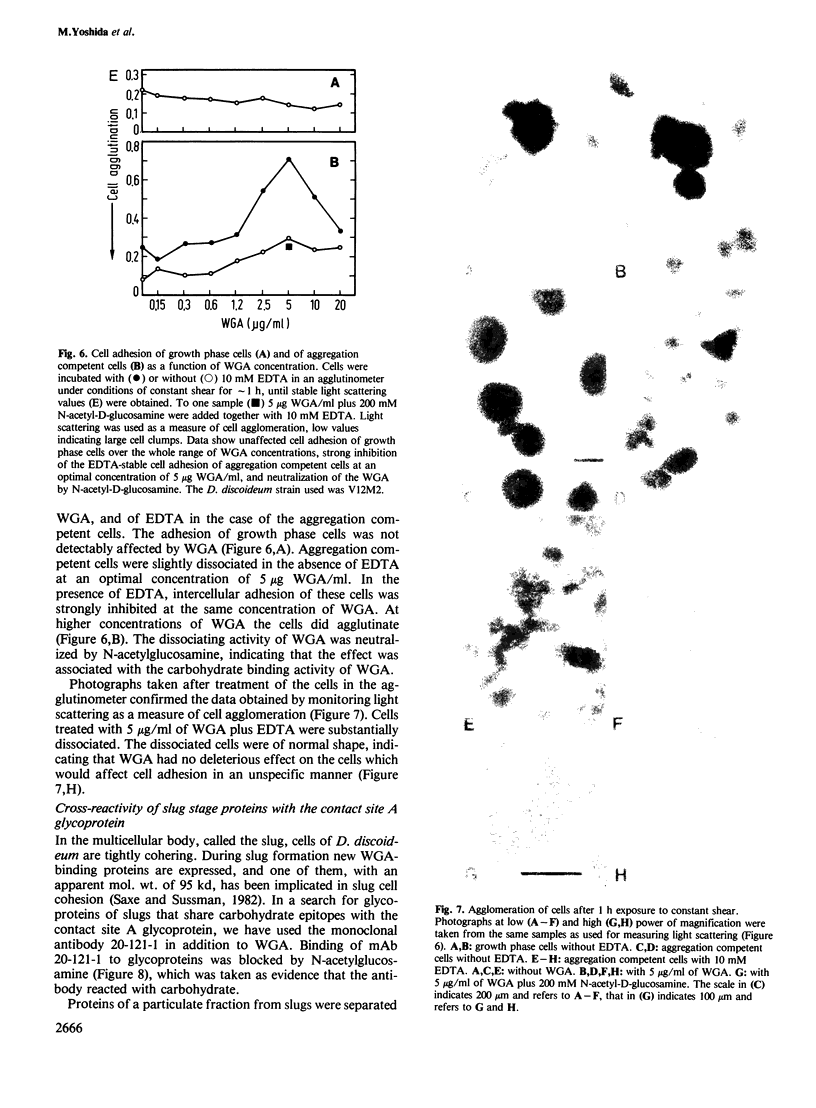
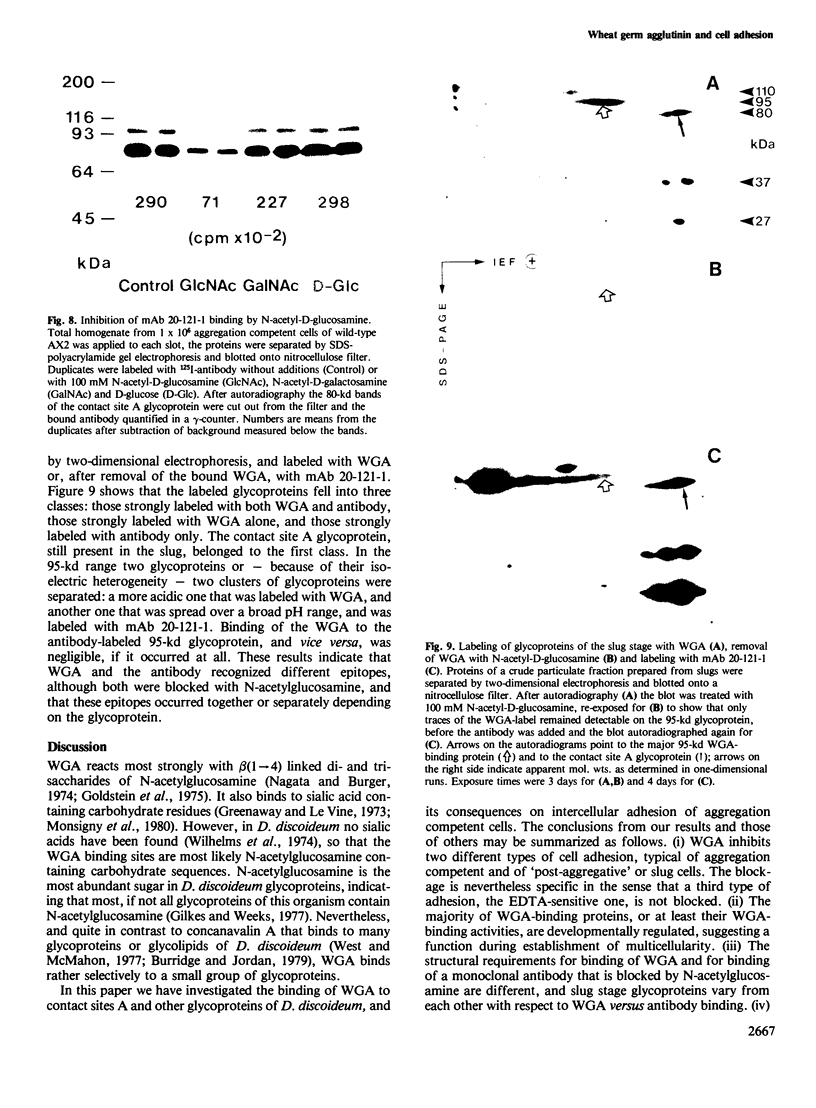
Abstract
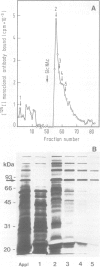
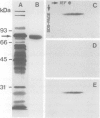
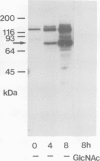
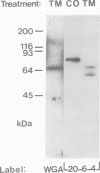
Wheat germ agglutinin (WGA), a lectin that primarily reacts with N-acetylglucosamine residues, specifically inhibits the EDTA-stable type of intercellular adhesion of aggregation competent Dictyostelium discoideum cells. The major WGA-binding protein of these cells is a developmentally-regulated glycolipoprotein of 80 kd apparent mol. wt., designated as contact site A. This glycoprotein is a target site of antibody fragments that block the EDTA-stable cell adhesion, and is characterized by sulfated carbohydrate residues. WGA does not significantly bind to glycoproteins of a mutant, HL220, which produces a 68-kd component in place of the 80-kd glycoprotein. Inhibition of N-glycosylation by tunicamycin causes wild-type cells to produce a WGA-binding but unsulfated 66-kd component and a non-binding 53-kd component. These results indicate that the 80-kd glycoprotein contains two classes of carbohydrate residues, a WGA-binding one that is defective in HL220, and another, sulfated, one that is absent from the 66-kd wild-type product; both are missing in the 53-kd protein. WGA and a monoclonal antibody that is blocked by N-acetylglucosamine were further used to probe for glycoproteins in the multicellular slug stage that share carbohydrate structures – and possibly functions – with the contact site A glycoprotein. Glycoproteins in the 95-kd range have previously been implicated in cell-to-cell adhesion during the slug stage. We distinguished a 95-kd glycoprotein that binds WGA from another one that binds antibody.
Keywords: lectins, cell differentiation, monoclonal antibody, cell surface carbohydrates
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beug H., Gerisch G. A micromethod for routine measurement of cell agglutination and dissociation. J Immunol Methods. 1972 Nov;2(1):49–57. doi: 10.1016/0022-1759(72)90017-8. [DOI] [PubMed] [Google Scholar]
- Beug H., Katz F. E., Gerisch G. Dynamics of antigenic membrane sites relating to cell aggregation in Dictyostelium discoideum. J Cell Biol. 1973 Mar;56(3):647–658. doi: 10.1083/jcb.56.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K. Direct identification of specific glycoproteins and antigens in sodium dodecyl sulfate gels. Methods Enzymol. 1978;50:54–64. doi: 10.1016/0076-6879(78)50007-4. [DOI] [PubMed] [Google Scholar]
- Burridge K., Jordan L. The glycoproteins of Dictyostelium discoideum. Changes during development. Exp Cell Res. 1979 Nov;124(1):31–38. doi: 10.1016/0014-4827(79)90254-4. [DOI] [PubMed] [Google Scholar]
- Finne J., Burger M. M., Prieels J. P. Enzymatic basis for a lectin-resistant phenotype: increase in a fucosyltransferase in mouse melanoma cells. J Cell Biol. 1982 Feb;92(2):277–282. doi: 10.1083/jcb.92.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finne J., Tao T. W., Burger M. M. Carbohydrate changes in glycoproteins of a poorly metastasizing wheat germ agglutinin-resistant melanoma clone. Cancer Res. 1980 Jul;40(7):2580–2587. [PubMed] [Google Scholar]
- GERISCH G. [Cell functions and change in cell function in the development of Dictyostelium discoideum. V. Stagespecific cell contact formation and its quantitative evaluation]. Exp Cell Res. 1961 Dec;25:535–554. doi: 10.1016/0014-4827(61)90189-6. [DOI] [PubMed] [Google Scholar]
- Gilkes N. R., Weeks G. The purification and characterization of Dictyostelium discoideum plasma membranes. Biochim Biophys Acta. 1977 Jan 4;464(1):142–156. doi: 10.1016/0005-2736(77)90377-7. [DOI] [PubMed] [Google Scholar]
- Goldstein I. J., Hammarström S., Sundblad G. Precipitation and carbohydrate-binding specificity studies on wheat germ agglutinin. Biochim Biophys Acta. 1975 Sep 9;405(1):53–61. doi: 10.1016/0005-2795(75)90313-x. [DOI] [PubMed] [Google Scholar]
- Greenaway P. J., LeVine D. Binding of N-acetyl-neuraminic acid by wheat-germ agglutinin. Nat New Biol. 1973 Feb 7;241(110):191–192. doi: 10.1038/newbio241191a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lam T. Y., Siu C. H. Inhibition of cell differentiation and cell cohesion by tunicamycin in Dictyostelium discoideum. Dev Biol. 1982 Aug;92(2):398–407. doi: 10.1016/0012-1606(82)90185-3. [DOI] [PubMed] [Google Scholar]
- MORTON R. K. Separation and purification of enzymes associated with insoluble particles. Nature. 1950 Dec 30;166(4235):1092–1095. doi: 10.1038/1661092a0. [DOI] [PubMed] [Google Scholar]
- Malchow D., Nägele B., Schwarz H., Gerisch G. Membrane-bound cyclic AMP phosphodiesterase in chemotactically responding cells of Dictyostelium discoideum. Eur J Biochem. 1972 Jun 23;28(1):136–142. doi: 10.1111/j.1432-1033.1972.tb01894.x. [DOI] [PubMed] [Google Scholar]
- Monsigny M., Roche A. C., Sene C., Maget-Dana R., Delmotte F. Sugar-lectin interactions: how does wheat-germ agglutinin bind sialoglycoconjugates? Eur J Biochem. 1980 Feb;104(1):147–153. doi: 10.1111/j.1432-1033.1980.tb04410.x. [DOI] [PubMed] [Google Scholar]
- Murray B. A., Wheeler S., Jongens T., Loomis W. F. Mutations affecting a surface glycoprotein, gp80, of Dictyostelium discoideum. Mol Cell Biol. 1984 Mar;4(3):514–519. doi: 10.1128/mcb.4.3.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray B. A., Yee L. D., Loomis W. F. Immunological analysis of glycoprotein (contact sites A) involved in intercellular adhesion of Dictyostelium discoideum. J Supramol Struct Cell Biochem. 1981;17(3):197–211. doi: 10.1002/jsscb.380170302. [DOI] [PubMed] [Google Scholar]
- Müller K., Gerisch G. A specific glycoprotein as the target site of adhesion blocking Fab in aggregating Dictyostelium cells. Nature. 1978 Aug 3;274(5670):445–449. doi: 10.1038/274445a0. [DOI] [PubMed] [Google Scholar]
- Müller K., Gerisch G., Fromme I., Mayer H., Tsugita A. A membrane glycoprotein of aggregating Dictyostelium cells with the properties of contact sites. Eur J Biochem. 1979 Sep;99(2):419–426. doi: 10.1111/j.1432-1033.1979.tb13271.x. [DOI] [PubMed] [Google Scholar]
- Nagata Y., Burger M. M. Wheat germ agglutinin. Molecular characteristics and specificity for sugar binding. J Biol Chem. 1974 May 25;249(10):3116–3122. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
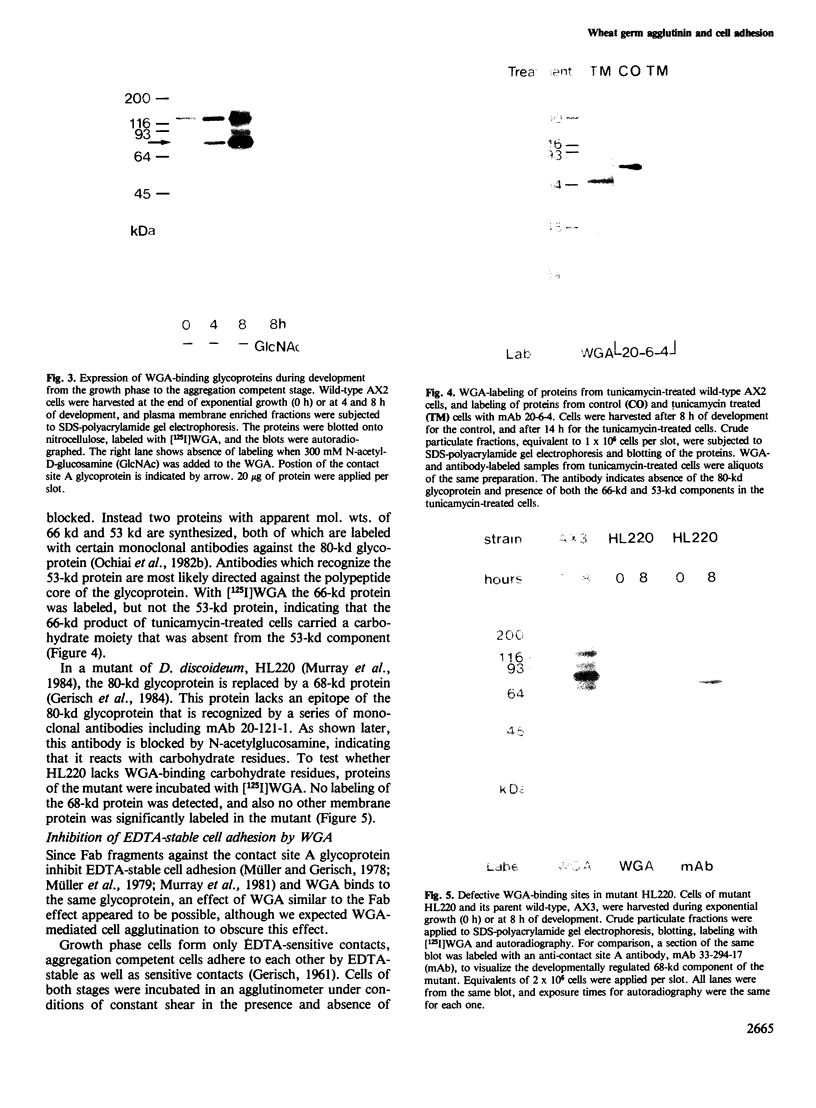
- Ochiai H., Schwarz H., Merkl R., Wagle G., Gerisch G. Stage-specific antigens reacting with monoclonal antibodies against contact site A, a cell-surface glycoprotein of Dictyostelium discoideum. Cell Differ. 1982 Jan;11(1):1–13. doi: 10.1016/0045-6039(82)90011-2. [DOI] [PubMed] [Google Scholar]
- Ochiai H., Stadler J., Westphal M., Wagle G., Merkl R., Gerisch G. Monoclonal antibodies against contact sites A of Dictyostelium discoideum: detection of modifications of the glycoprotein in tunicamycin-treated cells. EMBO J. 1982;1(8):1011–1016. doi: 10.1002/j.1460-2075.1982.tb01286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyama M., Okamoto K., Takeuchi I. Effects of cyclic AMP on contact formation and differentiation in Dictyostelium discoideum. J Cell Sci. 1982 Aug;56:223–232. doi: 10.1242/jcs.56.1.223. [DOI] [PubMed] [Google Scholar]
- Saxe C. L., 3rd, Sussman M. Induction of stage-specific cell cohesion in D. discoideum by a plasma-membrane-associated moiety reactive with wheat germ agglutinin. Cell. 1982 Jul;29(3):755–759. doi: 10.1016/0092-8674(82)90437-8. [DOI] [PubMed] [Google Scholar]
- Stadler J., Bordier C., Lottspeich F., Henschen A., Gerisch G. Improved purification and N-terminal amino acid sequence determination of the contact site A glycoprotein of Dictyostelium discoideum. Hoppe Seylers Z Physiol Chem. 1982 Aug;363(8):771–776. doi: 10.1515/bchm2.1982.363.2.771. [DOI] [PubMed] [Google Scholar]
- Stadler J., Gerisch G., Bauer G., Suchanek C., Huttner W. B. In vivo sulfation of the contact site A glycoprotein of Dictyostelium discoideum. EMBO J. 1983;2(7):1137–1143. doi: 10.1002/j.1460-2075.1983.tb01558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinemann C., Parish R. W. Evidence that a developmentally regulated glycoprotein is target of adhesion-blocking Fab in reaggregating Dictyostelium. Nature. 1980 Aug 7;286(5773):621–623. doi: 10.1038/286621a0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West C. M., McMahon D. Identification of concanavalin A receptors and galactose-binding proteins in purified plasma membranes of Dictyostelium discoideum. J Cell Biol. 1977 Jul;74(1):264–273. doi: 10.1083/jcb.74.1.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West C. M., McMahon D., Molday R. S. Identification of glycoproteins, using lectins as probes, in plasma membranes from Dictyostelium discoideum and human erythrocytes. J Biol Chem. 1978 Mar 10;253(5):1716–1724. [PubMed] [Google Scholar]
- Wilhelms O. H., Lüderitz O., Westphal O., Gerisch G. Glycosphingolipids and glycoproteins in the wild-type and in non-aggregating mutant of Dictyostelium discoideum. Eur J Biochem. 1974 Oct 1;48(1):89–101. doi: 10.1111/j.1432-1033.1974.tb03746.x. [DOI] [PubMed] [Google Scholar]