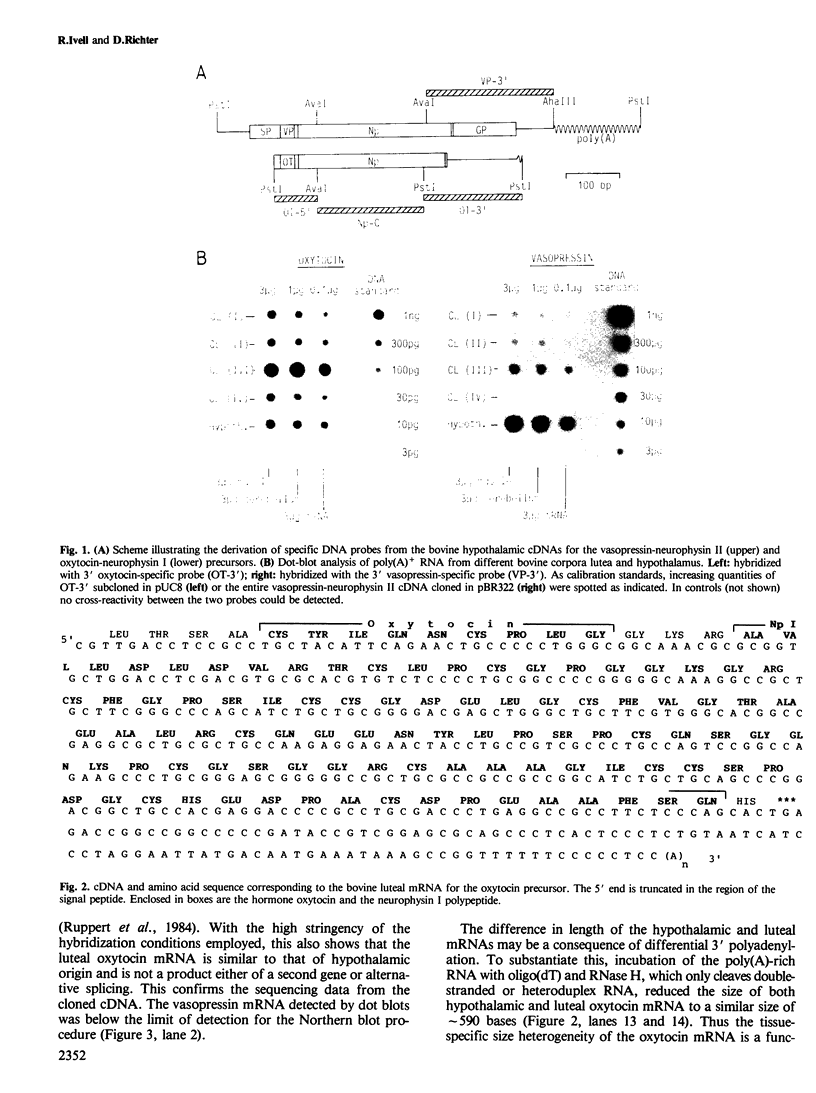
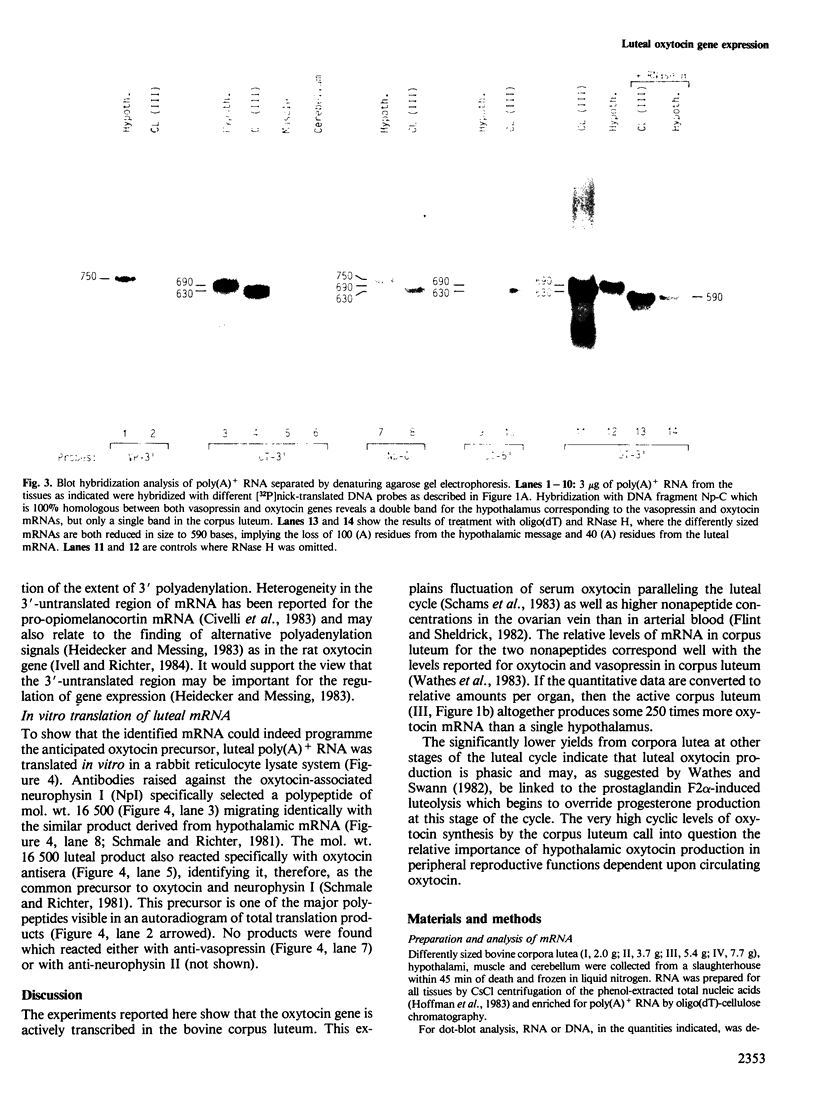
Expression of the vasopressin and oxytocin genes has been described so far only in the hypothalamus. We report here that at least the oxytocin gene is highly transcribed in the bovine corpus luteum during the mid-luteal phase of the oestrous cycle. Luteal cDNA sequence analysis as well as cell-free translation studies showed that the luteal mRNA is essentially similar to that in the hypothalamus, except that in the corpus luteum the poly(A) tail of this mRNA is shorter. When calculating the relative amounts per organ, the active corpus luteum produces approximately 250 times more oxytocin mRNA than a single hypothalamus.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amara S. G., Jonas V., Rosenfeld M. G., Ong E. S., Evans R. M. Alternative RNA processing in calcitonin gene expression generates mRNAs encoding different polypeptide products. Nature. 1982 Jul 15;298(5871):240–244. doi: 10.1038/298240a0. [DOI] [PubMed] [Google Scholar]
- Flint A. P., Sheldrick E. L. Ovarian secretion of oxytocin is stimulated by prostaglandin. Nature. 1982 Jun 17;297(5867):587–588. doi: 10.1038/297587a0. [DOI] [PubMed] [Google Scholar]
- Gal A., Nahon J. L., Sala-Trepat J. M. Detection of rare mRNA species in a complex RNA population by blot hybridization techniques: a comparative survey. Anal Biochem. 1983 Jul 1;132(1):190–194. doi: 10.1016/0003-2697(83)90446-3. [DOI] [PubMed] [Google Scholar]
- Heidecker G., Messing J. Sequence analysis of zein cDNAs obtained by an efficient mRNA cloning method. Nucleic Acids Res. 1983 Jul 25;11(14):4891–4906. doi: 10.1093/nar/11.14.4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann W., Bach T. C., Seliger H., Kreil G. Biosynthesis of caerulein in the skin of Xenopus laevis: partial sequences of precursors as deduced from cDNA clones. EMBO J. 1983;2(1):111–114. doi: 10.1002/j.1460-2075.1983.tb01390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivell R., Richter D. Fingerprint analysis of bovine hypothalamic preprosomatostatin. Identification of somatostatin-28 at the C terminus. Eur J Biochem. 1982 Dec;129(1):81–86. doi: 10.1111/j.1432-1033.1982.tb07023.x. [DOI] [PubMed] [Google Scholar]
- Ivell R., Richter D. Structure and comparison of the oxytocin and vasopressin genes from rat. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2006–2010. doi: 10.1073/pnas.81.7.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura N., Takagaki Y., Furuto S., Tanaka T., Nawa H., Nakanishi S. A single gene for bovine high molecular weight and low molecular weight kininogens. Nature. 1983 Oct 6;305(5934):545–549. doi: 10.1038/305545a0. [DOI] [PubMed] [Google Scholar]
- Land H., Grez M., Ruppert S., Schmale H., Rehbein M., Richter D., Schütz G. Deduced amino acid sequence from the bovine oxytocin-neurophysin I precursor cDNA. Nature. 1983 Mar 24;302(5906):342–344. doi: 10.1038/302342a0. [DOI] [PubMed] [Google Scholar]
- Land H., Schütz G., Schmale H., Richter D. Nucleotide sequence of cloned cDNA encoding bovine arginine vasopressin-neurophysin II precursor. Nature. 1982 Jan 28;295(5847):299–303. doi: 10.1038/295299a0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Nicholson H. D., Swann R. W., Burford G. D., Wathes D. C., Porter D. G., Pickering B. T. Identification of oxytocin and vasopressin in the testis and in adrenal tissue. Regul Pept. 1984 Mar;8(2):141–146. doi: 10.1016/0167-0115(84)90169-1. [DOI] [PubMed] [Google Scholar]
- Ruppert S., Scherer G., Schütz G. Recent gene conversion involving bovine vasopressin and oxytocin precursor genes suggested by nucleotide sequence. Nature. 1984 Apr 5;308(5959):554–557. doi: 10.1038/308554a0. [DOI] [PubMed] [Google Scholar]
- Schmale H., Heinsohn S., Richter D. Structural organization of the rat gene for the arginine vasopressin-neurophysin precursor. EMBO J. 1983;2(5):763–767. doi: 10.1002/j.1460-2075.1983.tb01497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmale H., Richter D. Immunological identification of a common precursor to arginine vasopressin and neurophysin II synthesized by in vitro translation of bovine hypothalamic mRNA. Proc Natl Acad Sci U S A. 1981 Feb;78(2):766–769. doi: 10.1073/pnas.78.2.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wathes D. C., Swann R. W., Birkett S. D., Porter D. G., Pickering B. T. Characterization of oxytocin, vasopressin, and neurophysin from the bovine corpus luteum. Endocrinology. 1983 Aug;113(2):693–698. doi: 10.1210/endo-113-2-693. [DOI] [PubMed] [Google Scholar]
- Wathes D. C., Swann R. W. Is oxytocin an ovarian hormone? Nature. 1982 May 20;297(5863):225–227. doi: 10.1038/297225a0. [DOI] [PubMed] [Google Scholar]