Abstract
Background
The purpose of this study was to examine the extent to which patterns of intensive end-of-life care explain geographic variation in end-of-life care expenditures among cancer decedents.
Methods
Using the SEER-Medicare database, we identified 90,465 decedents who were diagnosed with cancer in 2004–2011. Measures of intensive end-of-life care included chemotherapy received within 14 days of death; more than 1 emergency department visit, more than 1 hospitalization, or 1 or more intensive care unit (ICU) admissions within 30 days of death; in-hospital death; and hospice enrollment less than 3 days before death. Using hierarchical generalized linear models, we estimated risk-adjusted expenditures in the last month of life for each hospital referral region and identified key contributors to variation in expenditures.
Results
The mean expenditure per cancer decedent in the last month of life was $10,800, ranging from $8,300 to $15,400 in the lowest and highest expenditure quintile areas, respectively. There was considerable variation in the percentage of decedents receiving intensive end-of-life care intervention, with 41.7% of decedents receiving intensive care in the lowest quintile of expenditures versus 57.9% in the highest quintile. Regional patterns of late chemotherapy or late hospice use explained only approximately 1% of the expenditure difference between the highest and lowest quintile areas. In contrast, the proportion of decedents who had ICU admissions within 30 days of death was a major driver of variation, explaining 37.6% of the expenditure difference.
Conclusions
Promoting appropriate end-of-life care has the potential to reduce geographic variation in end-of-life care expenditures.
End-of-life care consumes a disproportionate share of Medicare expenditures, accounting for more than one-fourth of spending for the elderly.1–3 Cancer is the second leading cause of death,4 and the mean cost for end-of-life care for cancer deaths is substantially higher than for other causes of death.5 Furthermore, widespread differences in end-of-life health care spending exist between geographic regions among the general population,6 as well as among cancer decedents.7 For instance, mean end-of-life spending per cancer decedent in the highest quintile of national expenditures is approximately 40% more than spending in the lowest quintile.7
Quality of cancer care at the end of life, however, is suboptimal and often highly intensive.8 Prior studies have found high rates of intensive end-of-life care, including intensive care unit (ICU) admission, repeated hospitalization and emergency department (ED) visits in the last month of life, late chemotherapy and hospice enrollment, and in-hospital death.9–11 Such intensive care is often inconsistent with patient preferences and can lead to increased emotional and physical suffering as well as caregiver distress.12–15 To improve monitoring of palliative cancer care, the National Quality Forum has endorsed most of these intensive care patterns as quality measures.16 As with health care expenditures, there is wide variation in intensive end-of-life care patterns across geographic regions.11,17
As value-based health care has risen to the top of the health policy agenda,18,19 evaluation of end-of-life spending and the associated quality of care can provide important information to help transform medical practice. Conceptually, we would expect decedents who received intensive care to have higher expenditures than those who did not. However, the magnitude of the association between intensive care and end-of-life expenditures, as well as the specific services used, remains unclear. Our goal in this study was to first examine the extent to which intensive end-of-life care quality measures were linked with higher spending for individual patients. Second, we sought to pinpoint which quality indicators were the strongest predictors of geographic variation of health expenditures. Because the Centers for Medicare & Medicaid Services (CMS) is developing payment and delivery models designed to improve the effectiveness and efficiency of oncology care, such as the Oncology Care Model,20 our research could assist providers and policymakers in defining and targeting the key practices that have the greatest impact for improving care quality and reducing expenditure variation.
Methods
Data and Study Design
We used the SEER-Medicare database, a unique data source linking Medicare enrollment and claims records to tumor registries. SEER registries currently cover approximately 30% of the US population21 and collect information on patients’ sociodemographic and tumor characteristics. Use of health services and the corresponding expenditures were derived from Medicare claims. This study was reviewed by the Institutional Review Board of Yale University, which determined that this study did not directly involve human subjects.
Study Sample
We identified beneficiaries who had breast, prostate, lung, colorectal, pancreas, liver, kidney, or hematologic cancer or melanoma diagnosed in the years 2004–2011 and died within 3 years of diagnosis as a result of cancer by December 2011. We limited our sample to beneficiaries who were aged 66.5 to 94 years at death and had been enrolled in Medicare Parts A and B during the last 18 months of life. Patients were excluded if their diagnosis was reported only by death certificate or autopsy, they could not be assigned to a hospital referral region (HRR), or they lived less than 6 months after cancer diagnosis.
Measurement of Medicare Spending
Medicare claims files including inpatient, outpatient, physician, home health, durable medical equipment, and hospice services were used to estimate end-of-life expenditures. We calculated monthly spending in the last 6 months of life, but focused on the last month of spending because the quality measures are defined using that period. Unless otherwise specified, end-of-life expenditures represented spending in the last month of life. We adjusted for inflation22 and geographic price differences.23,24 All expenditures were expressed in 2011 US dollars. The sources of end-of-life expenditures were further categorized into inpatient, outpatient, home health, durable medical equipment, or hospice services.
Key Independent Variables
We used previously developed claims-based indicators of intensive end-of-life care,9,11 including (1) chemotherapy received within 14 days of death; (2) more than 1 ED visit within 30 days of death; (3) more than 1 hospitalization within 30 days of death; (4) 1 or more ICU admission within 30 days of death; (5) in-hospital death; and (6) hospice enrollment less than 3 days before death. We also created a composite measure of intensive end-of-life care, which was defined as the occurrence of at least one of the 6 indicators.
Other Control Variables
We included as candidate covariates patient age, race, Hispanic ethnicity, sex, year of death, marital status, SEER registry, metropolitan status of residence, and comorbidity.25 Income and education were derived from linked census-tract level data. We used Elixhauser comorbidity conditions between 7 and 18 months before death, adapting an approach that requires the diagnosis code to appear on an inpatient claim or 2 or more physician or outpatient claims greater than 30 days apart for the condition to be considered present.26 We also incorporated a measure of disability status, a claims-based indicator for services commonly needed by patients with poor functional or performance status.27 Tumor characteristics included tumor site, advanced stage at diagnosis, multiple-cancer diagnoses, and duration between cancer diagnosis and death, as reported by SEER.
Statistical Analysis
We conducted both individual and HRR-level analyses. First, at the individual patient level, we calculated end-of-life expenditures according to receipt of intensive end-of-life care. Second, we conducted HRR-level analyses, calculating the mean end-of-life expenditure for each HRR. We then categorized HRRs by quintiles based on expenditures and used chi-square tests to make pairwise comparisons between quintile 1 (lowest expenditure regions) and 5 (highest expenditure regions) regarding patient and tumor characteristics, as well as intensive care patterns.
In adjusted analyses, to account for the right-skewed spending data while accommodating the clustering of patients within HRRs, we used hierarchical generalized linear models (HGLMs) with a log link function and gamma distribution.28,29 To reduce variability caused by low HRR volumes, we excluded from the HGLM analysis HRRs that had less than 20 decedents during the study period. We winsorized data by truncating the end-of-life expenditures at the upper 97th percentile to eliminate the influence of extreme values.30–32 We calculated the adjusted mean end-of-life expenditure per decedent for HRRi as:
where and are the mean observed expenditure over all decedents in HRRi and in all HRRs, respectively; and is the mean expected expenditure over all decedents in HRRi, based on the predicted values of HGLM (details provided later).
To assess the relative contribution of patient demographics, tumor characteristics, and specific quality measures to regional variation in end-of-life expenditures, we estimated a series of HGLMs by adding these factors to the model sequentially. Model 0 included only an intercept and HRR random effects, and the difference in mean end-of-life expenditure between quintiles 1 and 5 represented unadjusted overall variation. Model 1 adjusted for patient demographics and tumor characteristics, and the difference in adjusted expenditures between quintiles 1 and 5 was calculated. We determined the percentage of variation that could be explained by these factors using the following formula: the denominator is unadjusted overall variation between quintiles 1 and 5 (based on Model 0), and the numerator is the difference between the denominator and the estimate from Model 1. We then estimated 6 models wherein for each model we added a dichotomous variable representing individual quality measures separately. Using a similar approach, we estimated the degree to which each quality measure explained the variation in addition to the variation explained by patient and tumor characteristics. Finally, we included all 6 sets of dichotomous variables that reflected end-of-life care quality. We further conducted 3 sensitivity analyses, including (1) creating a cohort of decedents who lived 1 to 36 months after cancer diagnosis; (2) using ordinal least squares (without log-transformation and winsorization); and (3) excluding decedents who did not use hospice and did not have provider visits during the last 30 days. All statistical analyses were completed using SAS, version 9.4 (SAS Institute, Cary, NC), and a 2-tailed P value of less than 0.05 was used to define statistical significance.
Results
The full study sample consisted of 90,465 decedents. A total of 49.2% had at least one intensive end-of-life care intervention. Compared with decedents who did not receive intensive end-of-life care treatment, decedents who received intensive end-of-life care treatment did not have substantially increased monthly spending in the last 2 to 6 months before death (Figure 1). In contrast, receiving at least one intensive end-of-life care intervention incurred substantially higher expenditures in the last month of life, compared with not receiving intensive care ($18,700 vs $4,200; P<.001). For decedents who received at least one intensive end-of-life care intervention, 71.3% of the end-of-life expenditures were for inpatient services. In contrast, the primary expenditure for decedents who did not receive any intensive end-of-life care was for hospice services (66.6%).
Figure 1.
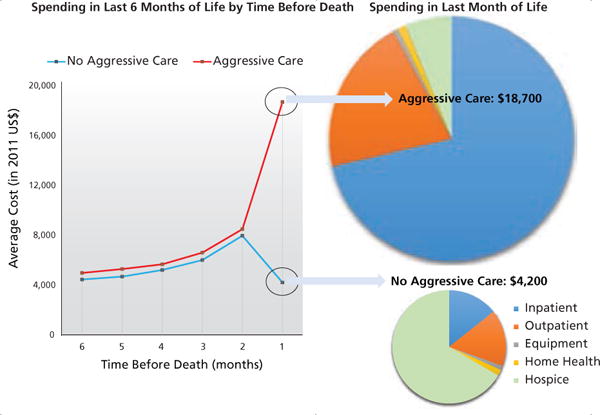
End-of-life spending according to care aggressiveness.
The observed mean end-of-life expenditure per beneficiary was $10,800, ranging from $6,600 in Dubuque, Iowa, to $15,600 in Los Angeles, California. The difference between the highest and lowest quintiles was $7,100 ($8,300 in quintile 1 and $15,400 in quintile 5). Distributions of patient demographics and tumor characteristics varied significantly across the quintiles. Patients in the highest expenditure areas were more likely to be older, female, and non-white; to reside in metropolitan or higher income areas; and to have longer times between cancer diagnosis and death (Table 1).
Table 1.
Patients’ Characteristics, According to Quintiles of Hospital Referral Region Medicare Spending in the Last Month of Life
| Q1 (Lowest Spending) |
Q2 | Q3 | Q4 | Q5 (Highest Spending) |
P Value | |
|---|---|---|---|---|---|---|
| Patient N=17,931 HRR N=33 | Patient N=17,418 HRR N=19 | Patient N=18,223 HRR N=15 | Patient N=18,246 HRR N=15 | Patient N=18,647 HRR N=10 | Q1 vs Q5 | |
| Average cost per patient (mean, SD) | $8,300 (9,600) | $9,400 (10,800) | $10,800 (12,100) | $12,500 (13,700) | $15,400 (16,300) | <.001 |
| Median cost per patient | $4,400 | $5,300 | $6,100 | $8,000 | $11,400 | |
| Age in years (mean, SD) | 76.5 (7.0) | 76.4 (7.0) | 76.6 (7.0) | 77.3 (7.0) | 77.2 (7.1) | <.001 |
| Female (%) | <.001 | |||||
| No | 45.2 | 45.9 | 46.5 | 50.1 | 49.1 | |
| Yes | 54.8 | 54.1 | 53.5 | 49.9 | 50.9 | |
| Race (%) | <.001 | |||||
| White | 91.5 | 86.8 | 89.1 | 89.0 | 78.2 | |
| Black | 6.7 | 7.8 | 7.5 | 6.5 | 13.6 | |
| Other | 1.9 | 5.4 | 3.4 | 4.4 | 8.2 | |
| Hispanic (%) | <.001 | |||||
| Non-Hispanic | 97.0 | 98.6 | 93.6 | 95.7 | 92.2 | |
| Hispanic | 3.0 | 1.4 | 6.4 | 4.3 | 7.8 | |
| Marital status (%) | <.001 | |||||
| Married | 54.8 | 52.2 | 52.2 | 49.9 | 49.1 | |
| Unmarried | 40.9 | 42.9 | 43.6 | 44.2 | 45.9 | |
| Unknown | 4.3 | 4.9 | 4.2 | 5.9 | 5.0 | |
| Metro (%) | <.001 | |||||
| Metro | 60.7 | 64.1 | 86.4 | 99.0 | 99.9 | |
| Nonmetro/unknown | 39.3 | 35.9 | 13.6 | 1.0 | 0.1 | |
| Income (%) | <.001 | |||||
| <$33,000 | 32.9 | 38.4 | 19.5 | 10.4 | 15.7 | |
| $33,000–$39,999 | 26.2 | 19.0 | 17.6 | 7.9 | 9.0 | |
| $40,000–$49,999 | 22.1 | 19.2 | 21.9 | 19.2 | 19.5 | |
| $50,000–$62,999 | 13.8 | 13.8 | 20.8 | 26.3 | 20.5 | |
| ≥$63,000 | 4.9 | 9.5 | 20.1 | 36.3 | 35.1 | |
| High school education (%) | <.001 | |||||
| <30% | 13.6 | 16.3 | 22.6 | 23.6 | 29.1 | |
| 30%–39% | 14.5 | 12.4 | 16.8 | 17.6 | 15.8 | |
| 40%–49% | 17.1 | 14.9 | 18.6 | 19.0 | 17.0 | |
| 50%–59% | 22.5 | 16.5 | 17.6 | 19.8 | 17.0 | |
| ≥60% | 32.2 | 39.9 | 24.3 | 19.9 | 21.0 | |
| Comorbidity (%) | <.001 | |||||
| None | 25.5 | 23.9 | 23.8 | 20.6 | 19.3 | |
| 1–2 | 41.5 | 40.8 | 41.5 | 39.2 | 38.1 | |
| >3 | 33.0 | 35.3 | 34.7 | 40.2 | 42.6 | |
| Disability status (%) | <.001 | |||||
| No | 91.7 | 91.3 | 91.4 | 90.5 | 89.2 | |
| Ye s | 8.3 | 8.7 | 8.6 | 9.5 | 10.8 | |
| Tumor site (%) | <.001 | |||||
| Breast | 7.6 | 7.1 | 7.3 | 8.1 | 8.3 | |
| Prostate | 8.1 | 7.2 | 7.8 | 6.6 | 7.0 | |
| Lung | 40.5 | 43.7 | 41.3 | 39.2 | 37.3 | |
| Colorectal | 14.6 | 13.7 | 14.2 | 15.6 | 15.8 | |
| Pancreas | 6.1 | 6.6 | 7.0 | 7.7 | 7.6 | |
| Liver | 2.2 | 2.1 | 2.5 | 2.8 | 3.6 | |
| Kidney | 3.6 | 3.3 | 3.5 | 3.1 | 2.8 | |
| Skin | 4.0 | 3.9 | 4.3 | 4.4 | 4.0 | |
| Hematologic cancer | 13.2 | 12.3 | 12.1 | 12.4 | 13.5 | |
| Stage IV at diagnosis (%) | .02 | |||||
| Not stage IV | 70.1 | 69.7 | 68.9 | 68.8 | 68.9 | |
| Stage IV | 29.9 | 30.3 | 31.1 | 31.2 | 31.1 | |
| Multiple cancers (%) | .25 | |||||
| No | 87.7 | 87.0 | 87.6 | 87.4 | 87.2 | |
| Yes | 12.3 | 13.0 | 12.4 | 12.6 | 12.8 | |
| Time between cancer diagnosis and death (%) | .003 | |||||
| 6 mo–1 y | 36.4 | 36.9 | 36.4 | 36.2 | 35.2 | |
| 1–2 y | 41.0 | 40.8 | 41.1 | 40.6 | 40.8 | |
| 2–3 y | 22.6 | 22.3 | 22.5 | 23.2 | 24.0 | |
| Total provider visits (%) | <.001 | |||||
| None | 13.8 | 13.1 | 11.9 | 9.8 | 8.8 | |
| 1–4 | 24.8 | 22.0 | 20.2 | 17.6 | 15.3 | |
| 5–10 | 25.4 | 24.8 | 25.2 | 23.7 | 22.2 | |
| ≥11 | 36.2 | 40.1 | 42.7 | 49.0 | 53.7 |
The percentage of decedents receiving at least one intensive end-of-life care intervention ranged from 26.9% in Cedar Rapids, Iowa, to 61.0% in Paterson, New Jersey. Across quintiles by end-of-life expenditures, the percentage still varied substantially, ranging from 41.7% in the lowest-spending quintile to 57.9% in the highest-spending quintile (P<.001; Figure 2). Compared with decedents living in the lowest-quintile areas, those living in the highest-quintile areas were more likely to receive late chemotherapy (4.7% vs 3.5%) and have repeated ED visits (40.2% vs 30.0%), repeated hospitalizations (17.9% vs 11.1%), ICU admission (26.5% vs 11.7%), and late hospice use (9.6% vs 7.0%; P<.001 for all comparisons).
Figure 2.
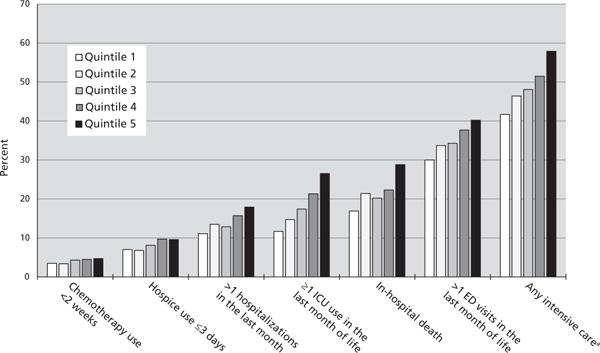
Variation in intensive end-of-life care patterns, according to quintiles of medicare spending.
Abbreviations: ED, emergency department; ICU, intensive care unit.
aAny of the 6 patterns of intensive end-of-life care.
Without adjusting for any patient or treatment characteristics (Model 0), the difference in mean end-of-life expenditure per beneficiary between quintiles 1 ($14,300) and 5 ($8,100) was $6,200. After adjusting for patient demographics and tumor characteristics (Model 1), the difference decreased to $5,300, indicating that patient characteristics were able to explain 14.7% of the expenditure variation. When we adjusted for each individual indicator of intensive end-of-life care, the percentage of expenditure variation explained between quintiles 1 and 5 differed substantially (Figure 3). Late chemotherapy or late hospice use did not significantly decrease mean expenditure differences between quintiles 1 and 5. After adjusting for repeated ED visits or hospitalizations, the mean expenditure difference between quintiles 1 and 5 was $4,100 or $4,000, respectively, showing that these 2 factors could account for approximately 20% of the expenditure variation. Furthermore, ICU admission appeared to be the major driver of end-of-life spending differences: the difference between quintiles 1 and 5 decreased to $2,900 after adjusting for ICU admission, indicating that ICU admission explained 37.6% of the expenditure variation. After adjusting for all intensive end-of-life care indicators, the mean expenditure difference between quintiles 1 and 5 was $1,400, which indicated that end-of-life care quality could explain 62.8% of the original difference. The results of the sensitivity analyses were qualitatively similar, with the same intensive end-of-life care indicators ranking least to most able to explain regional expenditure variation (see supplemental eAppendix 1, available with this article at JNCCN.org).
Figure 3.
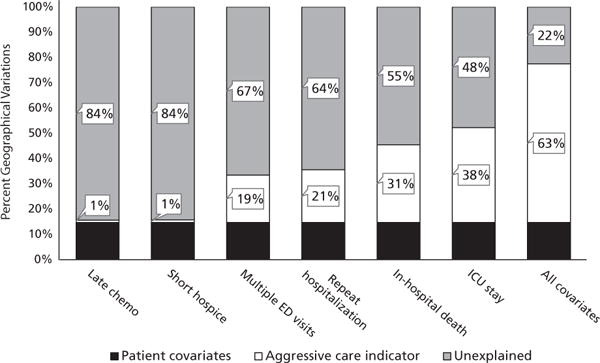
Regional expenditure variation (between the top and bottom quintiles) explained by intensive end-of-life care indicators.
Abbreviations: chemo, chemotherapy; ED, emergency department; ICU, intensive care unit.
Discussion
In this study, we examined associations between end-of-life quality measures and care expenditures for patients with cancer at both the individual and regional levels, building on prior work in several important ways. First, at the individual-level, we found a considerable difference in expenditures in the final month of life between decedents receiving and not receiving intensive end-of-life care (ie, $14,500). In fact, this difference is even larger than the observed mean expenditure per beneficiary ($10,800). Thus, efforts targeting improvements in end-of-life care as defined by these quality measures could produce substantial savings in health care spending, especially given that approximately 50% of decedents had at least one intensive end-of-life care intervention.
Second, our research confirms a problematic relationship between regional expenditures and end-of-life care quality. Following the results of a recently published study of patients with cancer,7 we found that decedents in high-spending areas were more likely to receive intensive end-of-life care interventions than those in low-spending regions. Importantly, previous findings showed that despite higher rates of intensive care interventions and spending, decedents in those regions did not experience any significant improvement in mean survival time, strengthening evidence that the quality measures examined in both studies are strong indicators of low-value care.
Third, we found that intensive end-of-life care patterns were able to explain nearly two-thirds of the extensive geographic variation in end-of-life expenditures known to exist.11 Furthermore, we quantified how specific care quality indicators contributed to expenditures and expenditure variation. Notably, late chemotherapy or late hospice use contributed less than 1% to the expenditure variation, whereas ICU admission was the main driver among the end-of-life care measures we examined, accounting for 38% of the observed variation. Prior research has shown that acute hospital care is the chief driver for expenditure variation among patients with advanced cancer.33 Indeed, at the HRR-level, ICU use and overall hospital use were highly correlated (see supplemental eAppendix 2). In combination, these data can support the design and implementation of increasingly targeted methods for cost and quality improvements. For instance, prompt palliative care consultation and communication about advance directives in the high expenditure areas could reduce ICU admission and thus decrease not only overall end-of-life care expenditures but also variation in expenditures.
Our findings that short hospice enrollment did not explain much of the geographic variation should be interpreted with caution. Hospice has been embraced as a means to control end-of-life expenditures and improve care quality.16 Previous literature has identified duration of hospice use as a key factor in determining cost savings.34–36 Because we followed the current recommended quality measures, which focus on aggressive care, we only included hospice enrollment of less than 3 days in our models. Future research should examine the timing of hospice initiation and its relationship with geographic variation in expenditures.
Consistent with prior literature,37 metropolitan areas accounted for almost all of the high-spending areas. Some researchers have speculated that under-use of certain care modalities may be due to barriers to access in rural areas.38 Nonetheless, patient characteristics (including metropolitan residence status) only explained 14% of the expenditure variation, whereas end-of-life care intensity measures explained a much larger proportion of the variation, highlighting the importance of monitoring quality of care to identify regions with the greatest potential for cost saving.
Our study has several limitations. Our analyses are based on the SEER-Medicare fee-for-service beneficiaries, which do not necessarily represent all Medicare beneficiaries. Second, we did not include patient preferences. Although patient demands have not been shown to be an important factor in expenditure variation,14 future investigations should explore patient experiences and their associations with expenditures. Third, we acknowledge that patients must be admitted to the hospital in order to receive ICU care; therefore, the quality measure of ICU admission also captures some of the hospitalization costs. The issue of how much variance is explained by ICU admission alone is outside the scope of this article, because our goals are to link quality metrics to variation in expenditures, but it warrants future research. Finally, our study assessed the association between an area’s spending on indicators of its quality of care but did not address other important factors, such as hospital and physician characteristics, on overall variations. For example, some research has suggested that physician beliefs are the most important for practice patterns.39
Conclusions
Our findings on expenditure variations provide insight for policymakers to develop a Medicare value-based payment system. We demonstrated that quality indicators were influential drivers of overall end-of-life expenditures, representing opportunities for considerably reducing these expenditures. Furthermore, ICU admission accounted for most of the geographic variation. These quality metrics, such as ICU admission, are important not only from a cost perspective but also as indicators for continuous quality improvement to assure patient-centered care. CMS is planning to pay providers for consultation related to advance care planning,40 which could promote conversations about treatment options when patients are approaching the end of life. Such incentives for appropriate end-of-life care can potentially decrease unnecessary use of resources and promote high-quality, compassionate care.
Supplementary Material
Acknowledgments
The collection of the California cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the NCI’s SEER Program under contract N01-PC-35136 awarded to the Northern California Cancer Center, contract N01-PC-35139 awarded to the University of Southern California, and contract N02-PC-15105 awarded to the Public Health Institute; and the CDC’s National Program of Cancer Registries, under agreement #U55/CCR921930-02 awarded to the Public Health Institute. The authors of this report are responsible for its content. The ideas and opinions expressed herein are those of the author(s) and endorsement by the State of California, Department of Public Health, the NCI, and the CDC or their contractors and subcontractors is not intended nor should be inferred. The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the SEER Program tumor registries in the creation of the SEER-Medicare database. The interpretation and reporting of the SEER-Medicare data are the sole responsibility of the authors.
Dr. Gross has disclosed that he receives grant/research support from Medtronic, Inc., Johnson & Johnson, Inc., 21st Century Oncology, and Pfizer; these sources of support were not used for any portion of the current manuscript. The remaining authors have disclosed that they have no financial interests, arrangements, affiliations, or commercial interests with the manufacturers of any products discussed in this article or their competitors. This study was supported by a Pilot Grant and a P30 Cancer Center Support Grant, both from Yale Comprehensive Cancer Center. Dr. Wang is supported by an AHRQ career development award (K01HS023900). Dr. Pollack is supported by an NCI career development award (K07CA151910).
Footnotes
See JNCCN.org for supplemental online content.
References
- 1.Lynn J, Adamson DM. White paper: living well at the end of life. Available at: https://www.rand.org/content/dam/rand/pubs/white_papers/2005/WP137.pdf. Accessed March 26, 2015.
- 2.Lubitz JD, Riley GF. Trends in Medicare payments in the last year of life. N Engl J Med. 1993;328:1092–1096. doi: 10.1056/NEJM199304153281506. [DOI] [PubMed] [Google Scholar]
- 3.Riley GF, Lubitz JD. Long-term trends in Medicare payments in the last year of life. Health Serv Res. 2010;45:565–576. doi: 10.1111/j.1475-6773.2010.01082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Leading causes of death. Available at: http://www.cdc.gov/nchs/fastats/leading-causes-of-death.htm. Assessed December 10, 2015.
- 5.Mariotto AB, Yabroff KR, Shao Y, et al. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst. 2011;103:117–128. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fisher ES, Wennberg DE, Stukel TA, et al. The implications of regional variations in Medicare spending. Part 1: the content, quality, and accessibility of care. Ann Int Med. 2003;138:273–287. doi: 10.7326/0003-4819-138-4-200302180-00006. [DOI] [PubMed] [Google Scholar]
- 7.Brooks GA, Li L, Sharma DB, et al. Regional variation in spending and survival for older adults with advanced cancer. J Natl Cancer Inst. 2013;105:634–642. doi: 10.1093/jnci/djt025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morden NE, Chang CH, Jacobson JO, et al. End-of-life care for Medicare beneficiaries with cancer is highly intensive overall and varies widely. Health Aff (Millwood) 2012;31:786–796. doi: 10.1377/hlthaff.2011.0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Earle CC, Park ER, Lai B, et al. Identifying potential indicators of the quality of end-of-life cancer care from administrative data. J Clin Oncol. 2003;21:1133–1138. doi: 10.1200/JCO.2003.03.059. [DOI] [PubMed] [Google Scholar]
- 10.Earle CC, Neville BA, Landrum MB, et al. Evaluating claims-based indicators of the intensity of end-of-life cancer care. Int J Qual Health Care. 2005;17:505–509. doi: 10.1093/intqhc/mzi061. [DOI] [PubMed] [Google Scholar]
- 11.Earle CC, Landrum MB, Souza JM, et al. Aggressiveness of cancer care near the end of life: is it a quality-of-care issue? J Clin Oncol. 2008;26:3860–3866. doi: 10.1200/JCO.2007.15.8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300:1665–1673. doi: 10.1001/jama.300.14.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steinhauser KE, Clipp EC, McNeilly M, et al. In search of a good death: observations of patients, families, and providers. Ann Int Med. 2000;132:825–832. doi: 10.7326/0003-4819-132-10-200005160-00011. [DOI] [PubMed] [Google Scholar]
- 14.Barnato AE, Herndon MB, Anthony DL, et al. Are regional variations in end-of-life care intensity explained by patient preferences?: a study of the US Medicare population. Med Care. 2007;45:386–393. doi: 10.1097/01.mlr.0000255248.79308.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wright AA, Keating NL, Balboni TA, et al. Place of death: correlations with quality of life of patients with cancer and predictors of bereaved caregivers’ mental health. J Clin Oncol. 2010;28:4457–4464. doi: 10.1200/JCO.2009.26.3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Quality Forum. NQF endorses cancer measures. Available at: http://www.qualityforum.org/News_And_Resources/Press_Releases/2012/NQF_Endorses_Cancer_Measures.aspx. Accessed September 15, 2014.
- 17.Wang SY, Hall J, Pollack CE, et al. Trends in end-of-life cancer care in the Medicare program. J Geriatr Oncol. 2016;7:116–125. doi: 10.1016/j.jgo.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Medicare.gov. Hospital value-based purchasing. Available at: https://www.medicare.gov/hospitalcompare/Data/hospital-vbp.html. Assessed September 24, 2015.
- 19.American Society of Clinical Oncology. Value in Cancer Care. Available at: https://www.asco.org/practice-guidelines/cancer-care-initiatives/value-cancer-care. Assessed September 24, 2015.
- 20.The Center for Medicare & Medicaid Services. Oncology Care Model. Available at: https://innovation.cms.gov/initiatives/Oncology-Care/. Accessed November 16, 2015.
- 21.National Cancer Institute. Surveillance, Epidemiology, and End Results Program. Overview of the SEER Program. Available at: http://seer.cancer.gov/about/overview.html. Accessed January 25, 2016.
- 22.Centers for Medicare & Medicaid Services. 2013 market basket data. Available at: http://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/MedicareProgramRatesStats/MarketBasketData.html. Accessed June 9, 2013.
- 23.Centers for Medicare & Medicaid Services. 2013 PFS Relative Value Files. Available at: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/PFS-Relative-Value-Files-Items/RVU13AR.html. Accessed July 8, 2016.
- 24.Centers for Medicare & Medicaid Services. FY 2013 Wage Index Home Page. Available at: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Wage-Index-Files-Items/CMS1252760.html. Accessed July 8, 2016.
- 25.Bach PB, Guadagnoli E, Schrag D, et al. Patient demographic and socioeconomic characteristics in the SEER-Medicare database applications and limitations. Med Care. 2002;40(Suppl 8):IV-19–25. doi: 10.1097/00005650-200208001-00003. [DOI] [PubMed] [Google Scholar]
- 26.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Davidoff AJ, Zuckerman IH, Pandya N, et al. A novel approach to improve health status measurement in observational claims-based studies of cancer treatment and outcomes. J Geriatr Oncol. 2013;4:157–165. doi: 10.1016/j.jgo.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manning WG, Basu A, Mullahy J. Generalized modeling approaches to risk adjustment of skewed outcomes data. J Health Econ. 2005;24:465–488. doi: 10.1016/j.jhealeco.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 29.Wang SY, Wang R, Yu JB, et al. Understanding regional variation in Medicare expenditures for initial episodes of prostate cancer care. Med Care. 2014;52:680–687. doi: 10.1097/MLR.0000000000000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dixon WJ, Yuen KK. Trimming and winsorization: a review. Stat Pap (Berl) 1974;15:157–170. [Google Scholar]
- 31.Adams JL, Mehrotra A, Thomas JW, McGlynn EA. Physician cost profiling-reliability and risk of misclassification. N Engl J Med. 2010;362:1014–1021. doi: 10.1056/NEJMsa0906323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jha AK, Orav EJ, Dobson A, et al. Measuring efficiency: the association of hospital costs and quality of care. Health Aff (Millwood) 2009;28:897–906. doi: 10.1377/hlthaff.28.3.897. [DOI] [PubMed] [Google Scholar]
- 33.Brooks GA, Li L, Uno H, et al. Acute hospital care is the chief driver of regional spending variation in Medicare patients with advanced cancer. Health Aff (Millwood) 2014;33:1793–1800. doi: 10.1377/hlthaff.2014.0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Obermeyer Z, Makar M, Abujaber S, et al. Association between the Medicare hospice benefit and health care utilization and costs for patients with poor-prognosis cancer. JAMA. 2014;312:1888–1896. doi: 10.1001/jama.2014.14950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taylor DH, Jr, Ostermann J, Van Houtven CH, et al. What length of hospice use maximizes reduction in medical expenditures near death in the US Medicare program? Soc Sci Med. 2007;65:1466–1478. doi: 10.1016/j.socscimed.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 36.Kelley AS, Deb P, Du Q, et al. Hospice enrollment saves money for Medicare and improves care quality across a number of different lengths-of-stay. Health Aff (Millwood) 2013;32:552–561. doi: 10.1377/hlthaff.2012.0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Long SH, Gibbs JO, Crozier JP, et al. Medical expenditures of terminal cancer patients during the last year of life. Inquiry. 1984;21:315–327. [PubMed] [Google Scholar]
- 38.Edelman MA, Menz BL. Selected comparisons and implications of a national rural and urban survey on health care access, demographics, and policy issues. J Rural Health. 1996;12:197–205. doi: 10.1111/j.1748-0361.1996.tb00794.x. [DOI] [PubMed] [Google Scholar]
- 39.Cutler D, Skinner J, Stern AD, Wennberg D. Physician Beliefs and Patient Preferences: A New Look at Regional Variation in Health Care Spending. doi: 10.1257/pol.20150421. Available at: http://econ.ohio-state.edu/seminar/papers/140116_Skinner.pdf. Accessed September 24, 2015. [DOI] [PMC free article] [PubMed]
- 40.Centers for Medicare & Medicaid Services. CMS Begins Implementation of Key Payment Legislation. Available at: https://www.cms.gov/Newsroom/MediaReleaseDatabase/Press-releases/2015-Press-releases-items/2015-07-08.html. Assessed September 24, 2015.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


