Abstract
DNA double-strand breaks (DSBs) are DNA lesions that must be accurately repaired in order to preserve genomic integrity and cellular viability. The response to DSBs reshapes the local chromatin environment and is largely orchestrated by the deposition, removal and detection of a complex set of chromatin-associated post-translational modifications. In particular, the nucleosome acts as a central signalling hub and landing platform in this process by organizing the recruitment of repair and signalling factors, while at the same time coordinating repair with other DNA-based cellular processes. While current research has provided a descriptive overview of which histone marks affect DSB repair, we are only beginning to understand how these marks are interpreted to foster an efficient DSB response. Here we review how the modified chromatin surrounding DSBs is read, with a focus on the insights gleaned from structural and biochemical studies.
This article is part of the themed issue ‘Chromatin modifiers and remodellers in DNA repair and signalling’.
Keywords: DNA repair, chromatin, post-translational modification, nucleosome, DNA double-strand breaks
1. Introduction
DNA double helices form the basic heritable genetic material of the cell and are under constant attack from both exogenous and endogenous sources of DNA damage [1]. One of the most toxic DNA lesions occurs when the backbone of the DNA is cut on both strands simultaneously, creating a DNA double-strand break (DSB). DSBs can form as a consequence of DNA replication stress and stalling, or chemical insults or from the absorption of ionizing radiation [1]. If DSBs are left unrepaired genetic material can be lost: if improperly repaired, DSBs cause chromosomal aberrations or copy number variations that are associated with cancer. As a result, multiple elegant and highly controlled pathways have evolved to detect, signal and repair DSBs. Repair of DSBs can be roughly divided into two separate mechanisms, namely end-joining and homology-directed repair (HDR). The canonical end-joining pathway, non-homologous end joining (NHEJ), occurs via direct re-ligation of breaks, after limited resection and end processing [2,3]. Most breaks are repaired faithfully by NHEJ, which is the major pathway used for repair of DSB in human cells [4]. Where another identical copy of DNA is present, such as the sister chromatid, HDR is preferentially used. As a result, the choice of HDR over NHEJ is temporally regulated across the cell cycle: HDR is favoured after DNA replication in late S, G2 and M phases, while NHEJ is used in interphase cells [5]. The state of chromatin around DSB profoundly shapes the response to DNA damage and the chromatin functions as more than just a simple barrier to repair enzymes [6–9].
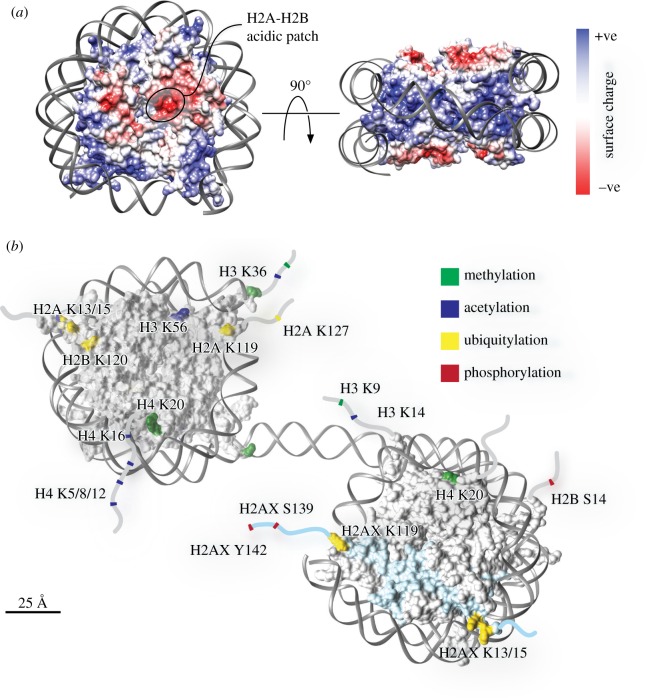
The size of eukaryotic genomes requires that they be packaged into stable, easily replicated and separable structures that are also sufficiently plastic to allow access to the genetic material. DNA and its associated histone proteins form chromatin, the fundamental unit of which is the nucleosome core particle (NCP). The NCP is an octamer of histone proteins composed of two copies each of histone H3-H4 and H2A-H2B, which wraps roughly 147 bp of DNA around its core [10]. The coin-like NCP wraps 1.65 turns of DNA around its equator, creating two symmetrical proteinaceous solvent-exposed faces (figure 1a).
Figure 1.
NCP modifications in the DNA damage response (DDR) pathway. (a) The structure of a NCP (PDB: 3LZ0) viewed along the DNA axis and the orthogonal direction, coloured according to coulombic surface charge. (b) Representation of two NCPs and linker DNA with critical solvent-exposed DDR modification sites coloured, created in UCSF Chimera. Model based on Xenopus laevis NCP crystal structures (PDB: 1AOI and 3LZ0) and labelled according to human amino acid residues. Disordered tails are indicated by lines and are not to scale. H2AX histone variant is coloured cyan. See text and references [11,12] for a description of the residues modified in the DDR.
The NCP is far more than just a packing material, it controls how the DNA in eukaryotic cells is replicated, read and used. NCPs provide a platform that becomes heavily decorated with post-translational modifications to control the myriad of DNA-related processes in the cell. The extended histone tails are the primary sites for modification [13,14]. Histone modifications can directly affect the chemistry of their modified residues and thereby the accessibility of chromatin [15,16]. However, histone modifications commonly mediate their function through chromatin-binding effector proteins, which can recognize both the modified residue and the surrounding protein sequence and structure of the NCP. To foster the appropriate response there is often cross talk between different modifications, either within the same histone tail or between different histones across a NCP [17–21]. Furthermore, chromatin-binding proteins often also form interactions with the unmodified histone surface to increase specificity and affinity [14,19,22,23]. Combinatorial histone mark recognition confers specificity, affinity and temporal control to finely tune responses to the chromatin post-translational modification status [17].
2. Post-translational modifications after DSBs: the DNA damage response pathway
In mammalian cells, DSBs elicit a cascade of signalling events that label DNA lesions in a process that is often collectively referred to as the DNA damage response (DDR) [7,24]. This pathway aids in the assembly of repair complexes, limits local transcription, and slows down or blocks cell cycle progression. Proteomic studies have revealed many targets of the DDR [25–28] but the most commonly modified set of proteins are histones (figure 1b).
After initial detection, the DSB signal is propagated on the chromatin around the break, up to megabases from the site of damage [29–31]. DNA damage-induced histone phosphorylation and ubiquitylation are both subject to positive feedback mechanisms, promoting their spreading along the chromatin, away from DSB sites. The resultant signals can be visualized as subnuclear foci by immunofluorescence microscopy.
PI3 K-like kinases DNA-PKcs, ATM and ATR phosphorylate Ser-139 of histone variant H2AX surrounding breaks, creating γ-H2AX [32–35]. This forms a binding platform for MDC1 [36] and leads to subsequent recruitment of E3 ligase RNF8 [37–39]. RNF8 potentiates the ubiquitylation cascade by extending constitutive mono-ubiquitylation on linker histone H1, forming Lys-63-linked chains [28]. RNF168 binds to the products of RNF8 ubiquitylation and amplifies the ubiquitin signal at DSBs [40–42]. RNF168 ubiquitylates multiple targets at DSB foci, but specifically ubiquitylates H2A at Lys-13 and Lys-15 (H2AK13/15ub) on NCPs [43,44].
The extensive ubiquitin signal at DSB sites leads to recruitment and retention of repair and signalling factors such as RAP80-BRCA1 and 53BP1 [41,42,45]. Despite both being recruited by RNF168-mediated ubiquitylation, BRCA1 and 53BP1 form an antagonistic pair at the hub of DSB repair choice [46]. 53BP1 and associated effector proteins promote NHEJ-based DSB repair and simultaneously inhibit the recruitment of BRCA1 to DSB sites in the G1 phase of the cell cycle [5]. In S/G2 phases, BRCA1 and DNA resection factors antagonize 53BP1, which in turns promotes HDR [47].
Outside of the canonical DDR signalling cascade, other histone post-translational modifications have been reported to be involved in the response to DSB [48] (figure 1b). Some marks are altered locally after DSBs, such as H4K20me1/2 [49–53], H3K36me2 [54,55], H3K9me3 [56–58], H2BK120ub [59,60], H2AK119ub [61–63], H2BS14ph [11], H3K14ac [64], H3K56ac [12,65] and H4K16ac [66–68]. Other already resident histone modifications are also used by DNA damage binding proteins, including H4K16ac [69], H4K20me2 [70,71] and H3K36me3 [72,73]. These pre-existing marks help to coordinate repair with other chromatin-related processes such as transcription and replication [74–76]. Alternatively, they may affect DNA pathway choice or increase the overall affinity of recruited proteins to DSB foci. The dynamic removal of DSB histone modifications is also crucial to repair. A number of phosphatases, de-ubiquitylating enzymes (DUBs) and histone deacetylases have been implicated in the repair of DSBs [65,77,78].
3. Recognition domains in the DNA damage response
Through biochemical and structural characterization of DNA damage-associated histone modifying and binding proteins, we are beginning to understand how the complex histone-signalling network is interpreted at DSBs. Many proteins in the DDR pathway contain dedicated post-translational modification recognition domains that are modular and transferable. In one example, the BRCT domains of MDC1 can independently localize to DSB sites [36] and they have been used to artificially recruit the E3 ligase activity of RNF168 in the absence of RNF8 [79]. However, context-specific chromatin reading often requires multiple protein domains, integrating multiple histone marks and nucleosomal elements in a multivalent fashion. Below we provide examples of the mechanisms by which proteins recognize modified chromatin to facilitate DSB repair (table 1), focusing on phosphorylation-, ubiquitin-, acetylation- and methylation-binding modules (figure 2).
Table 1.
Chromatin-binding protein domains in the DNA damage response (DDR) pathway. Summary of the reported chromatin interaction domains in the DDR. Abbreviations: PTM, post-translational modification; PHD, plant homeodomain; MBT, malignant brain tumour domain; PWWP, Pro-Trp-Trp-Pro domain; ANKD, ankyrin repeat domain; BRD, bromodomain; UDR, ubiquitination-dependent recruitment region; UIM, ubiquitin interaction motif; UDM, ubiquitin-dependent DSB recruitment module; MIU, motif interacting with ubiquitin; UBZ, ubiquitin-binding zinc-finger domain; RING, really interesting new gene; SIM, SUMO-interacting motif.
| domain | DDR protein | PTM | histone epitopes in the DDR | refs |
|---|---|---|---|---|
| BRCT | MDC1 | ph | H2AXS139ph | [36] |
| MCPH1 | ph | H2AXS139ph/Y142ph | [80] | |
| 53BP1 | ph | H2AXS139ph | [81,82] | |
| NBS1 | me | H3K36me2? | [55] | |
| PWWP | ZYMND8 | me | H3K36me0? | [20] |
| LEDGF/PSIP1 | me | H3K36me3 | [83] | |
| PHD | ZMYND8 | me | ? | [20] |
| PHRF1 | me | H3K36me2/3 | [84] | |
| tandem Tudor | 53BP1 | me | H4K20me1/2 | [85] |
| JMJD2A/KDM4A | me | H4K20me2/3, H3K4Me3 | [86] | |
| Chromo | TIP60/KAT5 | me | H3K9me3 | [57] |
| HP1 | me | H3K9me3 | [87] | |
| MBT | L3MBTL1 | me | H4K20me1/2 | [88] |
| MBTD1 | me | H4K20me1/2 | [89] | |
| BRD | BRD4 | ac | H3K9/14ac, H4K5/K8/K12/K16Ac | [90] |
| ZMYND8 | ac | H3K14ac, H4K5/K8/K12/K16Ac | [20,91] | |
| SMARCA2/BRM | ac | H3K18ac, H4K5/K8/K12/K16Ac? | [92] | |
| SMARCA4/BRG1 | ac | H3K9/14Ac, H4K8/K12/K16Ac? | [93] | |
| BAF180/PBMR1 | ac | H3K4/9/14/18/23Ac? | [94] | |
| UDR | 53BP1 | ub | NCP-H2AK15ub, acidic patch | [45] |
| UIM | RNF168 (UDM1) | ub | H1-ubK63, | [28] |
| RAP80 | ub | NCPub? | [95] | |
| MIU | RNF168 | ub | (UDM1)-H1-ubK63 | [28] |
| ub | (UDM2) NCP-H2AK13/15ub | [96–98] | ||
| RNF169 | ub | NCP-H2AK13/15ub | [96–98] | |
| CUE | SMARCAD1 | ub | H2A K127ub? | [99] |
| UBZ | RAD18 | ub | Ubiquitin chains, NCP-H2AK13/15ub | [96,97,100] |
| SIM | RAP80 | SUMO | Hybrid SUMO-ubK63 chains | [101] |
| RNF4 | SUMO | SUMO chains | [102] | |
| RING | RNF4 | — | DNA | [103] |
| RNF168 | — | ? | [104] | |
| RING1b-BMI1 | — | H2A-H2B-acidic patch | [105] | |
| BRCA1-BARD1 | — | H2A-H2B-acidic patch? | [105] | |
| ANKD | TONSL | — | H4K20me0 | [106] |
| BARD1 | — | ? | [106] |
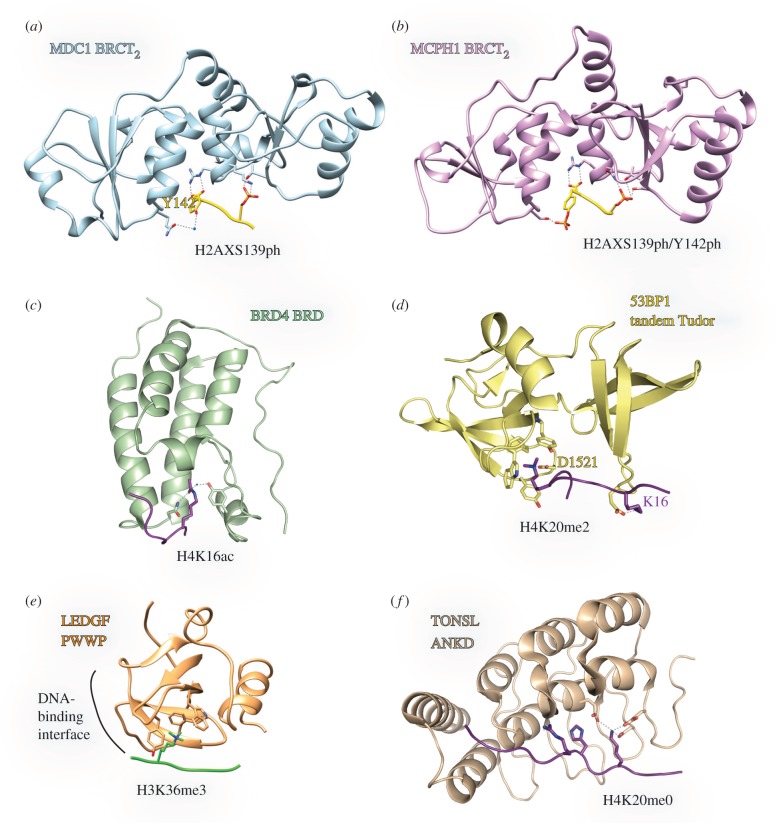
Figure 2.
Modified histone peptide reader domains in the DDR. Structures of chromatin reader domains referred to in the text, bound to their proposed DDR histone peptide targets. Structures were generated in Pymol or UCSF Chimera. Selected residues and hydrogen-bond/salt bridges are annotated. (a) MDC1 tandem BRCT bound to a Ser-139-phosphorylated H2AX peptide (PDB: 2AZM). (b) MCPH1 tandem BRCT domain with Ser-139-, Tyr-142-phosphorylated H2AX (PDB: 3U3Z). (c) BRD4 bromodomain bound to H4 acetylated Lys-12/16 (PDB: 3UVX). (d) Tandem Tudor domain of 53BP1 bound to a di-methylated H4K20 peptide (PDB: 2LVM). (e) PWWP domain of LEDGF bound to H3 tri-methylated Lys-36 (PDB: 3ZH1). (f) TONSL ankyrin repeat domain bound to an unmodified H4 peptide (PDB: 5JA4).
4. Recognition of γ-H2AX
The primary reader of γ-H2AX is the tandem BRCT domains of MDC1. BRCT domains are tandem repeats of approximately 100 amino acids that fold together in a head-to-tail configuration. The crystal structure of the BRCT domains of MDC1 in complex with the phosphorylated tail of H2AX was instrumental in explaining the recruitment and role of MDC1 in the DSB signalling cascade [36,107] (figure 2a). The tandem BRCT domain of MDC1 sandwiches the S139-phosphorylated H2AX peptide and specificity is conferred by recognition of a tyrosine residue (Tyr-142) after the ATM consensus SQ site. The elegant specificity of MDC1 to the short peptidic fragment is aided by enforced rigidity in the phospho-serine adjacent glutamine and the recognition of the free C-terminus of H2AX [108]. Super-resolution microscopy has revealed different γ-H2AX and MDC1 profiles at DSB foci in cells [109], suggesting MDC1 is not always associated with γ-H2AX. MDC1 can also engage in other non-γ-H2AX mediated interactions at DSBs, possibly stabilizing its chromatin interaction [110,111].
Although MDC1 is the primary reader of γ-H2AX, mouse cells lacking MDC1 still exhibit a partial recruitment of DDR factors [112]. Other proteins have been described to interact directly with γ-H2AX [80–82,113]. MCPH1, a protein involved in limiting cell cycle progression after DNA damage, can also bind γ-H2AX [80]. Interestingly, MCPH1 is recruited to DSBs prior to MDC1, but unlike MDC1, MCPH1 can accommodate the basal phosphorylation of H2AX Tyr-142 [114–116]. Phosphorylation at Tyr-142 is rapidly removed after DSB formation, allowing MDC1 binding. The structures of phosphorylated H2AX C-terminal peptides with BRCT2/3 of MCPH1 reveal a similar binding mode compared with the MDC1 BRCTs (figure 2b) [116,117]. However, phosphopeptide binding by MCPH1 is unusual; it is mediated through non-charged hydrogen bonding network rather than a charged basic residue. The two-phosphorylation sites on the H2AX peptide are bound by the two separate BRCT repeats and the overall electropositive surface of MCPH1 allows the accommodation of a second phosphate at Tyr-142. The tandem BRCT domains of 53BP1 have also been reported to bind to γ-H2AX (see below, section 6). In budding yeast the adaptor protein Rtt107 recognizes the phosphorylated tail of H2A via BRCT interaction [118,119]. However, despite a co-crystal structure of a γ-H2AX peptide and the BRCT repeats from PTIP [120]—the human homologue of Rtt107—this binding mode is not likely to be conserved between yeast and humans, as PTIP recruitment is reliant on binding to ATM-phosphorylated 53BP1 [121,122].
5. Recognition of histone acetylation in the DNA damage response
The role for histone acetylation in the DDR has been extensively reviewed recently [68,123,124]. Bromodomains (BRDs) are a diverse set of acetyl-binding modules composed of a left-handed bundle of four α-helices. Variable loops connecting the helices shape the acetyl-lysine binding pocket [21]. A recent study revealed that one-third of all BRD-containing proteins alter their subcellular localization after laser microirradiation-induced DNA damage [91].
The C-terminally truncated BRD4 isoform B limits the extent of DDR signalling by insulating chromatin from decompaction [90]. This requires the first BRD of BRD4, which binds to multiple acetyl residues on the H4 tail [21] (figure 2c). BRD4 may use its two BRDs to bind bivalently to NCPs via both acetylated H3 and H4 tails and nucleosomal DNA [125,126]. PBAF and BAF complexes are related SWI/SNF chromatin remodellers found to be recruited to sites of DSBs [91,127]. This recruitment is in part mediated via direct acetylated-histone tail recognition by BRDs in BAF180/PBMR1 [94], SMARCA2/BRM [92] and SMARCA4/BRG1 [93]. γ-H2AX promotes subsequent H3 N-terminal acetylation, which is read by a BRD within the ATPase subunit of the SWI/SNF complex, SMARCA4/BRG1 [93].
The multivalent reader ZMYND8 represses transcription in close proximity to DNA lesions and binds to acetylated histone peptides in vitro [20,91]. TIP60/NuA4-catalysed acetylation after DSBs promotes ZMYND8 recruitment in vivo leading to shutdown of local transcription and HDR, by promoting the recruitment of the NuRD complex deacetylase to chromatin flanking DNA lesions [91,128]. ZMYND8 contains a triple reader module, composed of a PHD-BRD-PWWP domain, which folds into one contiguous structure [20]. Intriguingly, the back face of this triple reader can also simultaneously bind DNA, and ZMYND8 relies on its three reader modules to be optimally recruited to DSB sites.
6. Recognition of multiple modified elements: the complex case of 53BP1
53BP1 was originally identified as a p53-interacting protein [129]. While 53BP1 is a bona fide modulator of p53 [130,131], it has a separate and better-characterized role in the repair of DSBs, as a key regulator of the NHEJ pathway [132,133]. 53BP1 is involved in both potentiating DSB signalling, and promoting NHEJ. In order to perform these functions 53BP1 must be recruited to sites of DSB and become phosphorylated by ATM/ATR, providing a landing platform for effector proteins [132,133].
53BP1 is recruited to chromatin by a combination of induced and pre-existing marks, as well as direct recognition of the NCP face. 53BP1 contains a tandem Tudor domain, which primarily engages H4 tails mono- and di-methylated at Lys-20 (H4K20me1/2) [85] (figure 2d). H4K20me1/2 is rapidly deposited after DNA replication [106] and 80% of all NCPs are methylated at H4 Lys-20 in asynchronously dividing cells. H4K20me1/2 is also induced locally at DSBs following DNA damage [49–53]. The crystal structure of the tandem Tudor domain of 53BP1 revealed that the first Tudor repeat of this domain forms an aromatic cage that specifies di-methyl lysine recognition [85]. The pocket is optimized to snugly accommodate a di-methylated lysine residue; smaller modifications would form weaker van der Waals and π-cation interactions within the aromatic cage. Conversely, tri-methylated lysines would break salt bridges and hydrogen bonding with 53BP1 residue Asp-1521. The H4 peptide spans a cleft between the tandem Tudor domain, with the four basic residues preceding H4 Lys-20 important for specificity [66] (figure 2d).
Our recent structure of 53BP1 bound to a larger substrate—a modified NCP—suggested that the Tudor domain of 53BP1 binds flexibly to its target [18] (figure 3). Poorly ordered density attributable to the tandem Tudor domain of 53BP1 was found over the NCP surface, tethered by the tail of H4. While recognizing the methylated H4 peptide tail, the tandem Tudor domain is free to move above the NCP surface, forming few other stable interactions with the rest of the NCP.
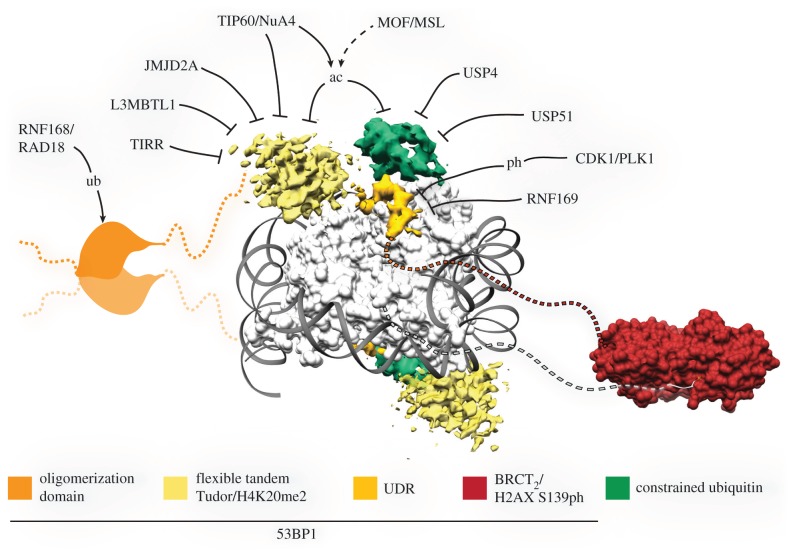
Figure 3.
53BP1 as a hub of regulation in the DNA damage response. Schematic representation of 53BP1 overlaid with known structures. Shown is the cryo-electron microscopy density for 53BP1 and ubiquitin (EMDB-8246) overlaid on the X. laevis NCP crystal structure (PDB: 3LZ0), and the crystal structure of the 53BP1 C-terminal tandem BRCT repeat domain bound to a γ-H2AX peptide (dashed grey line). Inhibitory modules referred to in the text are annotated.
The localization of 53BP1 to DSB sites cannot be solely explained by methyl-lysine binding. RNF168-mediated ubiquitylation is critical for the recruitment of 53BP1 [41,42]. While other models exist [86,88,134,135], the overwhelming evidence suggests that 53BP1 directly recognizes the RNF168-catalysed ubiquitylation of H2A at Lys-15 [43,45]. The minimal fragment required for 53BP1 recruitment to ionizing radiation-induced foci consists of a dimerized fragment of 53BP1 including the tandem Tudor domain and an extended 28-residue fragment termed the ubiquitination-dependent recruitment region (UDR) [45]. The UDR does not appear to be a prototypical ubiquitin-binding domain (UBD; described below); in isolation, the UDR has no measurable affinity for ubiquitin [45]. The cryo-electron microscopy (cryo-EM) structure of the tandem Tudor-UDR fragment bound to a site-specific ubiquitylated and methylated NCP revealed that 53BP1 contacts multiple elements on the modified nucleosome [18] (figure 3). The H2AK15ub nucleosomal specificity of 53BP1 is garnered by the UDR region. This short peptide motif is ordered in the cryo-EM structure and forms direct contacts with the NCP surface and bound ubiquitin. The ubiquitin tethered to Lys-15 is in a constrained state, folded over the nucleosomal face and forming direct contacts with the NCP surface itself. Indeed, mutation of NCP residues distal to the site of 53BP1-UDR-ubiquitin interaction inhibited 53BP1 binding in vitro, suggesting that the UDR promotes the constrained ubiquitin-bound conformation. RNF168 ubiquitylates H2A on both Lys-15 and Lys-13 [43], but 53BP1 preferentially binds to only H2AK15ub NCPs [45]. Surprisingly, the UDR does not contact the site of ubiquitin attachment to confer this preference; the constrained ubiquitin conformation is stabilized by nucleosomal and UDR contacts, allowed by ubiquitin tethered via a Lys-15 isopeptide bond. Increasing the distance from the NCP core by enforcing ubiquitylation at only Lys-13 prevents the correct positioning of the covalently attached ubiquitin that is required to form these interactions with 53BP1.
53BP1 also recognizes the NCP surface to help ensure specificity and affinity. The UDR snakes over the NCP face, interacting with a cleft formed between H2B and H4 as well as with a negatively charged region formed by residues in histone H2A and H2B, termed the acidic patch (figure 1a). The UDR contains multiple basic residues, which probably form electrostatic interactions with the negatively charged NCP acidic patch. Intriguingly, in the absence of any one of the three binding surfaces, the overall affinity of 53BP1 for the NCP is greatly reduced, suggesting that the 53BP1–NCP–ubiquitin–methylation interaction is truly multivalent.
The lack of any direct NCP–tandem Tudor interfaces outside of the H4 tail recognition may be important in 53BP1 Tudor domain function. The Tudor domain of 53BP1 can recognize a number of different methylated substrates [130,136,137], including methylated lysine marks on other histones in the NCP. The flexible association with only the peptidic methylated tail could allow 53BP1 to bind other modified histone tails, while still constrained by the rigid UDR.
In addition to the minimal dimerized tandem Tudor-UDR region required for 53BP1 binding, other reported chromatin-binding interfaces of 53BP1 may aid 53BP1 recruitment and subsequent DSB repair. 53BP1 has also been described to bind directly to DNA via a glycine-alanine-rich (GAR) region [138,139]. While charge-maintaining mutation of this motif does not seem to affect 53BP1 recruitment or function [140,141], the GAR motif may form stabilizing protein–DNA interactions on chromatin. Recent work has reported that the C-terminal BRCT repeats of 53BP1 bind to γ-H2AX [81,82] (figure 3). The BRCT domains bind in a similar manner to MDC1, albeit with lower affinity. While not absolutely essential for the recruitment or repair function of 53BP1 [140–142], mutations in the BRCT domain lead to persistent γ-H2AX signalling after damage, characteristic of impaired heterochromatin repair [82]. The exact nature of the oligomerization state of 53BP1 is unclear, although the oligomerization domain is absolutely required for recruitment to sites of DSBs [140]. While a dimeric 53BP1 is sufficient for recruitment in vitro and in vivo, higher order oligomerization may be required to efficiently promote 53BP1 repair functions [142].
7. Antagonism of 53BP1 recruitment to DSBs
53BP1 recruitment can be directly modulated by its own regulatory post-translational modifications [135,143–146] and other binding proteins [147,148] (figure 3). Other histone modifications can alter 53BP1 binding and concurrently alter DNA repair pathway choice. A number of reports have linked H4K16ac as a crucial modification for the HDR pathway [66,67]. Global H4K16ac is catalysed by the MOF/MSL complex and pre-existing H4K16ac is important for DSB repair [70,149]. Furthermore, at DSB sites, the N-terminal lysines of H4 and H2A are acetylated by the NuA4/TIP60 complex [67,68,89]. H4K16ac directly blocks the tandem Tudor domain of 53BP1 from engaging with H4K20me2 [66,69], possibly through breaking a salt bridge between H4 Lys-16 and one of a series of highly conserved acidic residues in the second Tudor domain [66]. The TIP60/NuA4 acetyltransferase also blocks 53BP1 directly. A recently identified MBT domain in the acetyltransferase complex can bind to H4K20me1/2 marks, competing with 53BP1 [89]. Adding insult to injury, the acetyltransferase can then acetylate the primary ubiquitin acceptor site H2A Lys-15, blocking the ubiquitylation by RNF168 and preventing ubiquitin-based recognition by 53BP1.
RNF20-mediated H2BK120ub inhibits 53BP1-mediated DNA damage signalling and is required for timely DSB repair [150,151]. H2BK120ub is in close proximity to the H2AK15ub site but does not directly block 53BP1 association with modified nucleosomes 53BP1 in vitro [18], suggesting that putative H2BK120ub-binding proteins may be involved in this antagonism. The extent of 53BP1 spreading and removal after DNA damage is also limited by the action of DUBs [77]. Multiple DUBs have been implicated in antagonizing RNF8/RNF168 signalling (reviewed in [77]). Interestingly, the same DUBs, USP44 and USP51, have been reported to antagonize both RNF168- and RNF20/40-mediated histone ubiquitylation [152–155].
Other proteins have also been reported to bind H4K20me2 marks in the absence of DNA damage (figure 3). A number of reports have shown that these proteins can outcompete 53BP1 and are removed from the vicinity of the break after the formation of a DSB. L3MBTL1 contains three MBT domains, which can simultaneously bridge multiple nucleosomes marked with H4K20me1/2, repressing transcription [156,157]. The MBT fold contains an aromatic cage and the second MBT repeat of L3MBTL1 specifically recognizes mono- and di-methyl marks [157]. At sites of DSBs, L3MBTL1 is rapidly ubiquitylated by RNF8/RNF168, leading to the recruitment of the AAA+ ATPase p97 and removal of L3MBTL1 from chromatin [88]. The DUB OTUB2 antagonizes L3MBTL1 ubiquitylation and therefore promotes the removal of L3MBTL1 from chromatin [158]. The lysine demethylases JMJD2A/KDM4A and JMJD2B/KDM4A can also bind to H4K20me2/3. The tandem Tudor domain of JMJD2A displays a very different fold and binding mode compared with the same domain in 53BP1 [159]; the two Tudor domains of JMJD2A share secondary structure to form a bilobed structure. JMJD2A is also ubiquitylated by RNF8/RNF168 and globally targeted for degradation to allow 53BP1 binding [86]. In addition to inhibiting 53BP1 focus formation, overexpression of JMJD2A catalyses the reduction in H3K36me3 levels leading to impaired HDR [72].
8. Ubiquitin recognition at chromatin surrounding DSBs
Ubiquitin recognition poses a unique problem to cells: ubiquitin-like proteins are much larger than other post-translational modifications, making it impractical for a single domain to bind to both the ubiquitin and the ubiquitylated protein. Ubiquitin-binding domains (UBDs) interact directly with only a ubiquitin moiety and possess a diverse number of different folds structured as either helical motifs or small globular domains [160]. However, site-specific readout of ubiquitylation state has been described for chromatin-binding proteins. UBDs tend to bind with low affinity in isolation and so coordinate their activity with other protein-binding motifs [96]. Panier and colleagues [96] identified small (less than 20 residue) fragments, termed LR motifs, which worked bivalently with UBDs to direct ligand binding for RAP80, RAD18, RNF168 and RNF169. The LR motifs are divergent in sequence so are likely to facilitate targeting of the readers to diverse ubiquitylated proteins at DSB sites, including ubiquitylated histones [96].
Outside of its N-terminal E3 ligase RING domain, RNF168 has two separable ubiquitylated-chromatin-binding modules, UDM1 and UDM2, which recognize different substrates on chromatin [28,96]. The UDM1 region comprises two helical UBDs and an LR motif [96] that interact with Lys-63 ubiquitylated histone H1 [28]. H1 is a linker histone that dynamically associates with chromatin to induce higher order chromatin compaction [161] and is polyubiquitylated by RNF8 near DSBs [28]. The UDM1 likely engages with H1 through electrostatic interactions, as this module is highly negatively charged. The extensive propagation of H2AK15ub on chromatin requires the second UDM of RNF168, which comprises a single helical MIU UBD flanked by another LR motif. Interestingly, UDM2 binds to the products of RNF168 ubiquitylation, leading to a positive feedback mechanism for RNF168 ubiquitin spreading [96–98].
Ubiquitin binding is used as a mechanism to inhibit 53BP1 binding. RNF169, a paralogue of RNF168, lacks a functional RING domain or UDM1 module, but has a conserved UDM2 module [162,163]. Overexpression of RNF169 reduces ionizing radiation-induced foci of ubiquitin, 53BP1 [96,162] and BRCA1 [163], but requires RNF168-mediated ubiquitylation for its own accumulation at DSBs. Recent structural studies have revealed that the UDM2 of RNF169 directly binds to NCPs ubiquitylated at Lys-13 and Lys-15, with appreciably higher affinity than either UDM2 of RNF168 or 53BP1 [97,98]. Interestingly, despite binding to both the ubiquitin and the NCP acidic patch RNF169 displays a different binding mode compared with 53BP1, with separable ubiquitin- and NCP-binding modules. Multiple residues in the LR motif of RNF169 directly contact the NCP surface, while the MIU stabilizes a DNA adjacent-conformation of ubiquitin [97,98]. While fragments of RNF169 can outcompete 53BP1 in vitro, the exact role of RNF169 in vivo is still unclear. RAD18 overexpression can also inhibit 53BP1 focal recruitment [164]. A fragment of RAD18 containing a UBZ UBD also engages specifically with H2AK13/15ub NCPs and may form contacts with both the NCP acidic patch and nucleosomal DNA [97].
Both RNF168-UDM2 and 53BP1 tandem Tudor-UDR minimally bind mono-ubiquitylated chromatin. While the UDM2 of RNF168 can tolerate binding to ubiquitin chains, it shows little chain type specificity [28]. Similarly, the structure of the UDR of 53BP1 could accommodate ubiquitin chains, but the UDR would be unlikely to interact directly with these additional ubiquitin moieties [18]. Nevertheless, RNF168 has been reported to build Lys-63- and Lys-27- based chains at DSB sites [41,42,165].
Histone ubiquitylation at DSBs is not limited to H2A Lys-13 and Lys-15. The repressive H2AK119ub mark is deposited by the RING1B-BMI1 E3 ligase components of the Polycomb complex PRC1 [166], which are recruited to sites of DNA damage [61–63,167,168] and aid in local transcriptional repression [61,74]. H2BK120ub and the enzymes responsible can also be visualized at sites of DNA damage [59,60,150]. H2BK120ub is normally associated with transcription elongation, but can also promote DNA repair, by encouraging local chromatin relaxation [60].
While understanding of how 53BP1 is recruited to chromatin is becoming clearer, how the canonical NHEJ antagonist BRCA1 is brought to DNA breaks in a timely fashion is still unclear. BRCA1 forms multiple complexes in the cell, each with different chromatin-binding properties [169]. BRCA1 is recruited to sites of DSB in an RNF168-dependent manner [41,42]. The BRCA1-A complex includes the ubiquitin-binding protein RAP80 [170–172]. The N-terminal 124 residues of RAP80 are sufficient for DSB focal recruitment [171,173] and contain two UIM UBDs, spaced to confer Lys-63 chain specificity [95,174]. In addition, RAP80 also contains a SIM motif [101,175,176] and an LR motif [96]. The identity of the protein harbouring the Lys-63 chains bound by RAP80 is still unclear. A recently identified zinc-finger protein, ZMYM3, can bind to both protein and DNA components of nucleosomes and helps to recruit BRCA1-A complex to sites of DSBs [177].
BRCA1 forms an obligate heterodimer with BARD1, creating an active E3 ligase [178,179]. This E3 targets H2A within chromatin, primarily at Lys-127 [180], possibly interacting with its NCP substrate via the acidic patch [105]. Recent work has shown that H2AK127ub helps to recruit the SMARCAD1 chromatin remodeller complex, which requires its two UBDs for this interaction [99,181]. SMARCAD1 promotes HDR and helps to relocalize 53BP1 to the periphery of the DSB-induced foci [99,182].
9. Recognition of underlying chromatin state
While some marks may not change following formation of a DSB, their accessibility may be altered due to topological rearrangement of chromatin or direct unmasking due to chromatin-binding protein removal (see above). For example, HP1 constitutively binds H3K9me3 at transcriptionally repressed loci [183]. HP1β rapidly mobilizes away from DNA lesions after DSBs [184], revealing H3K9me3-binding sites. This allows direct binding of the TIP60 chromodomain to H3K9me3 [57], activating TIP60 to locally acetylate both histone tails and ATM kinase [66,69,185,186]. Interestingly, HP1 is also recruited to DSB sites in a mechanism to spread H3K9me3 catalysed by SUV39H1 [56,57,87] and induce local chromatin condensation. This activity is not dependent upon the HP1 chromodomain.
While the H3K36me3 mark is not induced upon DNA damage, resident H3K36me3 is used to help promote HDR at sites of active transcription [72,73,187]. LEDGF/PSIP1 promotes HDR through its recruitment of CtIP to DSB sites [83,187] (figure 2e). LEDGF binds to chromatin using a PWWP domain [83], which binds bivalently to H3K36me3 and non-specifically to DNA on separate faces of the domain [188,189], thus conferring NCP specificity.
Unmodified elements of chromatin are used by DDR proteins to increase avidity in a multivalent manner. The H2A-H2B acidic patch is a commonly used feature for chromatin protein binding [23,190] (figure 1a). Available structures have revealed that DSB-implicated proteins 53BP1, TIP60/NuA4 and PRC1 also bind to NCPs via the acidic patch [18,19,105]. Other chromatin-binding modules such as the BRCA1-BARD1 RING domains and the UDM2 domains of RNF168 and RNF169 also bind to this same pocket on the NCP surface. Interestingly, mutations in the acidic patch do not measurably affect binding of the RING domain of RNF168 to the NCP [104], but an electronegative H2A-H2B acidic patch is required for optimal H2A ubiquitylation [191]. The SUMO-targeted E3 ligase, RNF4, is critical for regulating the removal and degradation of repair proteins [101,102,192,193]. RNF4 contains a patch of basic residues that may bind nucleosomal DNA, allowing H3 ubiquitylation in vitro [103]. It remains to be seen whether RNF4 interacts with NCPs and SUMO moieties at the same time and how the E3 ligase recruitment to NCPs is required to promote DSB repair.
Non-modified protein recognition has been co-opted as a means to recognize freshly deposited chromatin after DNA replication and help determine DNA repair pathway choice [106]. Newly synthesized histones are modified after replication in order to preserve epigenetic information. However, this process takes time and normally highly modified histone residues (such as H4 Lys-20) are a good marker of newly synthesized chromatin. 53BP1 is unable to bind non-modified H4K20 [85], excluding 53BP1 binding to newly deposited chromatin. The unmodified H4K20 tail can instead be recognized by an ankyrin repeat domain in the protein TONSL [106]. The crystal structure of the four ankyrin repeats revealed extensive contacts with residues of the H4 tail, principally with Arg-17, His-18 and Lys-20 (figure 2f). Lys-20 forms a hydrogen-bonding network, which would be disrupted by lysine modification. The MMS22-TONSL complex is involved in promoting HDR after DNA replication stress [194–196], and pre-recruitment by the TONSL ankyrin domain is critical for this function [106].
10. Conclusion and future perspectives
DSBs can form stochastically anywhere in the genome and at any stage of the cell cycle, but it is essential they are still repaired irrespective of the underlying chromatin state. The cell has evolved multiple mechanisms to deal with DSBs, which must be highly tailored to the specific incident. Chromatin plays an active role in this process and the deposition and reading of histone marks is essential for coordinating the DSB repair pathway with the local environment. We are only beginning to tease out the details of how chromatin signatures are read after DSB formation. The role for such extensive expansion of chromatin marks is unclear. Indeed, subnuclear damage foci are not homogeneous entities, different DDR proteins are not evenly distributed across foci [109,197–199] and there is different spatial and temporal distribution of DDR factors at DSBs. Each focus contains up to a few thousand copies of DNA repair and signalling proteins [200], but the requirement for such protein enrichment is still unclear.
With the advent of whole genome screening and proteomic approaches we have identified a wealth of proteins implicated in DSB repair and signalling. However, we are still lacking much mechanistic detail into how many of these proteins function in the signalling and repair of DSBs. Structural and biochemical studies using isolated fragments will be crucial in our understanding of chromatin reading at DNA DSBs. It is crucial to investigate and visualize these signalling interactions in the context of NCP structures and higher order chromatin assemblies. As outlined above, chromatin binding is multivalent and often involves multiple modified residues and the nucleosomal surface itself. We currently lack molecular understanding of how most chromatin-binding proteins recognize NCPs. Recent advances in structural biology techniques such as nuclear magnetic resonance (NMR) and cryo-EM will be instrumental in allowing the observation of previously intractable NCP-DDR protein complexes. Technical limitations have previously prevented the large-scale production of artificially modified chromatin. However, with the advent of improved chemical biology approaches [201,202] a better understanding of chromatin-based DSB repair is within reach through the reconstitution of increasingly larger protein assemblies that better model the chromatin environment at the break site.
Acknowledgements
The authors would like to thank Rachel Szilard for critical reading of the manuscript.
Data accessibility
This article has no additional data.
Author contributions
M.D.W. and D.D. conceived and wrote the manuscript.
Competing interests
M.D.W. declares no competing interests. D.D. is a paid advisor for CRISPR Therapeutics and Repare Therapeutics.
Funding
M.D.W. is supported by a Human Frontiers Long-Term Fellowship. Work in the Macromolecular Machines Laboratory is funded by the Francis Crick Institute (which receives its core funding from Cancer Research UK, the UK Medical Research Council and the Wellcome Trust) to Alessandro Costa. D.D. is a Canada Research Chair (Tier 1) in the Molecular Mechanisms of Genome Integrity. Funding for work in the D.D. laboratory relating to the regulation of DNA repair includes CIHR grant FDN143343 and a Grant-in-Aid from the Krembil Foundation.
References
- 1.Ciccia A, Elledge SJ. 2010. The DNA damage response: making it safe to play with knives. Mol. Cell 40, 179–204. ( 10.1016/j.molcel.2010.09.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biehs R, Steinlage M, Barton O, Juhász S, Künzel J, Spies J, Shibata A, Jeggo PA, Löbrich M. 2017. DNA double-strand break resection occurs during non-homologous end joining in G1 but is distinct from resection during homologous recombination. Mol. Cell 65, 671–684. ( 10.1016/j.molcel.2016.12.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riballo E, et al. 2004. A pathway of double-strand break rejoining dependent upon ATM, Artemis, and proteins locating to γ-H2AX foci. Mol. Cell 16, 715–724. ( 10.1016/j.molcel.2004.10.029) [DOI] [PubMed] [Google Scholar]
- 4.Karanam K, Kafri R, Loewer A, Lahav G. 2012. Quantitative live cell imaging reveals a gradual shift between DNA repair mechanisms and a maximal use of HR in mid S phase. Mol. Cell 47, 320–329. ( 10.1016/j.molcel.2012.05.052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hustedt N, Durocher D. 2016. The control of DNA repair by the cell cycle. Nat. Cell Biol. 19, 1–9. ( 10.1038/ncb3452) [DOI] [PubMed] [Google Scholar]
- 6.Polo SE, Almouzni G. 2015. Chromatin dynamics after DNA damage: the legacy of the access–repair–restore model. DNA Repair (Amst) 36, 114–121. ( 10.1016/j.dnarep.2015.09.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lukas J, Lukas C, Bartek J. 2011. More than just a focus: the chromatin response to DNA damage and its role in genome integrity maintenance. Nat. Cell Biol. 13, 1161–1169. ( 10.1038/ncb2344) [DOI] [PubMed] [Google Scholar]
- 8.Goodarzi AA, Noon AT, Deckbar D, Ziv Y, Shiloh Y, Lobrich M, Jeggo PA. 2008. ATM signaling facilitates repair of DNA double-strand breaks associated with heterochromatin. Mol. Cell 31, 167–177. ( 10.1016/j.molcel.2008.05.017) [DOI] [PubMed] [Google Scholar]
- 9.Noon AT, Shibata A, Rief N, Lobrich M, Stewart GS, Jeggo PA, Goodarzi AA. 2010. 53BP1-dependent robust localized KAP-1 phosphorylation is essential for heterochromatic DNA double-strand break repair. Nat. Cell Biol. 12, 177–184. ( 10.1038/ncb2017) [DOI] [PubMed] [Google Scholar]
- 10.Luger K, Mader AW, Richmond RK, Sargent DF, Richmond TJ. 1997. Crystal structure of the nucleosome core particle at 2.8 Å resolution. Nature 389, 251–260. ( 10.1038/38444) [DOI] [PubMed] [Google Scholar]
- 11.Fernandez-Capetillo O, Allis CD, Nussenzweig A. 2004. Phosphorylation of histone H2B at DNA double-strand breaks. J. Exp. Med. 199, 1671–1677. ( 10.1084/jem.20032247) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tjeertes JV, Miller KM, Jackson SP. 2009. Screen for DNA-damage-responsive histone modifications identifies H3K9Ac and H3K56Ac in human cells. EMBO J. 28, 1878–1889. ( 10.1038/emboj.2009.119) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bannister AJ, Kouzarides T. 2011. Regulation of chromatin by histone modifications. Cell Res. 21, 381–395. ( 10.1038/cr.2011.22) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ng MK, Cheung P. 2016. A brief histone in time: understanding the combinatorial functions of histone PTMs in the nucleosome context. Biochem. Cell Biol. 94, 33–42. ( 10.1139/bcb-2015-0031) [DOI] [PubMed] [Google Scholar]
- 15.Hansen JC, Tse C, Wolffe AP. 1998. Structure and function of the core histone N-termini: more than meets the eye. Biochemistry 37, 17 637–17 641. ( 10.1021/bi982409v) [DOI] [PubMed] [Google Scholar]
- 16.Shogren-Knaak M, Ishii H, Sun JM, Pazin MJ, Davie JR, Peterson CL. 2006. Histone H4-K16 acetylation controls chromatin structure and protein interactions. Science 311, 844–847. ( 10.1126/science.1124000) [DOI] [PubMed] [Google Scholar]
- 17.Ruthenburg AJ, Li H, Patel DJ, Allis CD. 2007. Multivalent engagement of chromatin modifications by linked binding modules. Nat. Rev. Mol. Cell Biol. 8, 983–994. ( 10.1038/nrm2298) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilson MD, et al. 2016. The structural basis of modified nucleosome recognition by 53BP1. Nature 536, 100–103. ( 10.1038/nature18951) [DOI] [PubMed] [Google Scholar]
- 19.Xu P, Li C, Chen Z, Jiang S, Fan S, Wang J, Dai J, Zhu P, Chen Z. 2016. The NuA4 core complex acetylates nucleosomal histone H4 through a double recognition mechanism. Mol. Cell 63, 965–975. ( 10.1016/j.molcel.2016.07.024) [DOI] [PubMed] [Google Scholar]
- 20.Savitsky P, et al. 2016. Multivalent histone and DNA engagement by a PHD/BRD/PWWP triple reader cassette recruits ZMYND8 to K14ac-rich chromatin. Cell Rep. 17, 2724–2737. ( 10.1016/j.celrep.2016.11.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Filippakopoulos P, et al. 2012. Histone recognition and large-scale structural analysis of the human bromodomain family. Cell 149, 214–231. ( 10.1016/j.cell.2012.02.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bartke T, Vermeulen M, Xhemalce B, Robson SC, Mann M, Kouzarides T. 2010. Nucleosome-interacting proteins regulated by DNA and histone methylation. Cell 143, 470–484. ( 10.1016/j.cell.2010.10.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agarwal P, Miller KM. 2016. The nucleosome: orchestrating DNA damage signaling and repair within chromatin. Biochem. Cell Biol. 94, 381–395. ( 10.1139/bcb-2016-0017) [DOI] [PubMed] [Google Scholar]
- 24.Dantuma NP, van Attikum H. 2016. Spatiotemporal regulation of posttranslational modifications in the DNA damage response. EMBO J. 35, 6–23. ( 10.15252/embj.201592595) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsuoka S, et al. 2007. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science 316, 1160–1166. ( 10.1126/science.1140321) [DOI] [PubMed] [Google Scholar]
- 26.Bennetzen MV, Larsen DH, Dinant C, Watanabe S, Bartek J, Lukas J, Andersen JS. 2013. Acetylation dynamics of human nuclear proteins during the ionizing radiation-induced DNA damage response. Cell Cycle 12, 1688–1695. ( 10.4161/cc.24758) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elia AE, et al. 2015. Quantitative proteomic atlas of ubiquitination and acetylation in the DNA damage response. Mol. Cell 59, 867–881. ( 10.1016/j.molcel.2015.05.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thorslund T, et al. 2015. Histone H1 couples initiation and amplification of ubiquitin signalling after DNA damage. Nature 527, 389–393. ( 10.1038/nature15401) [DOI] [PubMed] [Google Scholar]
- 29.Iacovoni JS, Caron P, Lassadi I, Nicolas E, Massip L, Trouche D, Legube G. 2010. High-resolution profiling of γH2AX around DNA double strand breaks in the mammalian genome. EMBO J. 29, 1446–1457. ( 10.1038/emboj.2010.38) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szilard RK, et al. 2010. Systematic identification of fragile sites via genome-wide location analysis of γ-H2AX. Nat. Struct. Mol. Biol. 17, 299–305. ( 10.1038/nsmb.1754) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seo J, et al. 2012. Genome-wide profiles of H2AX and γ-H2AX differentiate endogenous and exogenous DNA damage hotspots in human cells. Nucleic Acids Res. 40, 5965–5974. ( 10.1093/nar/gks287) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang H, Wang M, Wang H, Bocker W, Iliakis G. 2005. Complex H2AX phosphorylation patterns by multiple kinases including ATM and DNA-PK in human cells exposed to ionizing radiation and treated with kinase inhibitors. J. Cell. Physiol. 202, 492–502. ( 10.1002/jcp.20141) [DOI] [PubMed] [Google Scholar]
- 33.Ward IM, Chen J. 2001. Histone H2AX is phosphorylated in an ATR-dependent manner in response to replicational stress. J. Biol. Chem. 276, 47 759–47 762. ( 10.1074/jbc.C100569200) [DOI] [PubMed] [Google Scholar]
- 34.Reitsema T, Klokov D, Banath JP, Olive PL. 2005. DNA-PK is responsible for enhanced phosphorylation of histone H2AX under hypertonic conditions. DNA Repair (Amst) 4, 1172–1181. ( 10.1016/j.dnarep.2005.06.005) [DOI] [PubMed] [Google Scholar]
- 35.Celeste A, et al. 2002. Genomic instability in mice lacking histone H2AX. Science 296, 922–927. ( 10.1126/science.1069398) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stucki M, Clapperton JA, Mohammad D, Yaffe MB, Smerdon SJ, Jackson SP. 2005. MDC1 directly binds phosphorylated histone H2AX to regulate cellular responses to DNA double-strand breaks. Cell 123, 1213–1226. ( 10.1016/j.cell.2005.09.038) [DOI] [PubMed] [Google Scholar]
- 37.Kolas NK, et al. 2007. Orchestration of the DNA-damage response by the RNF8 ubiquitin ligase. Science 318, 1637–1640. ( 10.1126/science.1150034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huen MS, Grant R, Manke I, Minn K, Yu X, Yaffe MB, Chen J. 2007. RNF8 transduces the DNA-damage signal via histone ubiquitylation and checkpoint protein assembly. Cell 131, 901–914. ( 10.1016/j.cell.2007.09.041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mailand N, Bekker-Jensen S, Faustrup H, Melander F, Bartek J, Lukas C, Lukas J. 2007. RNF8 ubiquitylates histones at DNA double-strand breaks and promotes assembly of repair proteins. Cell 131, 887–900. ( 10.1016/j.cell.2007.09.040) [DOI] [PubMed] [Google Scholar]
- 40.Pinato S, Scandiuzzi C, Arnaudo N, Citterio E, Gaudino G, Penengo L. 2009. RNF168, a new RING finger, MIU-containing protein that modifies chromatin by ubiquitination of histones H2A and H2AX. BMC Mol. Biol. 10, 55 ( 10.1186/1471-2199-10-55) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stewart GS, et al. 2009. The RIDDLE syndrome protein mediates a ubiquitin-dependent signaling cascade at sites of DNA damage. Cell 136, 420–434. ( 10.1016/j.cell.2008.12.042) [DOI] [PubMed] [Google Scholar]
- 42.Doil C, et al. 2009. RNF168 binds and amplifies ubiquitin conjugates on damaged chromosomes to allow accumulation of repair proteins. Cell 136, 435–446. ( 10.1016/j.cell.2008.12.041) [DOI] [PubMed] [Google Scholar]
- 43.Mattiroli F, Vissers JH, van Dijk WJ, Ikpa P, Citterio E, Vermeulen W, Marteijn JA, Sixma TK. 2012. RNF168 ubiquitinates K13-15 on H2A/H2AX to drive DNA damage signaling. Cell 150, 1182–1195. ( 10.1016/j.cell.2012.08.005) [DOI] [PubMed] [Google Scholar]
- 44.Gatti M, Pinato S, Maspero E, Soffientini P, Polo S, Penengo L. 2012. A novel ubiquitin mark at the N-terminal tail of histone H2As targeted by RNF168 ubiquitin ligase. Cell Cycle 11, 2538–2544. ( 10.4161/cc.20919) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fradet-Turcotte A, et al. 2013. 53BP1 is a reader of the DNA-damage-induced H2A Lys 15 ubiquitin mark. Nature 499, 50–54. ( 10.1038/nature12318) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bunting SF, et al. 2010. 53BP1 inhibits homologous recombination in Brca1-deficient cells by blocking resection of DNA breaks. Cell 141, 243–254. ( 10.1016/j.cell.2010.03.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Escribano-Diaz C, et al. 2013. A cell cycle-dependent regulatory circuit composed of 53BP1-RIF1 and BRCA1-CtIP controls DNA repair pathway choice. Mol. Cell 49, 872–883. ( 10.1016/j.molcel.2013.01.001) [DOI] [PubMed] [Google Scholar]
- 48.Hunt CR, Ramnarain D, Horikoshi N, Iyengar P, Pandita RK, Shay JW, Pandita TK. 2013. Histone modifications and DNA double-strand break repair after exposure to ionizing radiations. Radiat. Res. 179, 383–392. ( 10.1667/RR3308.2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tuzon CT, Spektor T, Kong X, Congdon LM, Wu S, Schotta G, Rice JC. 2014. Concerted activities of distinct H4K20 methyltransferases at DNA double-strand breaks regulate 53BP1 nucleation and NHEJ-directed repair. Cell Rep. 8, 430–438. ( 10.1016/j.celrep.2014.06.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dulev S, Tkach J, Lin S, Batada NN. 2014. SET8 methyltransferase activity during the DNA double-strand break response is required for recruitment of 53BP1. EMBO Rep. 15, 1163–1174. ( 10.15252/embr.201439434) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pei H, et al. 2011. MMSET regulates histone H4K20 methylation and 53BP1 accumulation at DNA damage sites. Nature 470, 124–128. ( 10.1038/nature09658) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hajdu I, Ciccia A, Lewis SM, Elledge SJ. 2011. Wolf–Hirschhorn syndrome candidate 1 is involved in the cellular response to DNA damage. Proc. Natl Acad. Sci. USA 108, 13 130–13 134. ( 10.1073/pnas.1110081108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hartlerode AJ, Guan Y, Rajendran A, Ura K, Schotta G, Xie A, Shah JV, Scully R. 2012. Impact of histone H4 lysine 20 methylation on 53BP1 responses to chromosomal double strand breaks. PLoS ONE 7, e49211 ( 10.1371/journal.pone.0049211) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fnu S, et al. 2011. Methylation of histone H3 lysine 36 enhances DNA repair by nonhomologous end-joining. Proc. Natl Acad. Sci. USA 108, 540–545. ( 10.1073/pnas.1013571108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cao LL, et al. 2016. ATM-mediated KDM2A phosphorylation is required for the DNA damage repair. Oncogene 35, 301–313. ( 10.1038/onc.2015.81) [DOI] [PubMed] [Google Scholar]
- 56.Luijsterburg MS, et al. 2009. Heterochromatin protein 1 is recruited to various types of DNA damage. J. Cell Biol. 185, 577–586. ( 10.1083/jcb.200810035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sun Y, Jiang X, Xu Y, Ayrapetov MK, Moreau LA, Whetstine JR, Price BD. 2009. Histone H3 methylation links DNA damage detection to activation of the tumour suppressor Tip60. Nat. Cell Biol. 11, 1376–1382. ( 10.1038/ncb1982) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Young LC, McDonald DW, Hendzel MJ. 2013. Kdm4b histone demethylase is a DNA damage response protein and confers a survival advantage following γ-irradiation. J. Biol. Chem. 288, 21 376–21 388. ( 10.1074/jbc.M113.491514) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moyal L, et al. 2011. Requirement of ATM-dependent monoubiquitylation of histone H2B for timely repair of DNA double-strand breaks. Mol. Cell 41, 529–542. ( 10.1016/j.molcel.2011.02.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nakamura K, et al. 2011. Regulation of homologous recombination by RNF20-dependent H2B ubiquitination. Mol. Cell 41, 515–528. ( 10.1016/j.molcel.2011.02.002) [DOI] [PubMed] [Google Scholar]
- 61.Ginjala V, Nacerddine K, Kulkarni A, Oza J, Hill SJ, Yao M, Citterio E, van Lohuizen M, Ganesan S. 2011. BMI1 is recruited to DNA breaks and contributes to DNA damage-induced H2A ubiquitination and repair. Mol. Cell. Biol. 31, 1972–1982. ( 10.1128/MCB.00981-10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ismail IH, Andrin C, McDonald D, Hendzel MJ. 2010. BMI1-mediated histone ubiquitylation promotes DNA double-strand break repair. J. Cell Biol. 191, 45–60. ( 10.1083/jcb.201003034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ikura T, et al. 2007. DNA damage-dependent acetylation and ubiquitination of H2AX enhances chromatin dynamics. Mol. Cell. Biol. 27, 7028–7040. ( 10.1128/MCB.00579-07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tamburini BA, Tyler JK. 2005. Localized histone acetylation and deacetylation triggered by the homologous recombination pathway of double-strand DNA repair. Mol. Cell. Biol. 25, 4903–4913. ( 10.1128/MCB.25.12.4903-4913.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Miller KM, Tjeertes JV, Coates J, Legube G, Polo SE, Britton S, Jackson SP. 2010. Human HDAC1 and HDAC2 function in the DNA-damage response to promote DNA nonhomologous end-joining. Nat. Struct. Mol. Biol. 17, 1144–1151. ( 10.1038/nsmb.1899) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tang J, Cho NW, Cui G, Manion EM, Shanbhag NM, Botuyan MV, Mer G, Greenberg RA. 2013. Acetylation limits 53BP1 association with damaged chromatin to promote homologous recombination. Nat. Struct. Mol. Biol. 20, 317–325. ( 10.1038/nsmb.2499) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ikura T, Ogryzko VV, Grigoriev M, Groisman R, Wang J, Horikoshi M, Scully R, Qin J, Nakatani Y. 2000. Involvement of the TIP60 histone acetylase complex in DNA repair and apoptosis. Cell 102, 463–473. ( 10.1016/S0092-8674(00)00051-9) [DOI] [PubMed] [Google Scholar]
- 68.Dhar S, Gursoy-Yuzugullu O, Parasuram R, Price BD. 2017. The tale of a tail: histone H4 acetylation and the repair of DNA breaks. Phil. Trans. R. Soc. B 372, 20160284 ( 10.1098/rstb.2016.284) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hsiao KY, Mizzen CA. 2013. Histone H4 deacetylation facilitates 53BP1 DNA damage signaling and double-strand break repair. J. Mol. Cell Biol. 5, 157–165. ( 10.1093/jmcb/mjs066) [DOI] [PubMed] [Google Scholar]
- 70.Sharma GG, et al. 2010. MOF and histone H4 acetylation at lysine 16 are critical for DNA damage response and double-strand break repair. Mol. Cell. Biol. 30, 3582–3595. ( 10.1128/MCB.01476-09) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pesavento JJ, Yang H, Kelleher NL, Mizzen CA. 2008. Certain and progressive methylation of histone H4 at lysine 20 during the cell cycle. Mol. Cell. Biol. 28, 468–486. ( 10.1128/MCB.01517-07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pfister SX, et al. 2014. SETD2-dependent histone H3K36 trimethylation is required for homologous recombination repair and genome stability. Cell Rep. 7, 2006–2018. ( 10.1016/j.celrep.2014.05.026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Carvalho S, Vitor AC, Sridhara SC, Martins FB, Raposo AC, Desterro JM, Ferreira J, de Almeida SF. 2014. SETD2 is required for DNA double-strand break repair and activation of the p53-mediated checkpoint. eLife 3, e02482 ( 10.7554/eLife.02482) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shanbhag NM, Rafalska-Metcalf IU, Balane-Bolivar C, Janicki SM, Greenberg RA. 2010. ATM-dependent chromatin changes silence transcription in cis to DNA double-strand breaks. Cell 141, 970–981. ( 10.1016/j.cell.2010.04.038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brady CA, et al. 2011. Distinct p53 transcriptional programs dictate acute DNA-damage responses and tumor suppression. Cell 145, 571–583. ( 10.1016/j.cell.2011.03.035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Elkon R, Rashi-Elkeles S, Lerenthal Y, Linhart C, Tenne T, Amariglio N, Rechavi G, Shamir R, Shiloh Y.. 2005. Dissection of a DNA-damage-induced transcriptional network using a combination of microarrays, RNA interference and computational promoter analysis. Genome Biol. 6, R43 ( 10.1186/gb-2005-6-5-r43) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Citterio E. 2015. Fine-tuning the ubiquitin code at DNA double-strand breaks: deubiquitinating enzymes at work. Front. Genet. 6, 282 ( 10.3389/fgene.2015.00282) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zheng XF, Kalev P, Chowdhury D. 2015. Emerging role of protein phosphatases changes the landscape of phospho-signaling in DNA damage response. DNA Repair (Amst) 32, 58–65. ( 10.1016/j.dnarep.2015.04.014) [DOI] [PubMed] [Google Scholar]
- 79.Hodge CD, Ismail IH, Edwards RA, Hura GL, Xiao AT, Tainer JA, Hendzel MJ, Mark Glover JN. 2016. RNF8 E3 ubiquitin ligase stimulates Ubc13 E2 conjugating activity that is essential for DNA double strand break signaling and BRCA1 tumor suppressor recruitment. J. Biol. Chem. 291, 9396–9410. ( 10.1074/jbc.M116.715698) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wood JL, Singh N, Mer G, Chen J. 2007. MCPH1 functions in an H2AX-dependent but MDC1-independent pathway in response to DNA damage. J. Biol. Chem. 282, 35 416–35 423. ( 10.1074/jbc.M705245200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kleiner RE, Verma P, Molloy KR, Chait BT, Kapoor TM. 2015. Chemical proteomics reveals a γH2AX-53BP1 interaction in the DNA damage response. Nat. Chem. Biol. 11, 807–814. ( 10.1038/nchembio.1908) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Baldock RA, Day M, Wilkinson OJ, Cloney R, Jeggo PA, Oliver AW, Watts FZ, Pearl LH. 2015. ATM localization and heterochromatin repair depend on direct interaction of the 53BP1-BRCT2 domain with γH2AX. Cell Rep. 13, 2081–2089. ( 10.1016/j.celrep.2015.10.074) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Daugaard M, et al. 2012. LEDGF (p75) promotes DNA-end resection and homologous recombination. Nat. Struct. Mol. Biol. 19, 803–810. ( 10.1038/nsmb.2314) [DOI] [PubMed] [Google Scholar]
- 84.Chang CF, Chu PC, Wu PY, Yu MY, Lee JY, Tsai MD, Chang M-S. 2015. PHRF1 promotes genome integrity by modulating non-homologous end-joining. Cell Death Dis. 6, e1716 ( 10.1038/cddis.2015.81) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Botuyan MV, Lee J, Ward IM, Kim JE, Thompson JR, Chen J, Mer G. 2006. Structural basis for the methylation state-specific recognition of histone H4-K20 by 53BP1 and Crb2 in DNA repair. Cell 127, 1361–1373. ( 10.1016/j.cell.2006.10.043) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mallette FA, Mattiroli F, Cui G, Young LC, Hendzel MJ, Mer G, Sixma TK, Richard S. 2012. RNF8- and RNF168-dependent degradation of KDM4A/JMJD2A triggers 53BP1 recruitment to DNA damage sites. EMBO J. 31, 1865–1878. ( 10.1038/emboj.2012.47) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ayrapetov MK, Gursoy-Yuzugullu O, Xu C, Xu Y, Price BD. 2014. DNA double-strand breaks promote methylation of histone H3 on lysine 9 and transient formation of repressive chromatin. Proc. Natl Acad. Sci. USA 111, 9169–9174. ( 10.1073/pnas.1403565111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Acs K, Luijsterburg MS, Ackermann L, Salomons FA, Hoppe T, Dantuma NP. 2011. The AAA-ATPase VCP/p97 promotes 53BP1 recruitment by removing L3MBTL1 from DNA double-strand breaks. Nat. Struct. Mol. Biol. 18, 1345–1350. ( 10.1038/nsmb.2188) [DOI] [PubMed] [Google Scholar]
- 89.Jacquet K, et al. 2016. The TIP60 complex regulates bivalent chromatin recognition by 53BP1 through direct H4K20me binding and H2AK15 acetylation. Mol. Cell 62, 409–421. ( 10.1016/j.molcel.2016.03.031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Floyd SR, et al. 2013. The bromodomain protein Brd4 insulates chromatin from DNA damage signalling. Nature 498, 246–250. ( 10.1038/nature12147) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gong F, et al. 2015. Screen identifies bromodomain protein ZMYND8 in chromatin recognition of transcription-associated DNA damage that promotes homologous recombination. Genes Dev. 29, 197–211. ( 10.1101/gad.252189.114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ogiwara H, Ui A, Otsuka A, Satoh H, Yokomi I, Nakajima S, Yasui A, Yokota J, Kohno T. 2011. Histone acetylation by CBP and p300 at double-strand break sites facilitates SWI/SNF chromatin remodeling and the recruitment of non-homologous end joining factors. Oncogene 30, 2135–2146. ( 10.1038/onc.2010.592) [DOI] [PubMed] [Google Scholar]
- 93.Lee HS, Park JH, Kim SJ, Kwon SJ, Kwon J. 2010. A cooperative activation loop among SWI/SNF, γ-H2AX and H3 acetylation for DNA double-strand break repair. EMBO J. 29, 1434–1445. ( 10.1038/emboj.2010.27) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kakarougkas A, Ismail A, Chambers AL, Riballo E, Herbert AD, Kunzel J, Löbrich M, Jeggo PA, Downs JA. 2014. Requirement for PBAF in transcriptional repression and repair at DNA breaks in actively transcribed regions of chromatin. Mol. Cell 55, 723–732. ( 10.1016/j.molcel.2014.06.028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sato Y, Yoshikawa A, Mimura H, Yamashita M, Yamagata A, Fukai S. 2009. Structural basis for specific recognition of Lys 63-linked polyubiquitin chains by tandem UIMs of RAP80. EMBO J. 28, 2461–2468. ( 10.1038/emboj.2009.160) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Panier S, Ichijima Y, Fradet-Turcotte A, Leung CC, Kaustov L, Arrowsmith CH, Durocher D. 2012. Tandem protein interaction modules organize the ubiquitin-dependent response to DNA double-strand breaks. Mol. Cell 47, 383–395. ( 10.1016/j.molcel.2012.05.045) [DOI] [PubMed] [Google Scholar]
- 97.Hu Q, Botuyan MV, Cui G, Zhao D, Mer G. 2017. Mechanisms of ubiquitin-nucleosome recognition and regulation of 53BP1 chromatin recruitment by RNF168/169 and RAD18. Mol. Cell 66, 473–487.e9. ( 10.1016/j.molcel.2017.04.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kitevski-LeBlanc J, et al. 2017. The RNF168 paralog RNF169 defines a new class of ubiquitylated histone reader involved in the response to DNA damage. eLife 6, e23872. ( 10.7554/eLife.23872) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Densham RM, et al. 2016. Human BRCA1-BARD1 ubiquitin ligase activity counteracts chromatin barriers to DNA resection. Nat. Struct. Mol. Biol. 23, 647–655. ( 10.1038/nsmb.3236) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Huang J, Huen MS, Kim H, Leung CC, Glover JN, Yu X, Chen J. 2009. RAD18 transmits DNA damage signalling to elicit homologous recombination repair. Nat. Cell Biol. 11, 592–603. ( 10.1038/ncb1865) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Guzzo CM, Berndsen CE, Zhu J, Gupta V, Datta A, Greenberg RA, Wolberge C, Matunis MJ. 2012. RNF4-dependent hybrid SUMO-ubiquitin chains are signals for RAP80 and thereby mediate the recruitment of BRCA1 to sites of DNA damage. Sci. Signal. 5, ra88 ( 10.1126/scisignal.2003485) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Galanty Y, Belotserkovskaya R, Coates J, Jackson SP. 2012. RNF4, a SUMO-targeted ubiquitin E3 ligase, promotes DNA double-strand break repair. Genes Dev. 26, 1179–1195. ( 10.1101/gad.188284.112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Groocock LM, et al. 2014. RNF4 interacts with both SUMO and nucleosomes to promote the DNA damage response. EMBO Rep. 15, 601–608. ( 10.1002/embr.201338369) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mattiroli F, Uckelmann M, Sahtoe DD, van Dijk WJ, Sixma TK. 2014. The nucleosome acidic patch plays a critical role in RNF168-dependent ubiquitination of histone H2A. Nat. Commun. 5, 3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.McGinty RK, Henrici RC, Tan S. 2014. Crystal structure of the PRC1 ubiquitylation module bound to the nucleosome. Nature 514, 591–596. ( 10.1038/nature13890) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Saredi G, et al. 2016. H4K20me0 marks post-replicative chromatin and recruits the TONSL-MMS22 L DNA repair complex. Nature 534, 714–718. ( 10.1038/nature18312) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lee MS, Edwards RA, Thede GL, Glover JN. 2005. Structure of the BRCT repeat domain of MDC1 and its specificity for the free COOH-terminal end of the γ-H2AX histone tail. J. Biol. Chem. 280, 32 053–32 056. ( 10.1074/jbc.C500273200) [DOI] [PubMed] [Google Scholar]
- 108.Campbell SJ, Edwards RA, Glover JN. 2010. Comparison of the structures and peptide binding specificities of the BRCT domains of MDC1 and BRCA1. Structure 18, 167–176. ( 10.1016/j.str.2009.12.008) [DOI] [PubMed] [Google Scholar]
- 109.Nakamura AJ, Rao VA, Pommier Y, Bonner WM. 2010. The complexity of phosphorylated H2AX foci formation and DNA repair assembly at DNA double-strand breaks. Cell Cycle 9, 389–397. ( 10.4161/cc.9.2.10475) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Spycher C, Miller ES, Townsend K, Pavic L, Morrice NA, Janscak P, Stewart GS, Stucki M. 2008. Constitutive phosphorylation of MDC1 physically links the MRE11–RAD50–NBS1 complex to damaged chromatin. J. Cell Biol. 181, 227–240. ( 10.1083/jcb.200709008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chapman JR, Jackson SP. 2008. Phospho-dependent interactions between NBS1 and MDC1 mediate chromatin retention of the MRN complex at sites of DNA damage. EMBO Rep. 9, 795–801. ( 10.1038/embor.2008.103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lou Z, et al. 2006. MDC1 maintains genomic stability by participating in the amplification of ATM-dependent DNA damage signals. Mol. Cell 21, 187–200. ( 10.1016/j.molcel.2005.11.025) [DOI] [PubMed] [Google Scholar]
- 113.Kobayashi J, et al. 2002. NBS1 localizes to γ-H2AX foci through interaction with the FHA/BRCT domain. Curr. Biol. 12, 1846–1851. ( 10.1016/S0960-9822(02)01259-9) [DOI] [PubMed] [Google Scholar]
- 114.Cook PJ, Ju BG, Telese F, Wang X, Glass CK, Rosenfeld MG. 2009. Tyrosine dephosphorylation of H2AX modulates apoptosis and survival decisions. Nature 458, 591–596. ( 10.1038/nature07849) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Xiao A, et al. 2009. WSTF regulates the H2A.X DNA damage response via a novel tyrosine kinase activity. Nature 457, 57–62. ( 10.1038/nature07668) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Singh N, et al. 2012. Dual recognition of phosphoserine and phosphotyrosine in histone variant H2A.X by DNA damage response protein MCPH1. Proc. Natl Acad. Sci. USA 109, 14 381–14 386. ( 10.1073/pnas.1212366109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shao Z, et al. 2012. Specific recognition of phosphorylated tail of H2AX by the tandem BRCT domains of MCPH1 revealed by complex structure. J. Struct. Biol. 177, 459–468. ( 10.1016/j.jsb.2011.11.022) [DOI] [PubMed] [Google Scholar]
- 118.Li X, Liu K, Li F, Wang J, Huang H, Wu J, Shi Y. 2012. Structure of C-terminal tandem BRCT repeats of Rtt107 protein reveals critical role in interaction with phosphorylated histone H2A during DNA damage repair. J. Biol. Chem. 287, 9137–9146. ( 10.1074/jbc.M111.311860) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Leung GP, Brown JA, Glover JN, Kobor MS. 2016. Rtt107 BRCT domains act as a targeting module in the DNA damage response. DNA Repair (Amst) 37, 22–32. ( 10.1016/j.dnarep.2015.10.007) [DOI] [PubMed] [Google Scholar]
- 120.Yan W, Shao Z, Li F, Niu L, Shi Y, Teng M, Li X. 2011. Structural basis of γH2AX recognition by human PTIP BRCT5-BRCT6 domains in the DNA damage response pathway. FEBS Lett. 585, 3874–3879. ( 10.1016/j.febslet.2011.10.045) [DOI] [PubMed] [Google Scholar]
- 121.Callen E, et al. 2013. 53BP1 mediates productive and mutagenic DNA repair through distinct phosphoprotein interactions. Cell 153, 1266–1280. ( 10.1016/j.cell.2013.05.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Munoz IM, Jowsey PA, Toth R, Rouse J. 2007. Phospho-epitope binding by the BRCT domains of hPTIP controls multiple aspects of the cellular response to DNA damage. Nucleic Acids Res. 35, 5312–5322. ( 10.1093/nar/gkm493) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gong F, Chiu LY, Miller KM. 2016. Acetylation reader proteins: linking acetylation signaling to genome maintenance and cancer. PLoS Genet. 12, e1006272 ( 10.1371/journal.pgen.1006272) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chiu L-Y, Gong F, Miller KM. 2017. Bromodomain proteins: repairing DNA damage within chromatin. Phil. Trans. R. Soc. B 372, 20160286 ( 10.1098/rstb.2016.0286) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Miller TC, Simon B, Rybin V, Grotsch H, Curtet S, Khochbin S, Carlomagno T, Müller CW. 2016. A bromodomain-DNA interaction facilitates acetylation-dependent bivalent nucleosome recognition by the BET protein BRDT. Nat. Commun. 7, 13855 ( 10.1038/ncomms13855) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nguyen UT, Bittova L, Muller MM, Fierz B, David Y, Houck-Loomis B, Feng V, Dann GP, Muir TW. 2014. Accelerated chromatin biochemistry using DNA-barcoded nucleosome libraries. Nat. Methods 11, 834–840. ( 10.1038/nmeth.3022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Park JH, Park EJ, Lee HS, Kim SJ, Hur SK, Imbalzano AN, Kwon J. 2006. Mammalian SWI/SNF complexes facilitate DNA double-strand break repair by promoting γ-H2AX induction. EMBO J. 25, 3986–3997. ( 10.1038/sj.emboj.7601291) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Spruijt CG, et al. 2016. ZMYND8 co-localizes with NuRD on target genes and regulates poly(ADP-ribose)-dependent recruitment of GATAD2A/NuRD to sites of DNA damage. Cell Rep. 17, 783–798. ( 10.1016/j.celrep.2016.09.037) [DOI] [PubMed] [Google Scholar]
- 129.Iwabuchi K, Bartel PL, Li B, Marraccino R, Fields S. 1994. Two cellular proteins that bind to wild-type but not mutant p53. Proc. Natl Acad. Sci. USA 91, 6098–6102. ( 10.1073/pnas.91.13.6098) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kachirskaia I, Shi X, Yamaguchi H, Tanoue K, Wen H, Wang EW, Appella E, Gozani O. 2008. Role for 53BP1 Tudor domain recognition of p53 dimethylated at lysine 382 in DNA damage signaling. J. Biol. Chem. 283, 34 660–34 666. ( 10.1074/jbc.M806020200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cuella-Martin R, Oliveira C, Lockstone HE, Snellenberg S, Grolmusova N, Chapman JR. 2016. 53BP1 integrates DNA repair and p53-dependent cell fate decisions via distinct mechanisms. Mol. Cell 64, 51–64. ( 10.1016/j.molcel.2016.08.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zimmermann M, de Lange T. 2014. 53BP1: pro choice in DNA repair. Trends Cell Biol. 24, 108–117. ( 10.1016/j.tcb.2013.09.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Panier S, Boulton SJ. 2014. Double-strand break repair: 53BP1 comes into focus. Nat. Rev. Mol. Cell Biol. 15, 7–18. ( 10.1038/nrm3719) [DOI] [PubMed] [Google Scholar]
- 134.Kocylowski MK, Rey AJ, Stewart GS, Halazonetis TD. 2015. Ubiquitin-H2AX fusions render 53BP1 recruitment to DNA damage sites independent of RNF8 or RNF168. Cell Cycle 14, 1748–1758. ( 10.1080/15384101.2015.1010918) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bohgaki M, et al. 2013. RNF168 ubiquitylates 53BP1 and controls its response to DNA double-strand breaks. Proc. Natl Acad. Sci. USA 110, 20 982–20 987. ( 10.1073/pnas.1320302111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tong Q, et al. 2015. Structural plasticity of methyllysine recognition by the tandem Tudor domain of 53BP1. Structure 23, 312–321. ( 10.1016/j.str.2014.11.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Huyen Y, et al. 2004. Methylated lysine 79 of histone H3 targets 53BP1 to DNA double-strand breaks. Nature 432, 406–411. ( 10.1038/nature03114) [DOI] [PubMed] [Google Scholar]
- 138.Boisvert FM, Rhie A, Richard S, Doherty AJ. 2005. The GAR motif of 53BP1 is arginine methylated by PRMT1 and is necessary for 53BP1 DNA binding activity. Cell Cycle 4, 1834–1841. ( 10.4161/cc.4.12.2250) [DOI] [PubMed] [Google Scholar]
- 139.Iwabuchi K, et al. 2003. Potential role for 53BP1 in DNA end-joining repair through direct interaction with DNA. J. Biol. Chem. 278, 36 487–36 495. ( 10.1074/jbc.M304066200) [DOI] [PubMed] [Google Scholar]
- 140.Lottersberger F, Bothmer A, Robbiani DF, Nussenzweig MC, de Lange T. 2013. Role of 53BP1 oligomerization in regulating double-strand break repair. Proc. Natl Acad. Sci. USA 110, 2146–2151. ( 10.1073/pnas.1222617110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ward I, Kim JE, Minn K, Chini CC, Mer G, Chen J. 2006. The tandem BRCT domain of 53BP1 is not required for its repair function. J. Biol. Chem. 281, 38 472–38 477. ( 10.1074/jbc.M607577200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zgheib O, Pataky K, Brugger J, Halazonetis TD. 2009. An oligomerized 53BP1 Tudor domain suffices for recognition of DNA double-strand breaks. Mol. Cell. Biol. 29, 1050–1058. ( 10.1128/MCB.01011-08) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Orthwein A, et al. 2015. A mechanism for the suppression of homologous recombination in G1 cells. Nature 528, 422–426. ( 10.1038/nature16142) [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 144.Lee DH, et al. 2014. Dephosphorylation enables the recruitment of 53BP1 to double-strand DNA breaks. Mol. Cell 54, 512–525. ( 10.1016/j.molcel.2014.03.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Watanabe K, et al. 2009. RAD18 promotes DNA double-strand break repair during G1 phase through chromatin retention of 53BP1. Nucleic Acids Res. 37, 2176–2193. ( 10.1093/nar/gkp082) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Han X, et al. 2014. UbcH7 regulates 53BP1 stability and DSB repair. Proc. Natl Acad. Sci. USA 111, 17 456–17 461. ( 10.1073/pnas.1408538111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhang A, Peng B, Huang P, Chen J, Gong Z. 2017. The p53-binding protein 1-Tudor interacting repair regulator complex participates in the DNA damage response. J. Biol. Chem. 292, 6461–6467. ( 10.1074/jbc.M117.777474) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Drane P, et al. 2017. TIRR regulates 53BP1 by masking its histone methyl-lysine binding function. Nature 543, 211–216. ( 10.1038/nature21358) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Smith ER, Cayrou C, Huang R, Lane WS, Cote J, Lucchesi JC. 2005. A human protein complex homologous to the Drosophila MSL complex is responsible for the majority of histone H4 acetylation at lysine 16. Mol. Cell. Biol. 25, 9175–9188. ( 10.1128/MCB.25.21.9175-9188.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zeng M, et al. 2016. CRL4(Wdr70) regulates H2B monoubiquitination and facilitates Exo1-dependent resection. Nat. Commun. 7, 11364 ( 10.1038/ncomms11364) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ramachandran S, et al. 2016. The SAGA deubiquitination module promotes DNA repair and class switch recombination through ATM and DNAPK-mediated γH2AX formation. Cell Rep. 15, 1554–1565. ( 10.1016/j.celrep.2016.04.041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang Z, Zhang H, Liu J, Cheruiyot A, Lee JH, Ordog T, Lou Z, You Z, Zhang Z. 2016. USP51 deubiquitylates H2AK13,15ub and regulates DNA damage response. Genes Dev. 30, 946–959. ( 10.1101/gad.271841.115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Atanassov BS, et al. 2016. ATXN7L3 and ENY2 coordinate activity of multiple H2B deubiquitinases important for cellular proliferation and tumor growth. Mol. Cell 62, 558–571. ( 10.1016/j.molcel.2016.03.030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Fuchs G, et al. 2012. RNF20 and USP44 regulate stem cell differentiation by modulating H2B monoubiquitylation. Mol. Cell 46, 662–673. ( 10.1016/j.molcel.2012.05.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Mosbech A, Lukas C, Bekker-Jensen S, Mailand N. 2013. The deubiquitylating enzyme USP44 counteracts the DNA double-strand break response mediated by the RNF8 and RNF168 ubiquitin ligases. J. Biol. Chem. 288, 16 579–16 587. ( 10.1074/jbc.M113.459917) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Trojer P, et al. 2007. L3MBTL1, a histone-methylation-dependent chromatin lock. Cell 129, 915–928. ( 10.1016/j.cell.2007.03.048) [DOI] [PubMed] [Google Scholar]
- 157.Li H, Fischle W, Wang W, Duncan EM, Liang L, Murakami-Ishibe S, Allis CD, Patel DJ. 2007. Structural basis for lower lysine methylation state-specific readout by MBT repeats of L3MBTL1 and an engineered PHD finger. Mol. Cell 28, 677–691. ( 10.1016/j.molcel.2007.10.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kato K, Nakajima K, Ui A, Muto-Terao Y, Ogiwara H, Nakada S. 2014. Fine-tuning of DNA damage-dependent ubiquitination by OTUB2 supports the DNA repair pathway choice. Mol. Cell 53, 617–630. ( 10.1016/j.molcel.2014.01.030) [DOI] [PubMed] [Google Scholar]
- 159.Huang Y, Fang J, Bedford MT, Zhang Y, Xu RM. 2006. Recognition of histone H3 lysine-4 methylation by the double Tudor domain of JMJD2A. Science 312, 748–751. ( 10.1126/science.1125162) [DOI] [PubMed] [Google Scholar]
- 160.Husnjak K, Dikic I. 2012. Ubiquitin-binding proteins: decoders of ubiquitin-mediated cellular functions. Annu. Rev. Biochem. 81, 291–322. ( 10.1146/annurev-biochem-051810-094654) [DOI] [PubMed] [Google Scholar]
- 161.Song F, Chen P, Sun D, Wang M, Dong L, Liang D, Xu R-M, Zhu P, Li G. 2014. Cryo-EM study of the chromatin fiber reveals a double helix twisted by tetranucleosomal units. Science 344, 376–380. ( 10.1126/science.1251413) [DOI] [PubMed] [Google Scholar]
- 162.Poulsen M, Lukas C, Lukas J, Bekker-Jensen S, Mailand N. 2012. Human RNF169 is a negative regulator of the ubiquitin-dependent response to DNA double-strand breaks. J. Cell Biol. 197, 189–199. ( 10.1083/jcb.201109100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Chen J, Feng W, Jiang J, Deng Y, Huen MS. 2012. Ring finger protein RNF169 antagonizes the ubiquitin-dependent signaling cascade at sites of DNA damage. J. Biol. Chem. 287, 27 715–27 722. ( 10.1074/jbc.M112.373530) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Helchowski CM, Skow LF, Roberts KH, Chute CL, Canman CE. 2013. A small ubiquitin binding domain inhibits ubiquitin-dependent protein recruitment to DNA repair foci. Cell Cycle 12, 3749–3758. ( 10.4161/cc.26640) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gatti M, Pinato S, Maiolica A, Rocchio F, Prato MG, Aebersold R, Penengo L. 2015. RNF168 promotes noncanonical K27 ubiquitination to signal DNA damage. Cell Rep. 10, 226–238. ( 10.1016/j.celrep.2014.12.021) [DOI] [PubMed] [Google Scholar]
- 166.Wang H, Wang L, Erdjument-Bromage H, Vidal M, Tempst P, Jones RS, Zhang Y. 2004. Role of histone H2A ubiquitination in Polycomb silencing. Nature 431, 873–878. ( 10.1038/nature02985) [DOI] [PubMed] [Google Scholar]
- 167.Chagraoui J, Hebert J, Girard S, Sauvageau G. 2011. An anticlastogenic function for the Polycomb Group gene Bmi1. Proc. Natl Acad. Sci. USA 108, 5284–5289. ( 10.1073/pnas.1014263108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Facchino S, Abdouh M, Chatoo W, Bernier G. 2010. BMI1 confers radioresistance to normal and cancerous neural stem cells through recruitment of the DNA damage response machinery. J. Neurosci. 30, 10 096–10 111. ( 10.1523/JNEUROSCI.1634-10.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Her J, Soo Lee N, Kim Y, Kim H. 2016. Factors forming the BRCA1-A complex orchestrate BRCA1 recruitment to the sites of DNA damage. Acta Biochim. Biophys. Sin. (Shanghai) 48, 658–664. ( 10.1093/abbs/gmw047) [DOI] [PubMed] [Google Scholar]
- 170.Sobhian B, Shao G, Lilli DR, Culhane AC, Moreau LA, Xia B, Livingston DM, Greenberg RA. 2007. RAP80 targets BRCA1 to specific ubiquitin structures at DNA damage sites. Science 316, 1198–1202. ( 10.1126/science.1139516) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wang B, Matsuoka S, Ballif BA, Zhang D, Smogorzewska A, Gygi SP, Elledge SJ. 2007. Abraxas and RAP80 form a BRCA1 protein complex required for the DNA damage response. Science 316, 1194–1198. ( 10.1126/science.1139476) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kim H, Chen J, Yu X. 2007. Ubiquitin-binding protein RAP80 mediates BRCA1-dependent DNA damage response. Science 316, 1202–1205. ( 10.1126/science.1139621) [DOI] [PubMed] [Google Scholar]
- 173.van Wijk SJ, et al. 2012. Fluorescence-based sensors to monitor localization and functions of linear and K63-linked ubiquitin chains in cells. Mol. Cell 47, 797–809. ( 10.1016/j.molcel.2012.06.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sims JJ, Cohen RE. 2009. Linkage-specific avidity defines the lysine 63-linked polyubiquitin-binding preference of Rap80. Mol. Cell 33, 775–783. ( 10.1016/j.molcel.2009.02.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hu X, Paul A, Wang B. 2012. Rap80 protein recruitment to DNA double-strand breaks requires binding to both small ubiquitin-like modifier (SUMO) and ubiquitin conjugates. J. Biol. Chem. 287, 25 510–25 519. ( 10.1074/jbc.M112.374116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Anamika Spyracopoulos L. 2016. Molecular basis for phosphorylation-dependent SUMO recognition by the DNA repair protein RAP80. J. Biol. Chem. 291, 4417–4428. ( 10.1074/jbc.M115.705061) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Leung JW, et al. 2017. ZMYM3 regulates BRCA1 localization at damaged chromatin to promote DNA repair. Genes Dev. 31, 260–274. ( 10.1101/gad.292516.116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ruffner H, Joazeiro CA, Hemmati D, Hunter T, Verma IM. 2001. Cancer-predisposing mutations within the RING domain of BRCA1: loss of ubiquitin protein ligase activity and protection from radiation hypersensitivity. Proc. Natl Acad. Sci. USA 98, 5134–5139. ( 10.1073/pnas.081068398) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Polanowska J, Martin JS, Garcia-Muse T, Petalcorin MI, Boulton SJ. 2006. A conserved pathway to activate BRCA1-dependent ubiquitylation at DNA damage sites. EMBO J. 25, 2178–2188. ( 10.1038/sj.emboj.7601102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kalb R, Mallery DL, Larkin C, Huang JT, Hiom K. 2014. BRCA1 is a histone-H2A-specific ubiquitin ligase. Cell Rep. 8, 999–1005. ( 10.1016/j.celrep.2014.07.025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Rother MB, van Attikum H. 2017. DNA repair goes hip-hop: SMARCA and CHD chromatin remodellers join the break dance. Phil. Trans. R. Soc. B 372, 20160285 ( 10.1098/rstb.2016.0285) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Costelloe T, et al. 2012. The yeast Fun30 and human SMARCAD1 chromatin remodellers promote DNA end resection. Nature 489, 581–584. ( 10.1038/nature11353) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Nielsen PR, Nietlispach D, Mott HR, Callaghan J, Bannister A, Kouzarides T, Murzin AG, Murzina NV, Laue ED. 2002. Structure of the HP1 chromodomain bound to histone H3 methylated at lysine 9. Nature 416, 103–107. ( 10.1038/nature722) [DOI] [PubMed] [Google Scholar]
- 184.Ayoub N, Jeyasekharan AD, Bernal JA, Venkitaraman AR. 2008. HP1-β mobilization promotes chromatin changes that initiate the DNA damage response. Nature 453, 682–686. ( 10.1038/nature06875) [DOI] [PubMed] [Google Scholar]
- 185.Sun Y, Jiang X, Chen S, Fernandes N, Price BD. 2005. A role for the Tip60 histone acetyltransferase in the acetylation and activation of ATM. Proc. Natl Acad. Sci. USA 102, 13 182–13 187. ( 10.1073/pnas.0504211102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kaidi A, Jackson SP. 2013. KAT5 tyrosine phosphorylation couples chromatin sensing to ATM signalling. Nature 498, 70–74. ( 10.1038/nature12201) [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 187.Aymard F, et al. 2014. Transcriptionally active chromatin recruits homologous recombination at DNA double-strand breaks. Nat. Struct. Mol. Biol. 21, 366–374. ( 10.1038/nsmb.2796) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Eidahl JO, et al. 2013. Structural basis for high-affinity binding of LEDGF PWWP to mononucleosomes. Nucleic Acids Res. 41, 3924–3936. ( 10.1093/nar/gkt074) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.van Nuland R, van Schaik FM, Simonis M, van Heesch S, Cuppen E, Boelens R, Timmers HT, van Ingen H. 2013. Nucleosomal DNA binding drives the recognition of H3K36-methylated nucleosomes by the PSIP1-PWWP domain. Epigenetics Chromatin 6, 12 ( 10.1186/1756-8935-6-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.McGinty RK, Tan S. 2016. Recognition of the nucleosome by chromatin factors and enzymes. Curr. Opin. Struct. Biol. 37, 54–61. ( 10.1016/j.sbi.2015.11.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Leung JW, Agarwal P, Canny MD, Gong F, Robison AD, Finkelstein IJ, Durocher D, Miller KM. 2014. Nucleosome acidic patch promotes RNF168- and RING1B/BMI1-dependent H2AX and H2A ubiquitination and DNA damage signaling. PLoS Genet. 10, e1004178 ( 10.1371/journal.pgen.1004178) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Yin Y, Seifert A, Chua JS, Maure JF, Golebiowski F, Hay RT. 2012. SUMO-targeted ubiquitin E3 ligase RNF4 is required for the response of human cells to DNA damage. Genes Dev. 26, 1196–1208. ( 10.1101/gad.189274.112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Garvin AJ, Morris JR. 2017. SUMO, a small, but powerful, regulator of double-strand break repair. Phil. Trans. R. Soc. B 372, 20160281 ( 10.1098/rstb.2016.0281) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Piwko W, Olma MH, Held M, Bianco JN, Pedrioli PG, Hofmann K, Pasero P, Gerlich DW, Peter M. 2010. RNAi-based screening identifies the Mms22 L-Nfkbil2 complex as a novel regulator of DNA replication in human cells. EMBO J. 29, 4210–4222. ( 10.1038/emboj.2010.304) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Duro E, et al. 2010. Identification of the MMS22 L-TONSL complex that promotes homologous recombination. Mol. Cell 40, 632–644. ( 10.1016/j.molcel.2010.10.023) [DOI] [PubMed] [Google Scholar]
- 196.O'Donnell L, et al. 2010. The MMS22 L-TONSL complex mediates recovery from replication stress and homologous recombination. Mol. Cell 40, 619–631. ( 10.1016/j.molcel.2010.10.024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ochs F, Somyajit K, Altmeyer M, Rask MB, Lukas J, Lukas C. 2016. 53BP1 fosters fidelity of homology-directed DNA repair. Nat. Struct. Mol. Biol. 23, 714–721. ( 10.1038/nsmb.3251) [DOI] [PubMed] [Google Scholar]
- 198.Reindl J, Drexler GA, Girst S, Greubel C, Siebenwirth C, Drexler SE, Dollinger G, Friedl AA. 2015. Nanoscopic exclusion between Rad51 and 53BP1 after ion irradiation in human HeLa cells. Phys. Biol. 12, 066005 ( 10.1088/1478-3975/12/6/066005) [DOI] [PubMed] [Google Scholar]
- 199.Chapman JR, Sossick AJ, Boulton SJ, Jackson SP. 2012. BRCA1-associated exclusion of 53BP1 from DNA damage sites underlies temporal control of DNA repair. J. Cell Sci. 125, 3529–3534. ( 10.1242/jcs.105353) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Mok MT, Henderson BR. 2012. The in vivo dynamic interplay of MDC1 and 53BP1 at DNA damage-induced nuclear foci. Int. J. Biochem. Cell Biol. 44, 1398–1409. ( 10.1016/j.biocel.2012.05.025) [DOI] [PubMed] [Google Scholar]
- 201.Faggiano S, Pastore A. 2014. The challenge of producing ubiquitinated proteins for structural studies. Cells 3, 639–656. ( 10.3390/cells3020639) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Holt M, Muir T. 2015. Application of the protein semisynthesis strategy to the generation of modified chromatin. Annu. Rev. Biochem. 84, 265–290. ( 10.1146/annurev-biochem-060614-034429) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.