Abstract
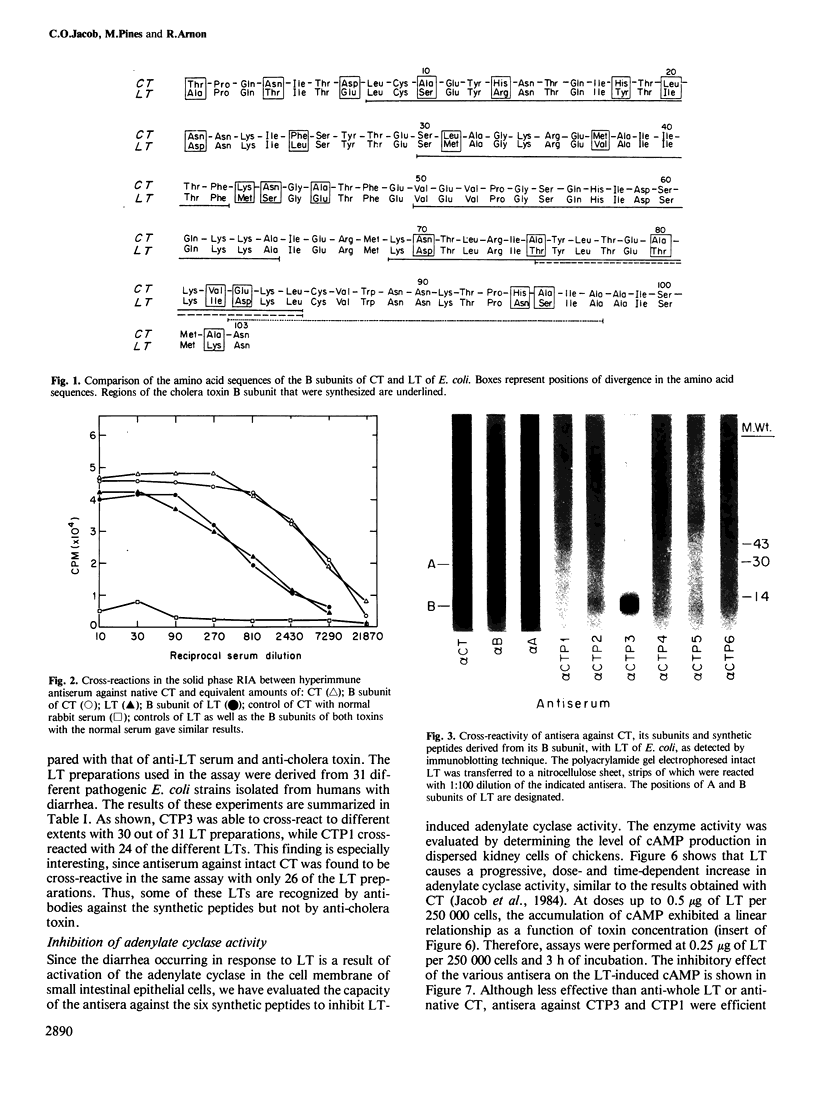
Antibodies elicited by six synthetic peptides corresponding to various fragments of B subunit of cholera toxin (CT) were evaluated for their cross-reactivity with heat-labile toxin (LT) of Escherichia coli. The antiserum directed towards the peptide CTP3 (residues 50-64) was found highly cross-reactive with the LT, in radioimmunoassay and immunoblotting. This peptide was also the most cross-reactive with intact CT. The antiserum against CTP1 (residues 8-20) was also cross-reactive with the two toxins, although to a much lower extent. Antisera to both CTP1 and CTP3, which are inhibitory towards CT, were found equally effective in neutralizing the biological activity of the E. coli LT. This was manifested by inhibition of both adenylate cyclase activity and fluid secretion into ligated ileal loops of rats. These results might indicate the potential of such synthetic peptides as the basis for a general vaccine against several types of infectious diarrhea.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bittle J. L., Houghten R. A., Alexander H., Shinnick T. M., Sutcliffe J. G., Lerner R. A., Rowlands D. J., Brown F. Protection against foot-and-mouth disease by immunization with a chemically synthesized peptide predicted from the viral nucleotide sequence. Nature. 1982 Jul 1;298(5869):30–33. doi: 10.1038/298030a0. [DOI] [PubMed] [Google Scholar]
- Black R. E., Merson M. H., Huq I., Alim A. R., Yunus M. Incidence and severity of rotavirus and Escherichia coli diarrhoea in rural Bangladesh. Implications for vaccine development. Lancet. 1981 Jan 17;1(8212):141–143. doi: 10.1016/s0140-6736(81)90719-4. [DOI] [PubMed] [Google Scholar]
- Clements J. D., Finkelstein R. A. Immunological cross-reactivity between a heat-labile enterotoxin(s) of Escherichia coli and subunits of Vibrio cholerae enterotoxin. Infect Immun. 1978 Sep;21(3):1036–1039. doi: 10.1128/iai.21.3.1036-1039.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements J. D., Flint D. C., Klipstein F. A. Immunological and physicochemical characterization of heat-labile enterotoxins isolated from two strains of Escherichia coli. Infect Immun. 1982 Nov;38(2):806–809. doi: 10.1128/iai.38.2.806-809.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallas W. S., Falkow S. Amino acid sequence homology between cholera toxin and Escherichia coli heat-labile toxin. Nature. 1980 Dec 4;288(5790):499–501. doi: 10.1038/288499a0. [DOI] [PubMed] [Google Scholar]
- Evans D. J., Jr, Chen L. C., Curlin G. T., Evans D. G. Stimulation of adenyl cyclase by Escherichia coli enterotoxin. Nat New Biol. 1972 Apr 5;236(66):137–138. doi: 10.1038/newbio236137a0. [DOI] [PubMed] [Google Scholar]
- Gill D. M., Clements J. D., Robertson D. C., Finkelstein R. A. Subunit number and arrangement in Escherichia coli heat-labile enterotoxin. Infect Immun. 1981 Sep;33(3):677–682. doi: 10.1128/iai.33.3.677-682.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper J. F., Brooker G. Femtomole sensitive radioimmunoassay for cyclic AMP and cyclic GMP after 2'0 acetylation by acetic anhydride in aqueous solution. J Cyclic Nucleotide Res. 1975;1(4):207–218. [PubMed] [Google Scholar]
- Holmgren J. Actions of cholera toxin and the prevention and treatment of cholera. Nature. 1981 Jul 30;292(5822):413–417. doi: 10.1038/292413a0. [DOI] [PubMed] [Google Scholar]
- Holmgren J. Comparison of the tissue receptors for Vibrio cholerae and Escherichia coli enterotoxins by means of gangliosides and natural cholera toxoid. Infect Immun. 1973 Dec;8(6):851–859. doi: 10.1128/iai.8.6.851-859.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren J., Söderlind O., Wadström T. Cross-reactivity between heat labile enterotoxins of Vibrio cholerae and Escherichia coli in neutralization tests in rabbit ileum and skin. Acta Pathol Microbiol Scand B Microbiol Immunol. 1973 Dec;81(6):757–762. doi: 10.1111/j.1699-0463.1973.tb02272.x. [DOI] [PubMed] [Google Scholar]
- Jacob C. O., Sela M., Arnon R. Antibodies against synthetic peptides of the B subunit of cholera toxin: crossreaction and neutralization of the toxin. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7611–7615. doi: 10.1073/pnas.80.24.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler E. M. Neonatal enteric colibacillosis of pigs and current research on immunization. J Am Vet Med Assoc. 1978 Sep 1;173(5 Pt 2):588–591. [PubMed] [Google Scholar]
- Lindholm L., Holmgren J., Wikström M., Karlsson U., Andersson K., Lycke N. Monoclonal antibodies to cholera toxin with special reference to cross-reactions with Escherichia coli heat-labile enterotoxin. Infect Immun. 1983 May;40(2):570–576. doi: 10.1128/iai.40.2.570-576.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchlewicz B. A., Finkelstein R. A. Immunological differences among the cholera/coli family of enterotoxins. Diagn Microbiol Infect Dis. 1983 Jun;1(2):129–138. doi: 10.1016/0732-8893(83)90042-1. [DOI] [PubMed] [Google Scholar]
- Moss J., Vaughan M. Activation of adenylate cyclase by choleragen. Annu Rev Biochem. 1979;48:581–600. doi: 10.1146/annurev.bi.48.070179.003053. [DOI] [PubMed] [Google Scholar]
- Takeda Y., Honda T., Sima H., Tsuji T., Miwatani T. Analysis of antigenic determinants in cholera enterotoxin and heat-labile enterotoxins from human and porcine enterotoxigenic Escherichia coli. Infect Immun. 1983 Jul;41(1):50–53. doi: 10.1128/iai.41.1.50-53.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Tamura T., Yokota T. Primary structure of heat-labile enterotoxin produced by Escherichia coli pathogenic for humans. J Biol Chem. 1984 Apr 25;259(8):5037–5044. [PubMed] [Google Scholar]



