Abstract
Objective:
We hypothesized that elevated parathyroid hormone (PTH) levels will be independently associated with 20-year cognitive decline in a large population-based cohort.
Methods:
We studied 12,964 middle-aged white and black ARIC participants without a history of prior stroke who, in 1990–1992 (baseline), had serum PTH levels measured and cognitive function testing, with repeat cognitive testing performed at up to 2 follow-up visits. Cognitive testing included the Delayed Word Recall, the Digit Symbol Substitution, and the Word Fluency tests, which were summed as a global Z score. Using mixed-effects models, we compared the relative decline in individual and global cognitive scores between each of the top 3 quartiles of PTH levels to the reference bottom quartile. We adjusted for demographic variables, education, vascular risk factors, and levels of calcium, phosphate, and vitamin D. We imputed missing covariate and follow-up cognitive data to account for attrition.
Results:
The mean (SD) age of our cohort was 57 (6) years, 57% were women, and 24% were black. There was no cross-sectional association of elevated PTH with cognitive global Z score at baseline (p > 0.05). Over a median of 20.7 years, participants in each PTH quartile showed a decline in cognitive function. However, there was no significant difference in cognitive decline between each of the top 3 quartiles and the lowest reference quartile (p > 0.05). In a subset, there was also no association of higher mid-life PTH levels with late-life prevalent adjudicated dementia (p > 0.05).
Conclusions:
Our work does not support an independent influence of PTH on cognitive decline in this population-based cohort study.
Elevated parathyroid hormone (PTH) levels may play a role in cognitive impairment, given that PTH receptors are present in the cerebral arteries.1,2 Prior work suggests that PTH may contribute to cerebrovascular diseases through endothelial dysfunction, atherosclerosis, and inflammation.3–7
Most prior studies examining the relationship between primary hyperparathyroidism and cognition have focused largely on short-term change in cognition after parathyroidectomy; limited data exist linking primary hyperparathyroidism with long-term dementia.8,9 Furthermore, whether more modest levels of PTH elevation are a cause of cognitive decline remains uncertain. A large systematic review examining the relationship between PTH and cognitive function from 27 studies (including only 1 randomized and prospective study) found only low-quality evidence for a weak link between PTH and cognition.9 In addition, none of these studies considered the effect of 25-hydroxyvitamin D [25(OH)D] in the PTH-cognition relationship. 25(OH)D has been independently linked to cognition in some studies,10,11 but not in others,12,13 and may be confounded by poorer health status.12 Therefore, there is a need to better examine the relationship of PTH and cognition, particularly in a prospective setting, with and without accounting for the influences of 25(OH)D.
We hypothesized that elevated PTH levels would be independently associated with cognitive decline in a population-based study. Using data from the Atherosclerosis Risk in Communities (ARIC) study,14 we examined the cross-sectional and longitudinal associations of PTH with cognitive function after adjustment for confounding and potential mediating variables, such as demographic and education, vascular risk factors, renal function, calcium, phosphate, and 25(OH)D.
METHODS
Population.
The ARIC study is a continuing, prospective, predominantly biracial, population-based cohort, which originally recruited 15,792 men and women aged 45–64 years from 4 US regions (suburban Minneapolis, MN; Washington County, MD; Forsyth County, NC; and Jackson, MS) during the years of 1987–1989 (visit 1).14
Initial cognitive evaluation and PTH measurement were performed at visit 2, which took place during 1990–1992; therefore, visit 2 represents the baseline for the current analysis. Participants participated in up to 2 repeat cognitive evaluations that were performed at visit 4 (conducted in 1996–1998) and at visit 5 (conducted in 2011–2013).
Visit 2 included 14,348 participants. We excluded nonwhites from Maryland or Minnesota (n = 49) and participants of nonwhite or nonblack race (n = 42), as numbers were too small to adjust for race/center groups. Participants who self-reported a prior stroke at visit 1 or had an incident adjudicated stroke before visit 2 were excluded (n = 272). Also, participants with missing PTH values due to insufficient stored serum (n = 975) or with extreme outlier values of either very low (<10 pg/mL) or very high (≥200 pg/mL) PTH (n = 24) were excluded. We also excluded those participants missing information on education status (n = 22). This gave a final analytic sample of 12,964 participants shown in figure e-1 at Neurology.org. The baseline characteristics of included vs excluded participants are shown in table e-1. Excluded participants had on average higher PTH levels, lower 25(OH)D levels, lower cognitive scores, and worse cardiovascular disease (CVD) risk profiles, as might be anticipated in this group enriched with prior stroke.
Standard protocol approvals, registrations, and patient consents.
The ARIC study was approved by the institutional review boards at each of the field centers, as well as the ARIC Coordinating Center. All participants provided written informed consent at each visit. Clinical trial registration is not applicable.
Laboratory tests.
At each visit, blood samples were obtained according to standard protocols and were then stored at −70°C. In 2012–2013, using stored samples from visit 2, we quantified serum intact PTH levels using Elecsys 2010 (Roche Diagnostics, Indianapolis, IN) at the Advanced Research and Diagnostic Laboratory, University of Minnesota.15 PTH measured by the Elecsys method is known to have very good long-term stability at −80°C.16 Comparing stored duplicate (split specimen) samples, we found the laboratory coefficient of variation (CV) to be 9.7% for PTH. We measured visit 2 calcium and phosphate levels by a colorimetric method through a Roche Modular P-Chemistry Analyzer (Roche Diagnostics). The interassay CV for calcium was 2.3% and for phosphate was 2.2%.
Also in 2012–2013, we measured 25(OH)D levels from stored visit 2 serum using liquid chromatography-tandem mass spectrometry (Waters Alliance e2795; Waters Corp., Milford, MA) at the University of Minnesota laboratory. The CV for 25(OH)D2 was 20.8% and for 25(OH)D3 was 6.9%. The total 25(OH)D level included the summation of 25(OH)D2 and 25(OH)D3. We adjusted 25(OH)D levels based on the month of blood draw, for each race separately, to account for variations in season.17
Lipid data at visit 2 were measured according to standard procedures.14 Plasma total cholesterol was measured by enzymatic approach, and high-density lipoprotein (HDL) cholesterol was determined after dextran-magnesium precipitation.
Cognitive function.
Using standardized protocols administered by trained examiners in a silent room, cognitive function was assessed using the Delayed Word Recall (DWR), Digit Symbol Substitution (DSS), and Word Fluency (WF) tests at visit 2 (1990–1992), visit 4 (1996–1998), and visit 5 (2011–2013).
The DWR tests verbal skills and short-term memory.18 Individuals learn 10 nouns and then must use them in 1 or 2 sentences. After 5 minutes of delay, they must repeat the nouns. The score represents the number of words the participant is able to remember with a maximum of 10. In a control group of similarly aged healthy ARIC participants, mean DWR scores varied from 5.2 to 6.7 based on race and educational background.19 The DSS tests for executive function and processing speed.20 Using a key, participants try to match symbols that correspond to numbers in 90 seconds with scores varying from 0 to 93 for each number of correct symbols. The ARIC normative controls had mean values ranging from 20.3 to 48.2.19 The WF appraises expressive language and executive function.21 Participants produce as many words that start with A, F, and S as possible in less than 1 minute. The total score represents the total words generated, with ARIC normative mean values varying from 19.4 to 39.5.19
A global Z score was calculated by the average of the Z scores from each of the 3 individual cognitive tests within each study visit (at visits 2, 4, and 5) and standardized using the visit 2 global Z-mean and SD. The global Z score was used as the primary outcome for our study.
In addition, participants of the ARIC Neurocognitive Study underwent comprehensive neuropsychological testing at visit 5,22 and the diagnosis of prevalent clinical dementia was adjudicated by 1 physician and 1 neuropsychologist based on the longitudinal cognitive testing performed at visits 2, 4, and 5, and the complete neurocognitive battery of tests performed at visit 5.22
Covariates.
Covariates were assessed using standardized question forms and interviews to obtain medication use, demographic, and behavioral factors.14 For this analysis, we used covariates measured at visit 2 except for physical activity and education, which were evaluated at visit 1.
In our models, we included age; sex; race/center (Minnesota whites, Maryland whites, North Carolina whites, North Carolina blacks, and Mississippi blacks); education attainment (<high school, high school, General Educational Development or vocational school, or college, graduate, or professional school); body mass index (BMI) (<25, 25 to <30, ≥30 kg/m2); diabetes mellitus (yes, no; defined as fasting glucose ≥126 mg/dL or nonfasting glucose ≥200 mg/dL, self-reported physician diagnosis, or use of oral hypoglycemic/insulin); self-reported history of alcohol use (current vs not current); smoking status (never, former, current); total cholesterol and HDL cholesterol (continuous; mg/dL); systolic and diastolic blood pressures (continuous; mm Hg); use of antihypertensive medications (yes, no); coronary heart disease (CHD) (yes, no, defined by standardized criteria and physician adjudication), physical activity (score range 1–5, using a modified Baecke Physical Activity Questionnaire23), and estimated glomerular filtration rate (eGFR) as calculated by the Chronic Kidney Disease-Epidemiology Collaboration (CKD-EPI) formula.24
Statistical analysis.
We grouped PTH levels into quartiles based on the overall population distribution at visit 2. Multivariable-adjusted linear regression models were used to examine cross-sectional differences in global cognitive scores between each of the top 3 quartiles of PTH concentration relative to the reference bottom quartile. To allow for a nonlinear association between PTH levels with cognitive function (assessed at visit 2), we used a restricted cubic spline model with an elevated PTH level of 65 pg/mL as the reference (the upper limit of normal used in clinical settings) and with knots at the 5th, 35th, 65th, and 95th percentiles of their sample distributions using model 2.
We found previously that those with elevated PTH levels had increased CVD risk factors and were less likely to return for the follow-up study.25 Therefore, for our prospective analysis, we imputed missing covariates and cognitive data with the multiple imputation by chained equation methods26 to account for attrition (see table e-2 for numbers imputed).
For prospective analyses, we used linear mixed-effects models with random intercepts and slopes and independent variance-covariance structure of the random effects for continuous outcomes to estimate the association between PTH quartiles and change in cognition over 20 years. We compared the relative decrease for the 3 individual and the global cognitive scores between each of the top 3 quartiles of PTH levels with the reference bottom quartile. Time in study was modeled using a linear spline with a knot at 6 years (approximately at the time of visit 4). Our primary coefficient of interest was the interaction of PTH with time spline terms.
For both cross-sectional and prospective analyses, we analyzed 4 progressively adjusted models as follows: model 1 adjusted for the demographics of age, race/center, and sex. Model 2 (our primary analytical model) additionally adjusted for behavioral and lifestyle confounders, including education, BMI, smoking, current alcohol use, and physical activity. Model 3 additionally adjusted for cardiovascular risk factors of systolic and diastolic blood pressures, use of antihypertensive medications, diabetes, total and HDL cholesterol, cholesterol-lowering medications, eGFR, and prevalent CHD. Model 4 additionally adjusted for biomarkers in the metabolic pathway, including 25(OH)D, calcium, and phosphate levels.
We formally tested for interaction by race; however, given the known racial differences in PTH concentrations,15 we planned ahead to show the overall results and those separated by race, regardless of whether a race interaction was present.
We performed several supplemental analyses. As the risk of elevated PTH might correlate better with a shorter-term decline rather than a longer-term decline as noted in a prior study,27 we assessed the association of PTH quartiles with 6-year cognitive decline between visits 2 and 4. We also used logistic regression to assess the relationship between PTH (at visit 2) and prevalent clinical dementia (at visit 5).
A 2-sided p value ≤0.05 was considered statistically significant. All statistical studies were analyzed using Stata version 12 (StataCorp, College Station, TX).
RESULTS
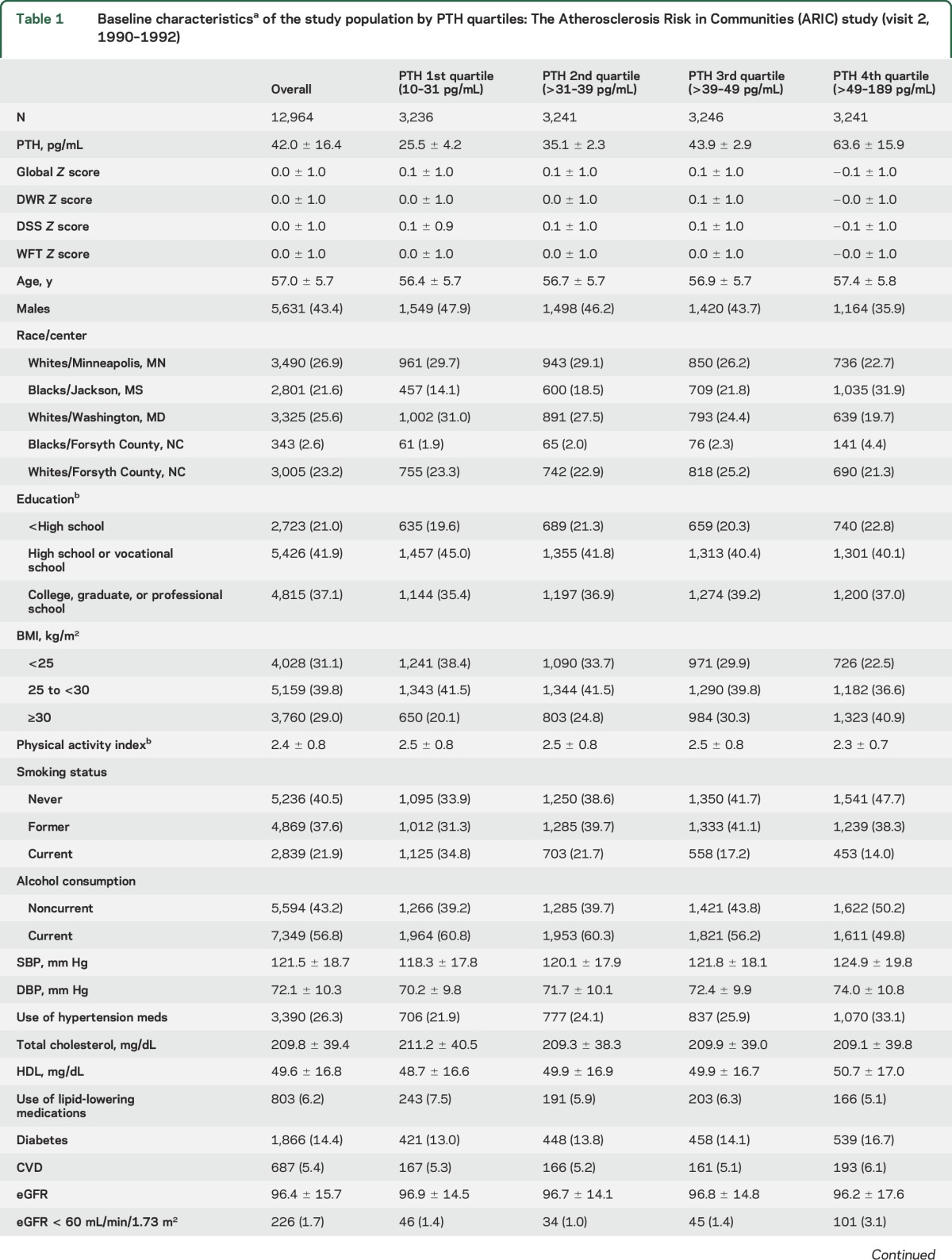
The baseline characteristics of the 12,964 participants by PTH quartiles are shown in table 1. The mean (SD) age was 57 (6) years, 57% were women, and 24% were black. The mean (SD) and median (25th–75th percentile) of PTH were 42.0 ± 16.4 and 39.2 (31.1–49.4) pg/mL, respectively. Those in the highest PTH quartile (63.6 ± 15.9 pg/mL) were somewhat older, were more likely to be women and black, and have lower cognitive Z scores, lower education, lower physical activity levels, higher blood pressures, higher prevalence of obesity, diabetes, and CKD (eGFR <60), and lower 25(OH)D and phosphate levels (all p < 0.05).
Table 1.
Baseline characteristicsa of the study population by PTH quartiles: The Atherosclerosis Risk in Communities (ARIC) study (visit 2, 1990–1992)
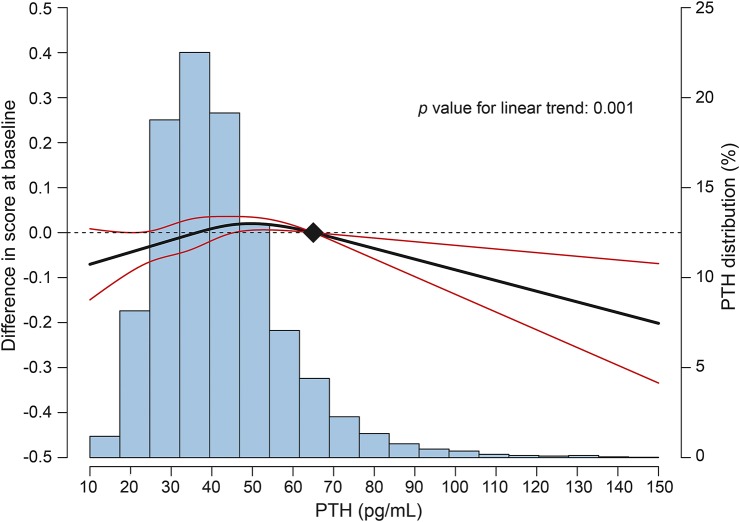
In a cross-sectional analysis, there were no statistically significant associations of higher PTH levels (quartiles 2–4) compared with the referent lowest quartile with a global cognitive score at baseline in any of the 4 models tested (p > 0.05 for all, data not shown). Figure 1 shows the multivariable-adjusted cross-sectional dose-response relationship of continuous PTH levels with a global cognitive score at visit 2. This spline model shows lower average cognitive scores for those with very elevated PTH, but there was also evidence for a possible U-shaped relationship with lower cognitive scores noted at low PTH values. Therefore, in sensitivity analyses, we repeated the cross-sectional quartile analysis using quartile 2 as the referent quartile, but there were still no statistically significant associations found.
Figure 1. Cross-sectional differences in global Z combination scores by parathyroid hormone (PTH) levels at visit 2.
Curves represent adjusted differences in the global Z combination score (black line) and their 95% confidence intervals (red lines) based on restricted cubic splines for PTH levels with knots at the 5th, 35th, 65th and 95th percentiles of their sample distributions. The reference value (filled diamond) was set at a clinically relevant threshold of 65 pg/mL. The model was adjusted for age, race/center, sex, education, smoking status, alcohol, body mass index, and physical activity (model 2).
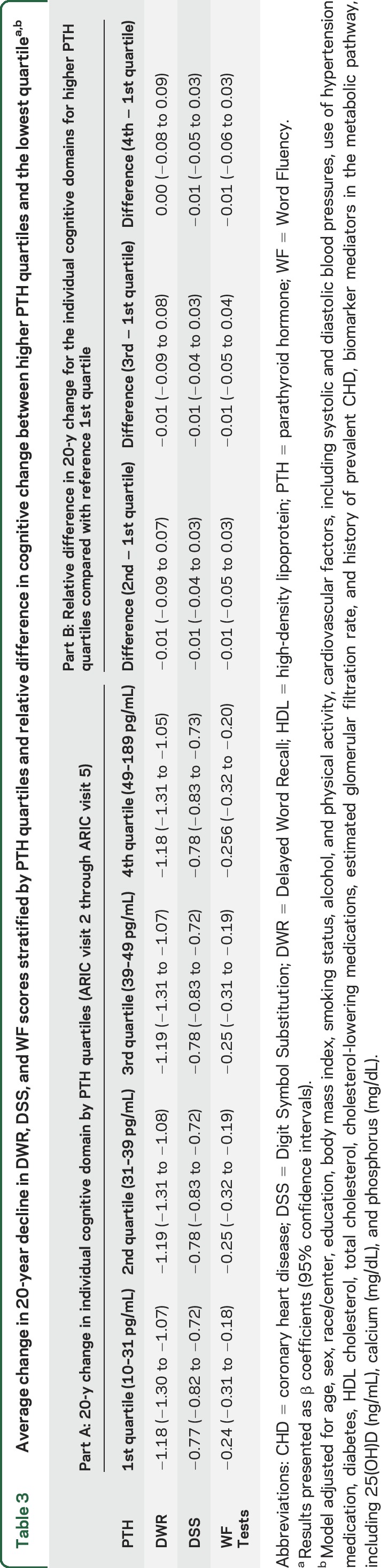
Over a median of 20.7 years, participants in all PTH quartiles showed a decline in global cognitive function from visits 2 through 5, with Z scores ranging from −0.94 to −0.96 after adjustment for lifestyle modifiers and demographics (model 2, table 2). However, global cognitive decline was not steeper in participants with PTH levels in the higher quartiles compared with participants in the lowest quartile. Findings were unaltered after further adjustment for cardiovascular risk factors, renal function, and markers of mineral metabolism. There was also no statistically significant difference between PTH quartiles for each of the 3 individual cognitive domains (model 4, table 3). There was no significant interaction by race; the race-stratified results are presented in table e-3.
Table 2.
Average change in 20-year global cognitive Z scoresa stratified by PTH quartiles and relative difference in cognitive change between higher PTH quartiles and lowest quartile
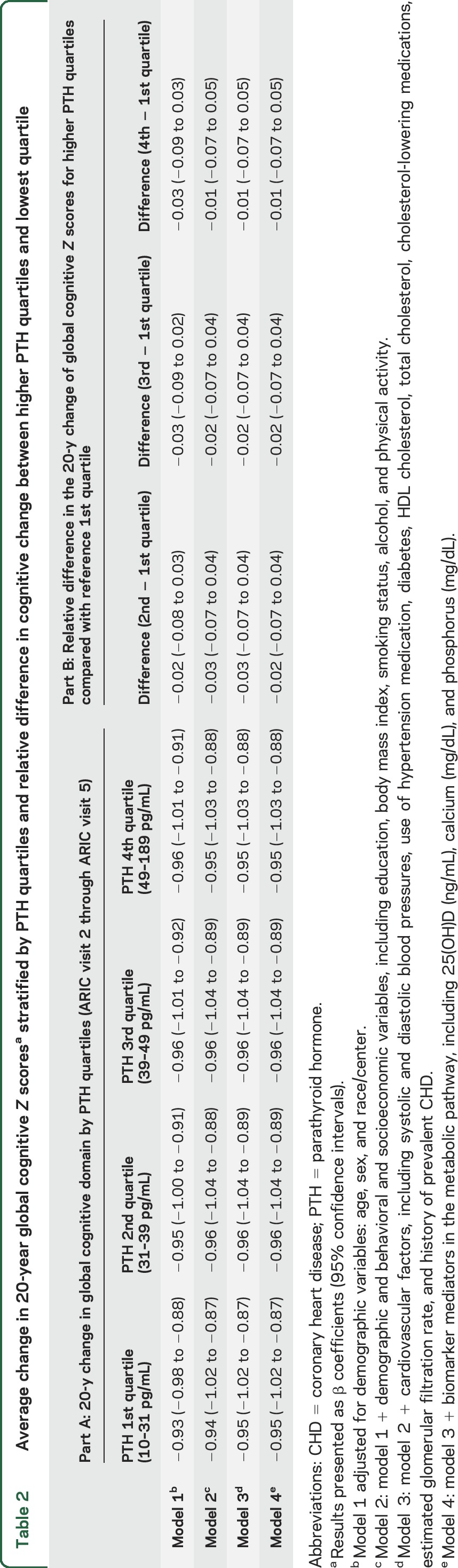
Table 3.
Average change in 20-year decline in DWR, DSS, and WF scores stratified by PTH quartiles and relative difference in cognitive change between higher PTH quartiles and the lowest quartilea,b
In sensitivity analysis, we also examined a short-term decline between visits 2 and 4. Elevated PTH levels were also not associated with a relatively steeper decline in global Z scores compared with lower PTH levels over a short term ∼6-year period (table e-4).
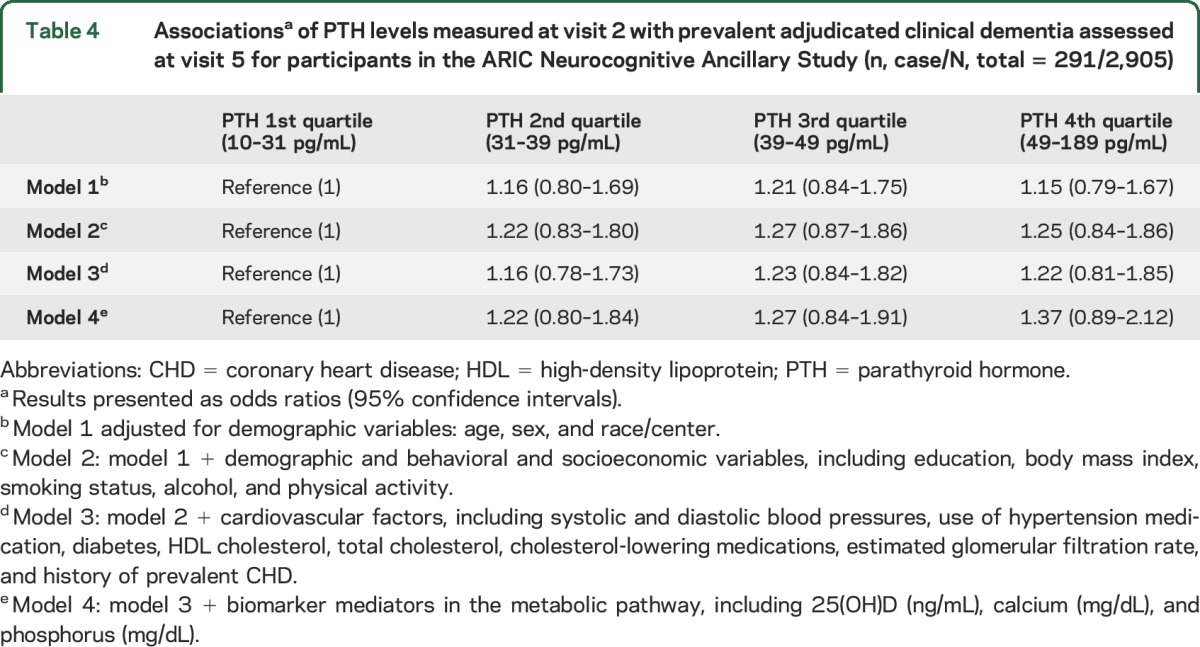
We also examined the relationship between PTH levels (visit 2) and the presence of clinical adjudicated dementia at visit 5. The odds ratios comparing the higher PTH quartiles to the reference quartile were not statistically significant in all the models tested (table 4).
Table 4.
Associationsa of PTH levels measured at visit 2 with prevalent adjudicated clinical dementia assessed at visit 5 for participants in the ARIC Neurocognitive Ancillary Study (n, case/N, total = 291/2,905)
DISCUSSION
In this large biracial population-based study, there was a decline in average cognitive function over 20 years. However, cognitive decline did not differ according to the baseline PTH concentration. In addition, in cross-sectional analyses and analyses with a shorter (6 year) follow-up, there were also no associations between PTH and cognition. Our work does not support an independent influence of PTH on short- or long-term cognitive decline in the general population.
Elevated PTH levels are considered to play a role in cognitive impairment, potentially via increased vascular calcification, elevated blood pressures, or endothelial dysfunction.4,6 However, the prior literature evaluating the association between PTH and cognitive function has considerable heterogeneity between study populations, and most of the literature studies were cross-sectional, which limits causal inference.9
Our results contrast with the one other prospective study that followed a smaller (n = 514) and much older cohort of adults (aged 75–85 years) for 1, 5, and 10 years. In that study, the authors did find an association between PTH and cognitive decline, but did not adjust for education, cardiovascular risk factors, or 25(OH)D levels.27 In our analyses, using a much larger cohort of younger individuals (mean age 57 years), we were able to consider numerous confounding and potential mediating variables, but did not find evidence for any associations. Also, in the prior study, elevated PTH levels were only associated with shorter 5-year cognitive decline but not with 10-year cognitive decline, although their sample size was greatly reduced by the time of their 10-year follow-up.27 We also examined a shorter time frame in a sensitivity analysis, but we did not discover a significant relationship between elevated PTH levels and 6-year cognitive decline.
Our results are similar to previous null results from the ARIC Brain MRI Study which showed that PTH was not independently associated with subclinical brain infarcts or white matter hyperintensities on brain MRI,25 which are indicators for subclinical cerebrovascular diseases and linked with reduced cognitive function.28 Our findings were also in accordance with another ARIC study that did not show a significant association between PTH and clinical cardiovascular events.15 These collective data suggest that perhaps elevated PTH levels may not independently contribute to the development of cognitive impairment.
Two relevant factors associated with PTH levels in cross-sectional analyses were 25(OH)D and obesity. A previous ARIC study evaluating the relationship between midlife metabolic syndrome with cognitive decline found modest associations for the metabolic syndrome and some of its individual components such as increased waist circumference.29 However, our prior work in ARIC did not find 25(OH)D to be independently associated with either 10-year or 20-year cognitive decline,12,13 or with subclinical cerebrovascular disease on brain MRI.30 In our current analyses, we adjusted for 25(OH)D levels and BMI/physical activity, but we still found no association of PTH with cognitive decline after considering these factors.
It is worth noting that the range of PTH in this study largely included PTH in the normal ranges with only a small fraction of patients with very elevated or depressed PTH. Sensitivity analyses comparing elevated PTH ≥65 vs <65 pg/mL were also null in our analysis. It is a possibility that PTH in huge excess can still induce cerebrovascular disease and subsequent dementia as demonstrated by certain animal models linking PTH excess with enhanced release of vasopressin and vasoconstriction.31
The major limitation of this study was attrition of participants over follow-up, which was higher among those with an elevated PTH who had increased CVD risk factors. In addition, those with poorer baseline cognitive function were also less likely to return. This differential loss to follow-up could potentially bias toward a null hypothesis, but we attempted to account for this by using imputation models. Another limitation was that we only had a single measure of PTH at the baseline examination and could not examine influences of changes in PTH levels over time. Also the prevalence of advanced CKD was low in our community-based cohort, so although we adjusted for renal function, we could not examine associations within this specific subgroup. CKD is associated with higher PTH levels, and the association between PTH and cognition may be different among those with significant renal failure. Finally, although our study sample is the largest population-based study that we are aware of examining the relationship between PTH and cognitive function, it may still be underpowered for prospective analysis. However, if any associations are present, the effect size is likely to be small.
The major strengths of our analyses are that, to date, this is the largest prospective cohort study that has analyzed the relationship between mid-life PTH levels and long-term cognitive decline, with rigorous adjustment for potentially confounding variables of demographics, education, behaviors, and lifestyle. Although our primary analytical model was null, we also included models adjusted for potential mediators, including CVD risk factors, renal function, and serum markers of bone mineral metabolism, such as 25(OH)D, calcium, and phosphate, which other studies have not done.
In summary, we did not find a significant independent association between elevated PTH levels and cognitive function or dementia. Our results challenge those of prior studies that have purported a causal relationship between elevated PTH and reduced cognitive function.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the staff and the participants of the ARIC study for their vital contributions.
GLOSSARY
- ARIC
Atherosclerosis Risk in Communities
- BMI
body mass index
- CHD
coronary heart disease
- CKD
chronic kidney disease
- CV
coefficient of variation
- CVD
cardiovascular disease
- DSS
Digit Symbol Substitution
- DWR
Delayed Word Recall
- eGFR
estimated glomerular filtration rate
- HDL
high-density lipoprotein
- PTH
parathyroid hormone
- WF
Word Fluency
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Dr. Kim and Dr. Michos designed the research. Dr. Zhao analyzed the data under the supervision of Dr. Guallar and Dr. Michos. Dr. Lutsey obtained funding for the PTH measurement. Dr. Kim wrote the first draft of the manuscript. Dr. Zhao, Dr. Schneider, Dr. Korada, Dr. Lutsey, Dr. Guallar, Dr. Alonso, Dr. Windham, Dr. Gottesman, and Dr. Michos reviewed the manuscript and provided critical scientific input. Dr. Kim and Dr. Michos had main responsibility for the final content of the manuscript. All the authors approved the final draft of the manuscript.
STUDY FUNDING
This work was supported by grants from NIH/National Institute of Neurological Disorders and Stroke (R01NS072243 to Dr. Michos), the NIH/NHLBI (R01HL103706 to Dr. Lutsey), and the NIH Office of Dietary Supplements (R01HL103706-S1 to Dr. Lutsey). The Atherosclerosis Risk in Communities Study is a collaborative research supported by the National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). ARIC-NCS was funded by U01 HL096812, HL096814, HL096899, HL096902, and HL096917, with additional support from the National Institute of Neurological Disorders and Stroke. Dr. Michos and Dr. Zhao are also supported by the Blumenthal Scholars Fund for Preventive Cardiology research.
DISCLOSURE
S.M. Kim, D. Zhao, A.L.C. Schneider, S.K. Korada, P.L. Lutsey, E. Guallar, A. Alonso, and B.G. Windham report no disclosures relevant to the manuscript. R.F. Gottesman is an Associate Editor for Neurology®. E.D. Michos reports receiving an honorarium from Siemens Healthcare Diagnostics for event adjudication in a clinical trial unrelated to this work. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Macdonald RL, Zhang ZD, Ono S, Komuro T. Up-regulation of parathyroid hormone receptor in cerebral arteries after subarachnoid hemorrhage in monkeys. Neurosurgery 2002;50:1083–1091; discussion 1091–1093. [DOI] [PubMed] [Google Scholar]
- 2.Usdin TB, Gruber C, Bonner TI. Identification and functional expression of a receptor selectively recognizing parathyroid hormone, the PTH2 receptor. J Biol Chem 1995;270:15455–15458. [DOI] [PubMed] [Google Scholar]
- 3.van Ballegooijen AJ, Kestenbaum B, Sachs MC, et al. Association of 25-hydroxyvitamin D and parathyroid hormone with incident hypertension: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol 2014;63:1214–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bosworth C, Sachs MC, Duprez D, et al. Parathyroid hormone and arterial dysfunction in the multi-ethnic study of atherosclerosis. Clin Endocrinol (Oxf) 2013;79:429–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hagstrom E, Hellman P, Larsson TE, et al. Plasma parathyroid hormone and the risk of cardiovascular mortality in the community. Circulation 2009;119:2765–2771. [DOI] [PubMed] [Google Scholar]
- 6.Hagstrom E, Ahlstrom T, Arnlov J, et al. Parathyroid hormone and calcium are independently associated with subclinical vascular disease in a community-based cohort. Atherosclerosis 2015;238:420–426. [DOI] [PubMed] [Google Scholar]
- 7.Hendy GN, Canaff L. Calcium-sensing receptor, proinflammatory cytokines and calcium homeostasis. Semin Cell Dev Biol 2016;49:37–43. [DOI] [PubMed] [Google Scholar]
- 8.Dotzenrath CM, Kaetsch AK, Pfingsten H, et al. Neuropsychiatric and cognitive changes after surgery for primary hyperparathyroidism. World J Surg 2006;30:680–685. [DOI] [PubMed] [Google Scholar]
- 9.Lourida I, Thompson-Coon J, Dickens CM, et al. Parathyroid hormone, cognitive function and dementia: a systematic review. PLoS One 2015;10:e0127574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Annweiler C, Dursun E, Feron F, et al. Vitamin D and cognition in older adults: international consensus guidelines. Geriatr Psychol Neuropsychiatr Vieil 2016;14:265–273. [DOI] [PubMed] [Google Scholar]
- 11.Landel V, Annweiler C, Millet P, Morello M, Feron F. Vitamin D, cognition and Alzheimer's disease: the therapeutic benefit is in the D-Tails. J Alzheimers Dis 2016;53:419–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schneider AL, Lutsey PL, Alonso A, et al. Vitamin D and cognitive function and dementia risk in a biracial cohort: the ARIC Brain MRI Study. Eur J Neurol 2014;21:1211–1218, e1269–e1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schneider AL, Zhao D, Lutsey PL, et al. Abstract 14260: mid-life vitamin D levels are not associated with 20-year cognitive decline in whites and blacks: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation 2016;134:A14260. [Google Scholar]
- 14.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol 1989;129:687–702. [PubMed] [Google Scholar]
- 15.Folsom AR, Alonso A, Misialek JR, et al. Parathyroid hormone concentration and risk of cardiovascular diseases: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J 2014;168:296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cavalier E, Delanaye P, Hubert P, Krzesinski JM, Chapelle JP, Rozet E. Estimation of the stability of parathyroid hormone when stored at −80 degrees C for a long period. Clin J Am Soc Nephrol 2009;4:1988–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McKibben RA, Zhao D, Lutsey PL, et al. Factors associated with change in 25-hydroxyvitamin D levels over longitudinal follow-up in the ARIC study. J Clin Endocrinol Metab 2015;101:33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knopman DS, Ryberg S. A verbal memory test with high predictive accuracy for dementia of the Alzheimer type. Arch Neurol 1989;46:141–145. [DOI] [PubMed] [Google Scholar]
- 19.Schneider AL, Sharrett AR, Gottesman RF, et al. Normative data for 8 neuropsychological tests in older blacks and whites from the atherosclerosis risk in communities (ARIC) study. Alzheimer Dis Assoc Disord 2015;29:32–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sudarshan NJ, Bowden SC, Saklofske DH, Weiss LG. Age-related invariance of abilities measured with the Wechsler adult intelligence scale-IV. Psychol Assess 2016;28:1489–1501. [DOI] [PubMed] [Google Scholar]
- 21.Benton AL, Hamsher KD, Sivan AB. Multilingual Aphasia Examination: Manual of Instructions. Iowa City: AJA Assoc; 1994. [Google Scholar]
- 22.Knopman DS, Gottesman RF, Sharrett AR, et al. Mild cognitive impairment and dementia prevalence: the Atherosclerosis Risk in Communities Neurocognitive Study (ARIC-NCS). Alzheimers Dement (Amst) 2016;2:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr 1982;36:936–942. [DOI] [PubMed] [Google Scholar]
- 24.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 2012;367:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Korada SK, Zhao D, Gottesman RF, et al. Parathyroid hormone and subclinical cerebrovascular disease: the Atherosclerosis Risk in Communities Brain Magnetic Resonance Imaging Study. J Stroke Cerebrovasc Dis 2016;25:883–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377–399. [DOI] [PubMed] [Google Scholar]
- 27.Bjorkman MP, Sorva AJ, Tilvis RS. Does elevated parathyroid hormone concentration predict cognitive decline in older people? Aging Clin Exp Res 2010;22:164–169. [DOI] [PubMed] [Google Scholar]
- 28.Mosley TH Jr, Knopman DS, Catellier DJ, et al. Cerebral MRI findings and cognitive functioning: the Atherosclerosis Risk in Communities study. Neurology 2005;64:2056–2062. [DOI] [PubMed] [Google Scholar]
- 29.Dearborn JL, Knopman D, Sharrett AR, et al. The metabolic syndrome and cognitive decline in the Atherosclerosis Risk in Communities study (ARIC). Dement Geriatr Cogn Disord 2014;38:337–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Michos ED, Carson KA, Schneider AL, et al. Vitamin D and subclinical cerebrovascular disease: the Atherosclerosis Risk in Communities brain magnetic resonance imaging study. JAMA Neurol 2014;71:863–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khudaverdian DN, Asratian AA. Effects of parathormone on 45Ca2+ accumulation in neurosecretory cells and contents of vasopressin in blood after administration of parathyroidin in parathyroid gland insufficiency [in Russian]. Biull Eksp Biol Med 1992;113:230–232. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.