Abstract
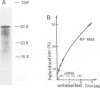
A crude membrane-bound replication complex isolated from encephalomyocarditis virus-infected cells is able to initiate the synthesis of viral RNA. Both the formation of the primer, VPg-pU, and its utilization for the initiation of RNA chains take place in this system. A significant amount of the synthesized VPg-pU is found in the free form. The predominant product of the de novo initiation is represented by short phenol-soluble VPg oligonucleotide species, and only a small percentage of the latter appear to be elongated into longer RNA chains.
Full text
PDF
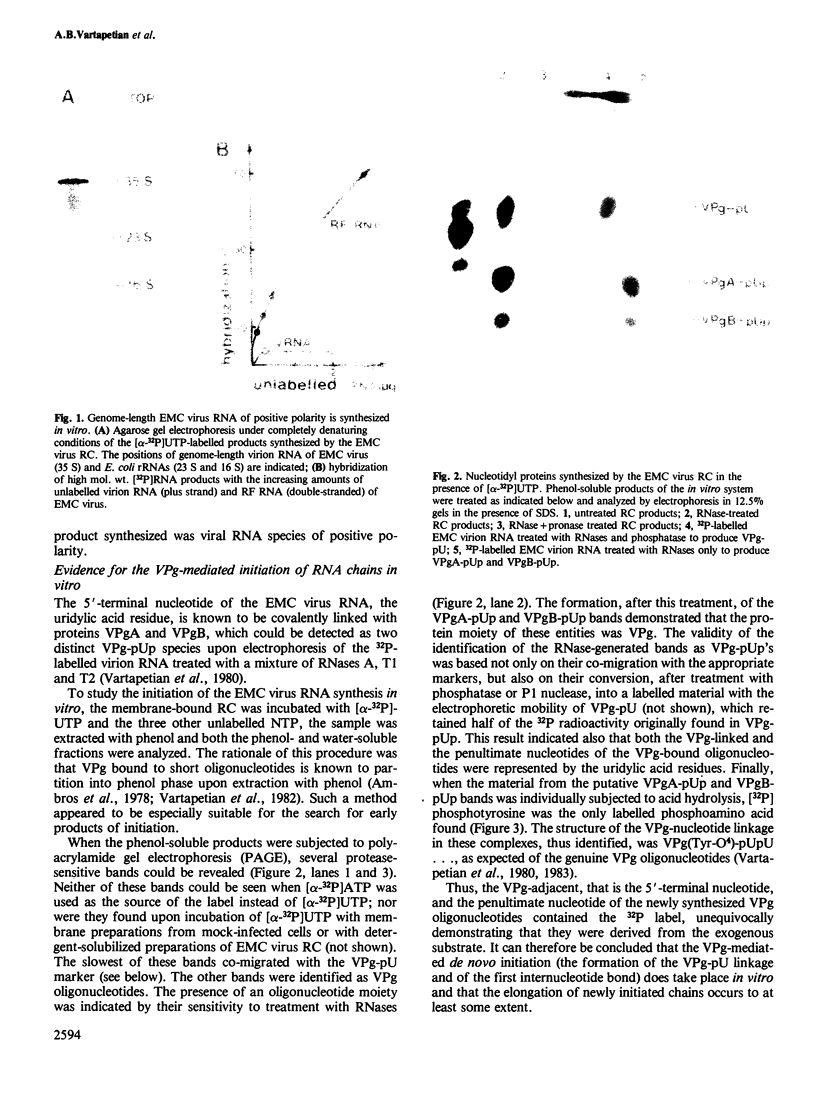



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler C. J., Elzinga M., Wimmer E. The genome-linked protein of picornaviruses. VIII. Complete amino acid sequence of poliovirus VPg and carboxy-terminal analysis of its precursor, P3-9. J Gen Virol. 1983 Feb;64(Pt 2):349–355. doi: 10.1099/0022-1317-64-2-349. [DOI] [PubMed] [Google Scholar]
- Ambros V., Pettersson R. F., Baltimore D. An enzymatic activity in uninfected cells that cleaves the linkage between poliovirion RNA and the 5' terminal protein. Cell. 1978 Dec;15(4):1439–1446. doi: 10.1016/0092-8674(78)90067-3. [DOI] [PubMed] [Google Scholar]
- Baron M. H., Baltimore D. Antibodies against the chemically synthesized genome-linked protein of poliovirus react with native virus-specific proteins. Cell. 1982 Feb;28(2):395–404. doi: 10.1016/0092-8674(82)90357-9. [DOI] [PubMed] [Google Scholar]
- Carpousis A. J., Gralla J. D. Cycling of ribonucleic acid polymerase to produce oligonucleotides during initiation in vitro at the lac UV5 promoter. Biochemistry. 1980 Jul 8;19(14):3245–3253. doi: 10.1021/bi00555a023. [DOI] [PubMed] [Google Scholar]
- Chumakov K. M. Izuchenie plavleniia i reassotsiatsii dvuspiral'noi RNK virusa entsefalomiokardita. Biokhimiia. 1979 Jan;44(1):57–66. [PubMed] [Google Scholar]
- Crawford N. M., Baltimore D. Genome-linked protein VPg of poliovirus is present as free VPg and VPg-pUpU in poliovirus-infected cells. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7452–7455. doi: 10.1073/pnas.80.24.7452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dmitrieva T. M., Agol V. I. Selective inhibition of the synthesis of single-stranded RNA of encephalomyocarditis virus by 2-(alpha-hydroxybenzyl)-benzimidazole in cell-free systems. Arch Gesamte Virusforsch. 1974;45(1-2):17–26. doi: 10.1007/BF01240538. [DOI] [PubMed] [Google Scholar]
- Drygin Iu F., Vartapetian A. B., Chumakov K. M. Kovalentnoe soedinenie RNK i belka v viruse zntsefalomnokardita. Mol Biol (Mosk) 1979 Jul-Aug;13(4):777–787. [PubMed] [Google Scholar]
- Easton D. M., Lipner H., Hines J., Leif R. C. Fluorescence monitoring of unstained protein bands in acrylamide gel. Anal Biochem. 1971 Feb;39(2):478–486. doi: 10.1016/0003-2697(71)90437-4. [DOI] [PubMed] [Google Scholar]
- Flanegan J. B., Petterson R. F., Ambros V., Hewlett N. J., Baltimore D. Covalent linkage of a protein to a defined nucleotide sequence at the 5'-terminus of virion and replicative intermediate RNAs of poliovirus. Proc Natl Acad Sci U S A. 1977 Mar;74(3):961–965. doi: 10.1073/pnas.74.3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. A., Walseth T. F. The enzymatic preparation of [alpha-32P]ATP, [alpha-32P]GTP, [32P]cAMP, and [32P]cGMP, and their use in the assay of adenylate and guanylate cyclases and cyclic nucleotide phosphodiesterases. Adv Cyclic Nucleotide Res. 1979;10:135–167. [PubMed] [Google Scholar]
- Kerr I. M., Martin E. M. Simple method for the isolation of encephalomyocarditis virus ribonucleic acid. J Virol. 1972 Mar;9(3):559–561. doi: 10.1128/jvi.9.3.559-561.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koonin E. V., Agol V. I. Encephalomyocarditis virus replication complexes that prefer nucleoside diphosphates as substrates for viral RNA synthesis. Virology. 1983 Sep;129(2):309–318. doi: 10.1016/0042-6822(83)90170-8. [DOI] [PubMed] [Google Scholar]
- Matsumoto K., Saito T., Hirokawa H. In vitro initiation of bacteriophage phi 29 and M2 DNA replication: genes required for formation of a complex between the terminal protein and 5'dAMP. Mol Gen Genet. 1983;191(1):26–30. doi: 10.1007/BF00330885. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Morrow C. D., Dasgupta A. Antibody to a synthetic nonapeptide corresponding to the NH2 terminus of poliovirus genome-linked protein VPg reacts with native VPg and inhibits in vitro replication of poliovirus RNA. J Virol. 1983 Nov;48(2):429–439. doi: 10.1128/jvi.48.2.429-439.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomoto A., Detjen B., Pozzatti R., Wimmer E. The location of the polio genome protein in viral RNAs and its implication for RNA synthesis. Nature. 1977 Jul 21;268(5617):208–213. doi: 10.1038/268208a0. [DOI] [PubMed] [Google Scholar]
- Pettersson R. F., Ambros V., Baltimore D. Identification of a protein linked to nascent poliovirus RNA and to the polyuridylic acid of negative-strand RNA. J Virol. 1978 Aug;27(2):357–365. doi: 10.1128/jvi.27.2.357-365.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillman B. W. The replication of adenovirus DNA with purified proteins. Cell. 1983 Nov;35(1):7–9. doi: 10.1016/0092-8674(83)90201-5. [DOI] [PubMed] [Google Scholar]
- Takegami T., Kuhn R. J., Anderson C. W., Wimmer E. Membrane-dependent uridylylation of the genome-linked protein VPg of poliovirus. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7447–7451. doi: 10.1073/pnas.80.24.7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsugita A., Scheffler J. J. A rapid method for acid hydrolysis of protein with a mixture of trifluoroacetic acid and hydrochloric acid. Eur J Biochem. 1982 Jun;124(3):585–588. doi: 10.1111/j.1432-1033.1982.tb06634.x. [DOI] [PubMed] [Google Scholar]
- Vartapetian A. B., Drygin Y. F., Chumakov K. M., Bogdanov A. A. The structure of the covalent linkage between proteins and RNA in encephalomyocarditis virus. Nucleic Acids Res. 1980 Aug 25;8(16):3729–3742. doi: 10.1093/nar/8.16.3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartapetian A. B., Kunin E. V., Chumakov K. M., Bogdanov A. A., Agol V. I. Initsiatsiia sinteza RNK virusa éntsefalomiokardita v beskletochnoi sisteme i vozmozhnoe uchastie v étom protsesse belka VPg. Dokl Akad Nauk SSSR. 1982;267(4):963–965. [PubMed] [Google Scholar]
- Vartapetian A. B., Mankin A. S., Skripkin E. A., Chumakov K. M., Smirnov V. D., Bogdanov A. A. The primary and secondary structure of the 5'-end region of encephalomyocarditis virus RNA. A novel approach to sequencing long RNA molecules. Gene. 1983 Dec;26(2-3):189–195. doi: 10.1016/0378-1119(83)90189-0. [DOI] [PubMed] [Google Scholar]
- Wimmer E. Genome-linked proteins of viruses. Cell. 1982 Feb;28(2):199–201. doi: 10.1016/0092-8674(82)90335-x. [DOI] [PubMed] [Google Scholar]
- Yamakawa M., Furuichi Y., Nakashima K., LaFiandra A. J., Shatkin A. J. Excess synthesis of viral mRNA 5-terminal oligonucleotides by reovirus transcriptase. J Biol Chem. 1981 Jun 25;256(12):6507–6514. [PubMed] [Google Scholar]