Abstract
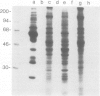
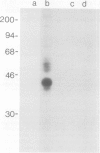
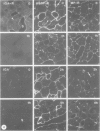
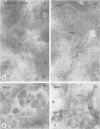
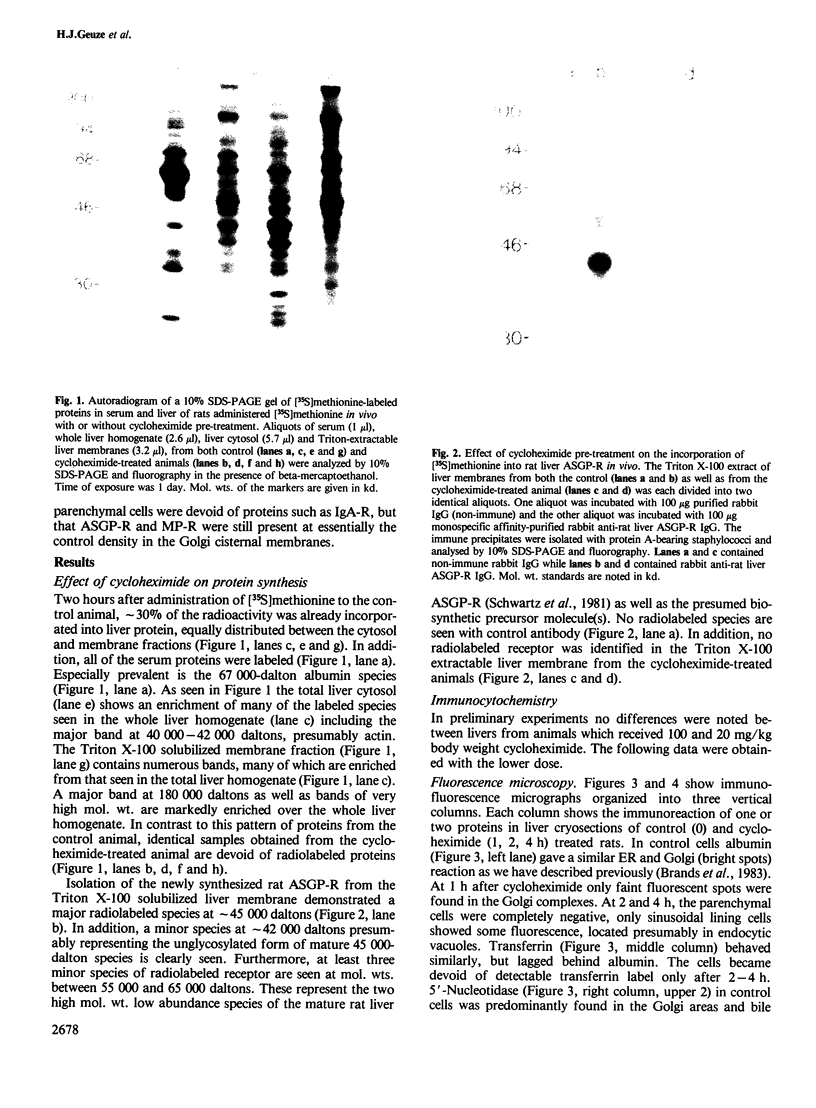
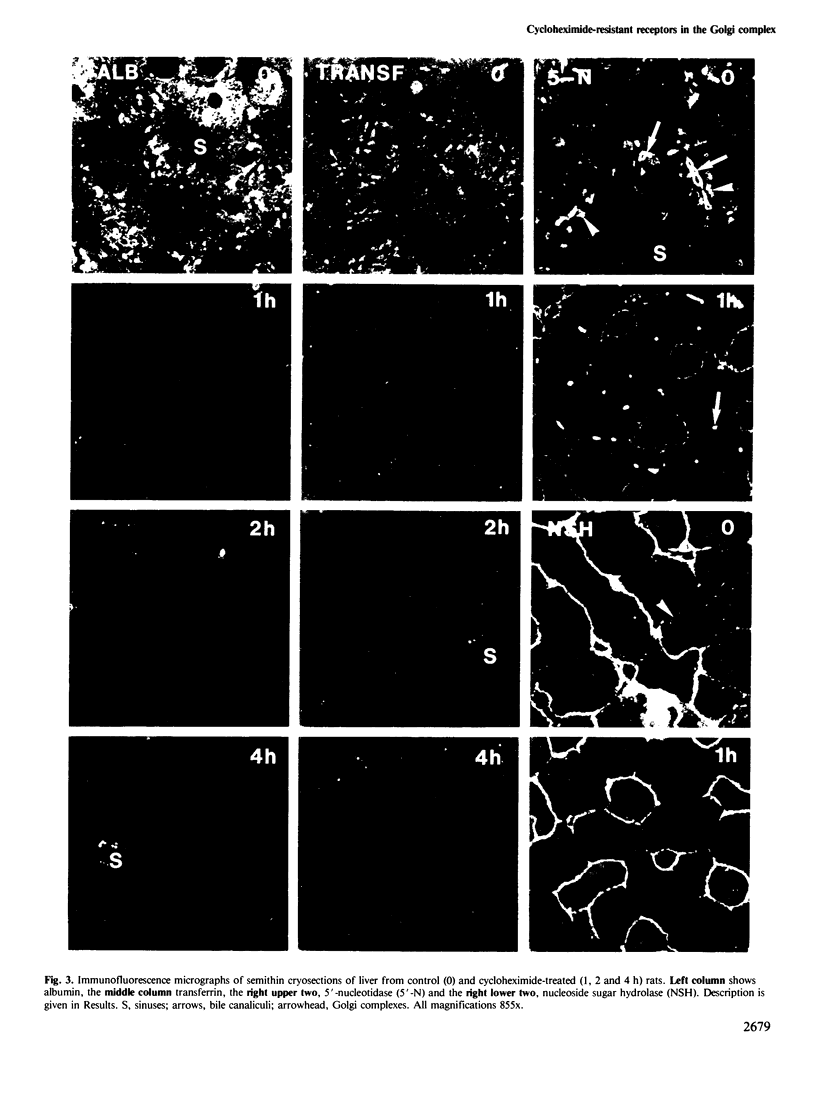
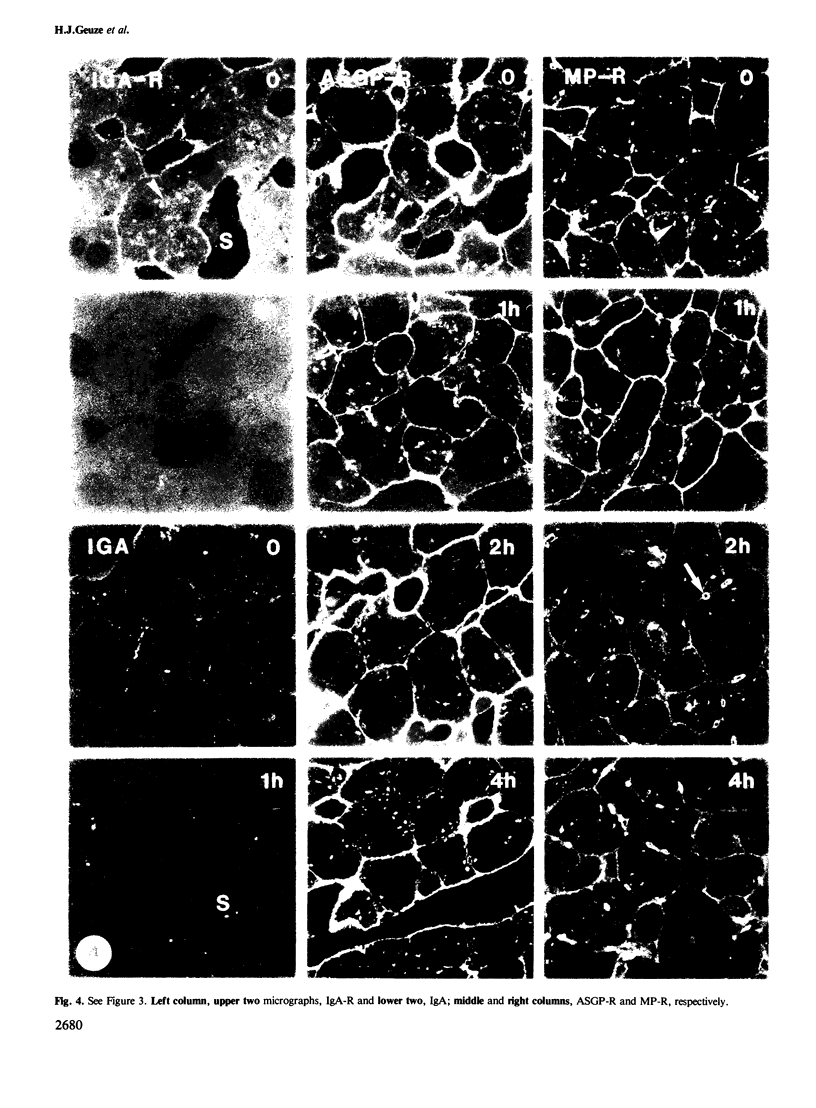
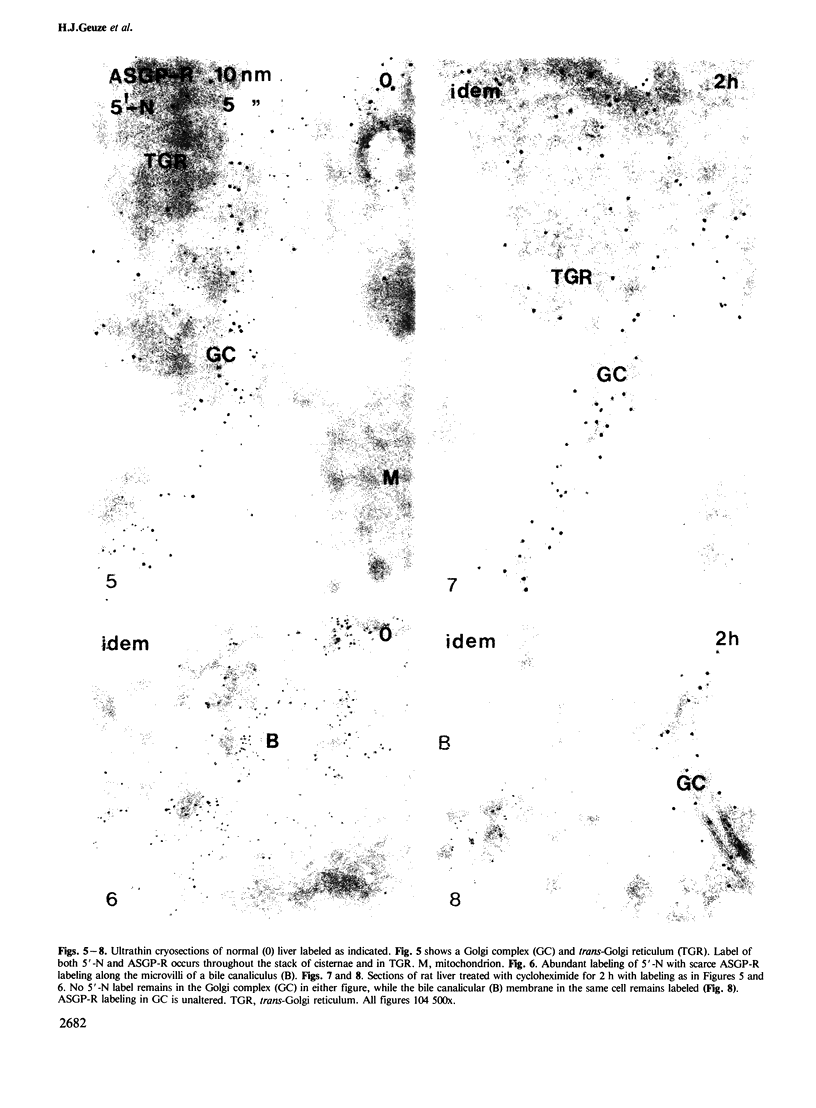
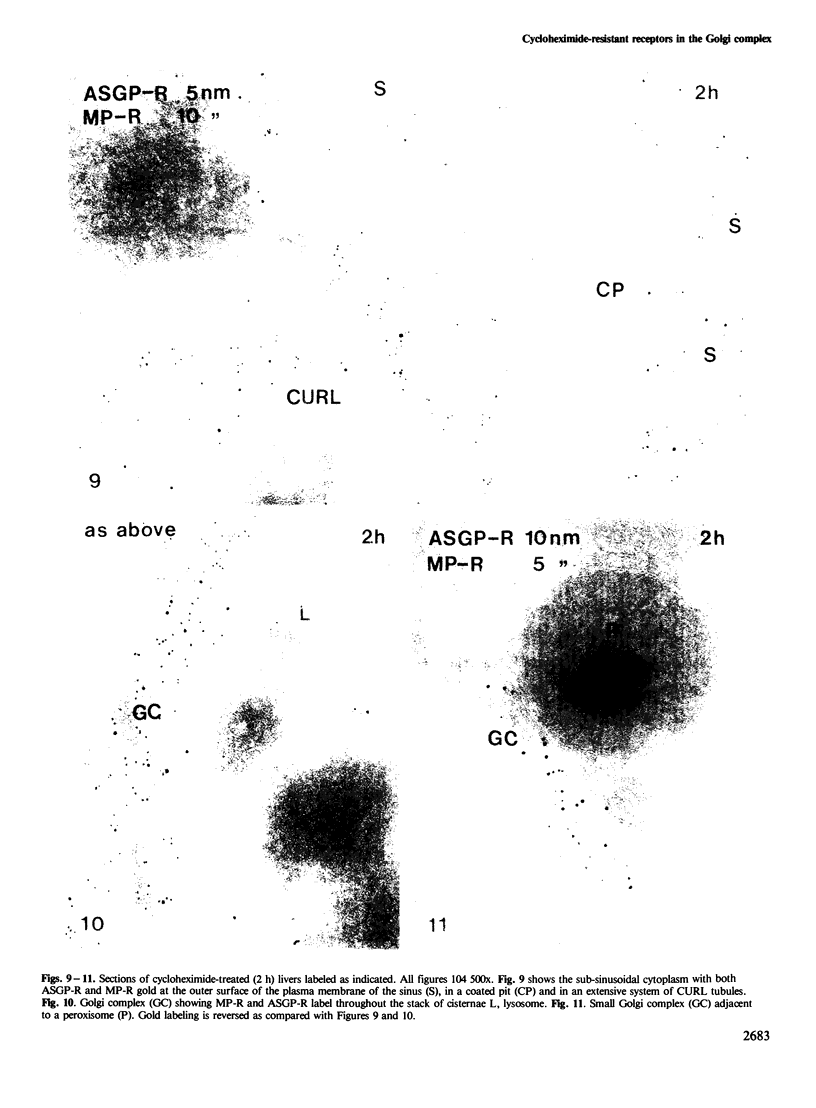
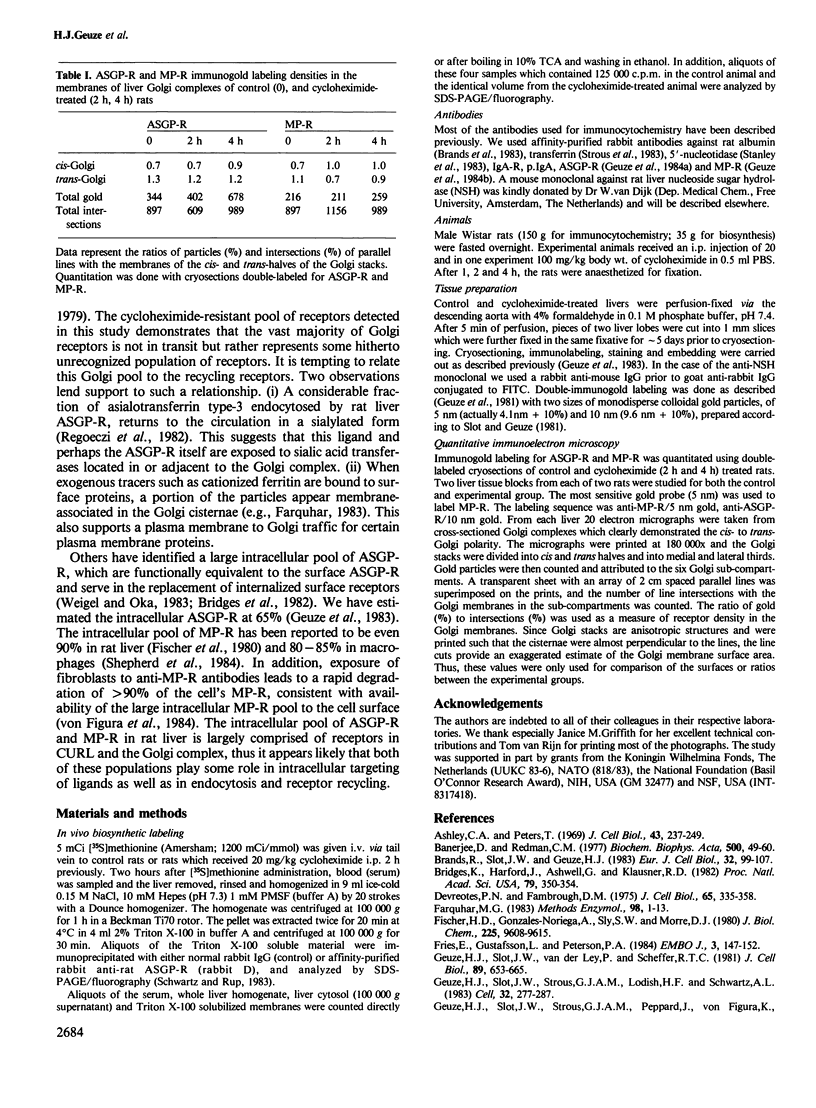
Following in vivo administration of cycloheximide (20 mg/kg body weight i.p.) protein synthesis was completely inhibited (99%) in rat liver. No newly synthesized asialoglycoprotein receptor (ASGP-R) could be detected by metabolic labeling. Fluorescence immunocytochemistry of several secretory proteins and plasma membrane proteins, including the receptors for polymeric IgA (IgA-R), demonstrated a rapid loss from the Golgi complex following cycloheximide administration. On the other hand, two membrane proteins, the receptors for ASGP-R and mannose 6-phosphate (MP-R), were not altered in their cellular localization including the Golgi. Using quantitative immunoelectron microscopy with colloidal gold, we found that 2 h and 4 h after cycloheximide administration, the densities of ASGP-R and MP-R in the membranes of the Golgi complex were unaltered compared with control liver. Similarly, there was no significant effect of cycloheximide on the receptor labeling in coated vesicles and compartment of uncoupling receptors and ligands (CURL). These observations are consistent with an involvement of the Golgi and CURL pools of the receptors in intracellular trafficking, endocytosis and receptor recycling.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashley C. A., Peters T., Jr Electron microscopic radioautographic detection of sites of protein synthesis and migration in liver. J Cell Biol. 1969 Nov;43(2):237–249. doi: 10.1083/jcb.43.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee D., Redman C. M. Effect of local anesthetics on plasma protein secretion by rat hepatocytes. Biochim Biophys Acta. 1977 Nov 7;500(1):49–60. doi: 10.1016/0304-4165(77)90045-9. [DOI] [PubMed] [Google Scholar]
- Brands R., Slot J. W., Geuze H. J. Albumin localization in rat liver parenchymal cells. Eur J Cell Biol. 1983 Nov;32(1):99–107. [PubMed] [Google Scholar]
- Bridges K., Harford J., Ashwell G., Klausner R. D. Fate of receptor and ligand during endocytosis of asialoglycoproteins by isolated hepatocytes. Proc Natl Acad Sci U S A. 1982 Jan;79(2):350–354. doi: 10.1073/pnas.79.2.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devreotes P. N., Fambrough D. M. Acetylcholine receptor turnover in membranes of developing muscle fibers. J Cell Biol. 1975 May;65(2):335–358. doi: 10.1083/jcb.65.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar M. G. Intracellular membrane traffic: pathways, carriers, and sorting devices. Methods Enzymol. 1983;98:1–13. doi: 10.1016/0076-6879(83)98134-x. [DOI] [PubMed] [Google Scholar]
- Fischer H. D., Gonzalez-Noriega A., Sly W. S., Morré D. J. Phosphomannosyl-enzyme receptors in rat liver. Subcellular distribution and role in intracellular transport of lysosomal enzymes. J Biol Chem. 1980 Oct 25;255(20):9608–9615. [PubMed] [Google Scholar]
- Fries E., Gustafsson L., Peterson P. A. Four secretory proteins synthesized by hepatocytes are transported from endoplasmic reticulum to Golgi complex at different rates. EMBO J. 1984 Jan;3(1):147–152. doi: 10.1002/j.1460-2075.1984.tb01775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuze H. J., Slot J. W., Strous G. J., Lodish H. F., Schwartz A. L. Intracellular site of asialoglycoprotein receptor-ligand uncoupling: double-label immunoelectron microscopy during receptor-mediated endocytosis. Cell. 1983 Jan;32(1):277–287. doi: 10.1016/0092-8674(83)90518-4. [DOI] [PubMed] [Google Scholar]
- Geuze H. J., Slot J. W., Strous G. J., Peppard J., von Figura K., Hasilik A., Schwartz A. L. Intracellular receptor sorting during endocytosis: comparative immunoelectron microscopy of multiple receptors in rat liver. Cell. 1984 May;37(1):195–204. doi: 10.1016/0092-8674(84)90315-5. [DOI] [PubMed] [Google Scholar]
- Geuze H. J., Slot J. W., van der Ley P. A., Scheffer R. C. Use of colloidal gold particles in double-labeling immunoelectron microscopy of ultrathin frozen tissue sections. J Cell Biol. 1981 Jun;89(3):653–665. doi: 10.1083/jcb.89.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green J., Griffiths G., Louvard D., Quinn P., Warren G. Passage of viral membrane proteins through the Golgi complex. J Mol Biol. 1981 Nov 15;152(4):663–698. doi: 10.1016/0022-2836(81)90122-4. [DOI] [PubMed] [Google Scholar]
- Gurd J. W., Evans W. H. Distribution of liver plasma membrane 5' nucleotidase as indicated by its reaction with anti-plasma membrane serum. Arch Biochem Biophys. 1974 Sep;164(1):305–311. doi: 10.1016/0003-9861(74)90035-6. [DOI] [PubMed] [Google Scholar]
- Jamieson J. D., Palade G. E. Intracellular transport of secretory proteins in the pancreatic exocrine cell. 3. Dissociation of intracellular transport from protein synthesis. J Cell Biol. 1968 Dec;39(3):580–588. doi: 10.1083/jcb.39.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodish H. F., Kong N., Snider M., Strous G. J. Hepatoma secretory proteins migrate from rough endoplasmic reticulum to Golgi at characteristic rates. Nature. 1983 Jul 7;304(5921):80–83. doi: 10.1038/304080a0. [DOI] [PubMed] [Google Scholar]
- Mollay C., Vilas U., Kreil G. Cleavage of honeybee prepromelittin by an endoprotease from rat liver microsomes: identification of intact signal peptide. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2260–2263. doi: 10.1073/pnas.79.7.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novikoff P. M., Tulsiani D. R., Touster O., Yam A., Novikoff A. B. Immunocytochemical localization of alpha-D-mannosidase II in the Golgi apparatus of rat liver. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4364–4368. doi: 10.1073/pnas.80.14.4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters T., Jr, Peters J. C. The biosynthesis of rat serum albumin. VI. Intracellular transport of albumin and rates of albumin and liver protein synthesis in vivo under various physiological conditions. J Biol Chem. 1972 Jun 25;247(12):3858–3863. [PubMed] [Google Scholar]
- Pohlmann R., Waheed A., Hasilik A., von Figura K. Synthesis of phosphorylated recognition marker in lysosomal enzymes is located in the cis part of Golgi apparatus. J Biol Chem. 1982 May 25;257(10):5323–5325. [PubMed] [Google Scholar]
- Roth J., Berger E. G. Immunocytochemical localization of galactosyltransferase in HeLa cells: codistribution with thiamine pyrophosphatase in trans-Golgi cisternae. J Cell Biol. 1982 Apr;93(1):223–229. doi: 10.1083/jcb.93.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahagian G. G., Neufeld E. F. Biosynthesis and turnover of the mannose 6-phosphate receptor in cultured Chinese hamster ovary cells. J Biol Chem. 1983 Jun 10;258(11):7121–7128. [PubMed] [Google Scholar]
- Schwartz A. L., Marshak-Rothstein A., Rup D., Lodish H. F. Identification and quantification of the rat hepatocyte asialoglycoprotein receptor. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3348–3352. doi: 10.1073/pnas.78.6.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz A. L., Rup D. Biosynthesis of the human asialoglycoprotein receptor. J Biol Chem. 1983 Sep 25;258(18):11249–11255. [PubMed] [Google Scholar]
- Shepherd V. L., Freeze H. H., Miller A. L., Stahl P. D. Identification of mannose 6-phosphate receptors in rabbit alveolar macrophages. J Biol Chem. 1984 Feb 25;259(4):2257–2261. [PubMed] [Google Scholar]
- Slot J. W., Geuze H. J. Sizing of protein A-colloidal gold probes for immunoelectron microscopy. J Cell Biol. 1981 Aug;90(2):533–536. doi: 10.1083/jcb.90.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sly W. S., Fischer H. D. The phosphomannosyl recognition system for intracellular and intercellular transport of lysosomal enzymes. J Cell Biochem. 1982;18(1):67–85. doi: 10.1002/jcb.1982.240180107. [DOI] [PubMed] [Google Scholar]
- Strous G. J., Berger E. G. Biosynthesis, intracellular transport, and release of the Golgi enzyme galactosyltransferase (lactose synthetase A protein) in HeLa cells. J Biol Chem. 1982 Jul 10;257(13):7623–7628. [PubMed] [Google Scholar]
- Strous G. J., Lodish H. F. Intracellular transport of secretory and membrane proteins in hepatoma cells infected by vesicular stomatitis virus. Cell. 1980 Dec;22(3):709–717. doi: 10.1016/0092-8674(80)90547-4. [DOI] [PubMed] [Google Scholar]
- Strous G. J., Van Kerkhof P., Willemsen R., Geuze H. J., Berger E. G. Transport and topology of galactosyltransferase in endomembranes of HeLa cells. J Cell Biol. 1983 Sep;97(3):723–727. doi: 10.1083/jcb.97.3.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sztul E. S., Howell K. E., Palade G. E. Intracellular and transcellular transport of secretory component and albumin in rat hepatocytes. J Cell Biol. 1983 Nov;97(5 Pt 1):1582–1591. doi: 10.1083/jcb.97.5.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe T., Pricer W. E., Jr, Ashwell G. Subcellular membrane topology and turnover of a rat hepatic binding protein specific for asialoglycoproteins. J Biol Chem. 1979 Feb 25;254(4):1038–1043. [PubMed] [Google Scholar]
- Tauber R., Park C. S., Reutter W. Intramolecular heterogeneity of degradation in plasma membrane glycoproteins: evidence for a general characteristic. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4026–4029. doi: 10.1073/pnas.80.13.4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel P. H., Oka J. A. The large intracellular pool of asialoglycoprotein receptors functions during the endocytosis of asialoglycoproteins by isolated rat hepatocytes. J Biol Chem. 1983 Apr 25;258(8):5095–5102. [PubMed] [Google Scholar]